Abstract
Sirtuins compose a family of NAD+-dependent deacetylases and/or ADP-ribosyltransferases, which have been implicated in aging, metabolism and tolerance to oxidative stress. Many of the biological processes regulated by sirtuins result from the adaptation of complex gene-expression programs to the energetic state of the cell, sensed through NAD+ levels. To that respect, sirtuins, and particularly the founding member of the family Sirt1, have emerged as important regulators of transcription, which they modulate both positively and negatively by targeting histones and transcriptional complex regulatory proteins. This review will focus on recent advances that have started deciphering how mammalian sirtuins regulate transcriptional networks and thereby control physiology.
In mammals, sirtuins (Sirt) constitute a family of energy sensors which mediate NAD-dependent deacetylation and/or O-ADP-ribosylation in response to a rise in the cellular NAD+/NADH ratio (Fig. 1; see [1-3] for review). Sirt1, the founding member of the family, was initially discovered in yeast, where it is named Sir2 and regulates gene silencing and longevity. While these functions extend to higher eukaryotes, sirtuins have emerged as broader integrators of mammalian physiology, which regulate metabolism and homeostasis by coordinating complex gene expression programs through deacetylation of histones, transcription factors and coregulators (Fig. 2). This review will focus on the implication of sirtuins in transcriptional regulation, but it should be noted that sirtuins can also impact on whole-body homeostasis, independently of transcriptional regulation, by directly modulating the activity of enzymes or structural proteins [1-3].
Figure 1. Sirtuins catalyze deacetylation and/or O-ADP-ribosylation reactions.
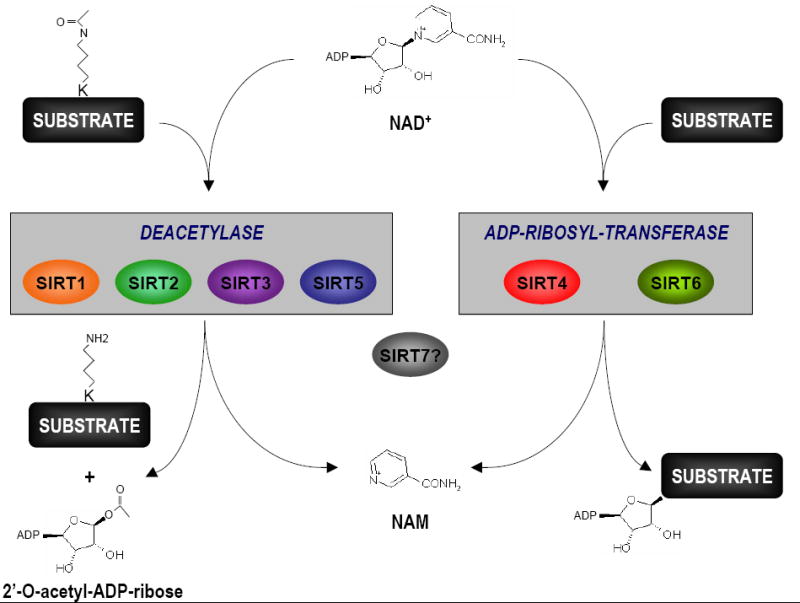
The two enzymatic reactions catalyzed by sirtuins use nicotinamide-adenine-dinucleotide (NAD+) as a cofactor and produce nicotinamide (NAM). In the deacetylation reaction, the acetyl group of a target lysine residue is transferred to the ADP-ribose moiety of NAD+ to generate 2’-O-acetyl-ADP-ribose. Sirtuins can also catalyze the mono ADP-ribosylation of a non-acetylated protein substrate by transferring the ADP-ribose moiety of NAD+ to the target protein. The principal reaction of each sirtuin is depicted but it should be noted that most sirtuins harbor both activities to different degrees. For example, Sirt6 also has a weak deacetylase activity while Sirt1, Sirt2 and Sirt3 can catalyze O-ADP-ribosylation. The reactions catalyzed by Sirt7 remain to be determined.
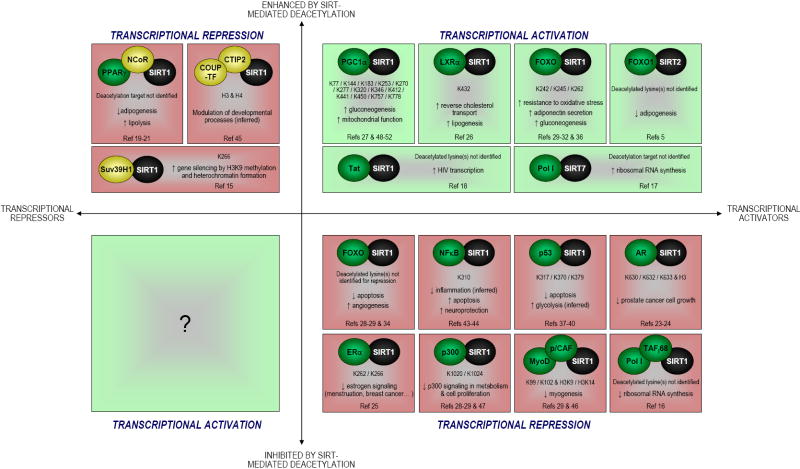
Figure 2. Transcriptional targets of sirtuins.
The main molecular mechanisms through which sirtuins regulate gene expression are depicted according to the nature of the transcriptional regulator interacting with sirtuins, and to the action that sirtuins exert on the activity of this factor. Transcriptional activation mediated by sirtuins (indicated by green boxes) can result both from the enhanced activity of a transcriptional activator and from the inhibition of a repressor, although examples of this latter case have not been documented to date. In contrast, Sirt-mediated transcriptional repression (indicated by red boxes) is caused both by enhanced repressor activity and from the inhibition of an activator. Abbreviations are PPAR: peroxisome proliferator-activated receptor; NCoR: nuclear corepressor; COUP-TF: chicken ovalbumin upstream promoter transcription factor; CTIP2: COUP-TF-interacting protein 2; Suv39H1: suppressor of variegation 3–9 homologue 1; PGC-1α: PPARγ coactivator 1 α; LXR: liver X receptor; FOXO: Forkhead Box class O; Tat: transactivator; Pol I: RNA polymerase I; NFκB: nuclear factor κ B; AR: androgen receptor; ER: estrogen receptor; MyoD: myoblast determination protein 1; p/CAF: p300/CBP-associated factor; TAFI68: TATA box binding protein-associated factor I 68.
Sub-cellular localization of sirtuins
The duplication of sirtuin genes in higher eukaryotes has been associated with a divergence of the sub-cellular localization of the proteins they encode, to fulfill specialized functions. The localization of sirtuins within the cell determines therefore the action that these proteins can exert on transcription, which occurs predominantly in the nucleus. Consistent with a strong role in the regulation of chromatin structure and gene expression, Sirt1, Sirt6 and Sirt7 are nuclear proteins, which are enriched in the nucleoplasm, in heterochromatin and in nucleoli, respectively [4]. Sirt2 is predominantly cytoplasmic, but it can affect gene expression by deacetylating transcription factors which shuttle from the cytoplasm to the nucleus [5], and it can impact on chromatin structure upon disassembly of the cell nucleus during mitosis [6]. In contrast, Sirt3, Sirt4 and Sirt5 are predominantly mitochondrial proteins [4,7,8], and could therefore modulate the transcription of the mitochondrial genome. Since Sirt3 induces global deacetylation of mitochondrial proteins [9], it is possible that also mitochondrial transcription factors are targeted. The static view of sirtuin compartmentalization should, however, be taken with some caution considering that regulatory proteins often dynamically exchange between cellular compartments. This has been recently illustrated by the nucleo-cytoplasmic shuttling of Sirt1 [10], and the observation that Sirt3 can be present in the nucleus and translocate to mitochondria in response to cellular stress [11].
Sirtuins and histone modifications
The complex post-translational modifications (PTM) of histone tails, commonly referred to as the histone code, regulate gene expression by modulating the compaction and the epigenetic state of chromatin [12]. As acetylation of histones strongly correlates with active chromatin, which facilitates transcription, it is logical that the histone deacetylase (HDAC) activity of the sirtuins has been linked to gene silencing. The yeast Sirt1 homologue Sir2 associates with inactive telomeric chromatin and silences the transcription of ribosomal DNA and of mating-type loci [1]. In mammals, only Sirt1-Sirt3 and Sirt5 have a conserved deacetylase domain [1,2], and HDAC activity seems restricted to Sirt1-Sirt3. Sirt1 can affect the acetylation of the four core histones in vitro, but seems to preferentially deacetylate histone 3 on lysines 9 and 14 (H3K9 and H3K14) and histone 4 on lysine 16 (H4K16) [13,14]. This direct histone deacetylation activity of Sirt1 synergizes with facilititated tri-methylation of H3K9 [13], a well established mark of facultative heterochromatin and transcriptional repression. The mechanistic basis of the positive action of Sirt1 on H3K9 methylation to further repress transcription involves the activation of the histone methyltransferase Suv39H1 by Sirt1-mediated deacetylation [15].
Despite its cytoplasmic localization, Sirt2 can deacetylate H4K16, and to a lesser extent H3K9, during mitosis when the nuclear envelope disassembles [6]. These results suggest, therefore, that Sirt2 could promote cell cycle progression by favoring the condensation of chromatin prior to chromosome segregation during mitosis. Finally, Sirt3 also exhibits HDAC activity directed towards H3K9 and H4K16 in vitro [11]. The normal pattern of histone acetylation of Sirt3-deficient cells suggests, however, that the deacetylase activity of Sirt3, if relevant in vivo, is most probably restricted to a small proportion of chromatin which remains undetectable at the genome wide level [11]. Altogether, these studies have demonstrated that histones are targets of sirtuin-mediated deacetylation facilitating heterochromatin formation. Further work will, however, be required to understand how the promoter-specific deacetylation of histones by sirtuins can selectively favor the transcriptional silencing of given loci in mammals.
Sirtuins and the basal transcriptional machinery
Sirtuins are major regulators of RNA polymerase (Pol) II transcribed genes encoding messenger RNAs, which they regulate either negatively or positively by deacetylating histones (see above) and transcription factors and coregulators (see below). To our knowledge, sirtuins have until now, however, not been implicated in the regulation of transcriptional initiation by the Pol II basal transcriptional machinery. Transcriptional regulation by sirtuins, however, affects Pol I-mediated transcription of ribosomal RNAs (rRNAs). Sirt1 inhibits Pol I transcription by deacetylating the TATA box-binding protein-associated factor TAFI68 [16]. In contrast, the nucleolar Sirt7 stimulates rRNA transcription by directly interacting with Pol I and stimulating its activity [17]. Finally, sirtuins also regulate viral transcription. Sirt1-Sirt3 can deacetylate the human immunodeficiency virus (HIV) 1 transactivator Tat, thereby promoting viral transcription [18]. Interestingly, the observation that Sirt1 inhibitors can reduce HIV transcription suggests that Sirt1 antagonists could prove useful to combat viral infection.
Transcription factors and coregulators as targets of sirtuins
Nuclear receptors (NRs)
Several NRs are regulated by acetylation and Sirt1-mediated deacetylation plays an important role in adapting the activity of NRs implicated in the maintenance of whole body homeostasis, to the cellular energetic status that is sensed through NAD+ levels. By promoting transcriptional repression by the NR corepressor NCoR, Sirt1 inhibits adipocyte differentiation and adiponectin secretion, two processes controlled by the Peroxisome Proliferator-Activated Receptor γ (PPARγ) [19,20]. Interestingly, the action of Sirt1 in adipocytes seems restricted to a subset of PPARγ targets [21], which could potentially result from the selective regulation of PPARγ by corepressors. The glucocorticoid-mediated activation of the uncoupling protein 3 (UCP3) promoter is also inhibited by Sirt1, which prevents acetylation of histones 3 and 4 [22]. The observation that the Sirt1-mediated repression of glucocorticoid action is restricted to certain promoters suggests that the action of Sirt1 results from promoter-specific epigenetic events rather than from a deacetylation of the glucocorticoid receptor itself. The androgen receptor is another NR whose activity is directly inhibited by Sirt1-mediated deacetylation [23,24]. Sirt1 also impedes the transcriptional activity of the estrogen receptor α (Erα), by inhibiting its binding to target DNA after deacetylation of lysines 266 and 268 [25]. Interestingly, many NRs have conserved lysine residues in the proximity of their DNA-binding domain [25], suggesting that regulation by acetylation could be a hallmark of the family.
Sirt1-mediated deacetylation can also stimulate the activity of NRs. Sirt1 regulates cholesterol homeostasis by deacetylating the lysine 432 of the liver X receptor (LXR), which subsequently induces the ubiquitination, destabilization and hence activation of LXR [26]. This direct regulation of LXR most likely synergizes with increased coactivation of LXRs by the PPARγ Coactivator 1α (PGC-1α) [27], which is also activated by Sirt1-mediated deacetylation (see below).
Forkhead Box class O (FOXO) transcription factors
Sirtuins can modulate the activity of at least three of the four mammalian FOXOs to regulate cell survival and metabolism. Sirt1 deacetylates FOXO 1, 3 and 4, resulting in most cases in the repression of FOXO-mediated transcription [28-32]. Deacetylation by Sirt1 requires the LXXLL motif of FOXO1 [33], and transcriptional repression of FOXO by Sirt1 synergizes with the LIM domain FOXO1 corepressor FHL2 [34]. Altered FOXO signaling by Sirt1-dependent deacetylation inhibits forkhead-dependent apoptosis [28,29] and promotes vascular growth by reducing the anti-angiogenic actions of FOXO1 [35]. In some cases, however, Sirt1 can stimulate FOXO activity. This is for example the case for FOXO1, following deacetylation of lysines 242, 245 and 262 [30], or for a subset of FOXO3 and FOXO4 genes controlling resistance to oxidative stress [29,31]. Sirt1-dependent activation of FOXO1 has important metabolic consequences such as the stimulation of adiponectin production in adipocytes [36] and the induction of gluconeogenic genes in hepatocytes [32].
FOXO signaling relies on a nucleo-cytoplasmic shuttling mechanism where phosphorylation reduces FOXO transcriptional activity by inducing its cytoplasmic retention. Post-transtional modifications often occur in an inter-dependent manner and emerging evidence suggests that acetylation can impact on protein phosphorylation. While Sirt1 most likely cross-talks with FOXO signaling when this last transcription factor is localized in the nucleus, the predominantly cytoplasmic Sirt2 can regulate FOXO signaling from the cytoplasm by affecting its state of phosphorylation. By interacting with and deacetylating FOXO1 in adipocytes, Sirt2 inhibits the insulin/akt-dependent phosphorylation of FOXO1 [5]. This leads subsequently to the activation of FOXO1 signaling by inducing its nuclear accumulation, thereby inhibiting adipogenesis.
p53
Sirt1 is a well recognized regulator of p53 activity, which it represses by the direct deacetylation of lysines 317, 370 and 379 [37]. Sirt1-mediated deacetylation inhibits p53-dependent apoptosis in response to DNA damage and oxidative stress [37-39]. At the molecular level, this regulation relies, at least in part, on the recruitment of Sirt1 to promyelocytic leukemia (PML) bodies, a sub-compartment of the nucleus where p53 is enriched [40]. p53 has also recently emerged as a metabolic regulator, which among other functions, inhibits glycolysis and promotes oxidative metabolism through the induction of the TP53-induced glycolysis and apoptosis regulator (TIGAR) and of the synthesis of cytochrome c oxidase 2 (SCO2) protein [41]. It is therefore worth exploring whether Sirt1 can affect metabolic homeostasis by inhibiting the metabolic actions of p53.
Other transcription factors
Sirt1 also exerts anti-apoptotic functions by deacetylating and inhibiting the pro-apoptotic factors p73 and E2F1 and by preventing Bax-dependent apoptosis through Ku70 deacetylation (see [1] for review). In addition, the deacetylation of SMAD7 by Sirt1 promotes its ubiquitination and degradation and thereby inhibits TGFβ-dependent apoptosis [42].
Sirt1 also antagonizes inflammatory mechanisms by inhibiting NF-κB activity through the deacetylation of p65/RelA on lysine 310 [43]. Interestingly, this action of Sirt1 on NF-κB signaling promotes apoptosis induced by TNFα [43], and inhibits the neurotoxicity of amyloid β peptides [44].
Coregulators
As outlined above, Sirt1 interacts with NCoR and SMRT to repress PPARγ activity [19]. It is, however, currently unclear whether corepressors are directly deacetylated by Sirt1 or whether they merely cooperate with Sirt1 to repress PPARγ-dependent gene expression through histone deacetylation. In addition, it remains to be determined whether the interplay of Sirt1 with these pleiotropic corepressors extends to pathways regulated by other transcription factors. Similarly, transcriptional repression by the COUP-TF corepressor, CTIP2, is also enhanced by Sirt1-mediated deacetylation of histones 3 and 4 [45].
Sirt1 also represses gene expression by inhibiting the activity of a number of coactivators. Sirt1 interacts with the acetyltransferases pCAF and GCN5 to prevent their auto-acetylation as well as that of the muscle-specific transcription factor MyoD [29,46], which was shown to inhibit skeletal muscle differentiation [46]. Another example is the coactivator p300, which is inhibited by Sirt1-dependent deacetylation of lysine residues 1020 and 1024 [28,29,47], most probably because these residues become available for SUMOylation following deacetylation [47].
Interestingly, sirtuins have recently also emerged as positive regulators of gene expression. Perhaps one of the best known examples of this is provided by the activation of the PPARγ coactivator 1α (PGC-1α), the master regulator of mitochondrial function which strongly impacts metabolic homeostasis [48]. During fasting, the hepatic stimulation of Sirt1 activity by elevated NAD+ levels promotes the transcriptional activity of PGC-1α through the direct deacetylation of 13 lysine residues. This Sirt1-dependent deacetylation and activation of PGC-1α contributes to the stimulation of gluconeogenesis and subsequent restoration of glucose levels after fasting [27,49]. The activation of PGC-1α by Sirt1-mediated deacetylation also extends to gene expression programs controlling mitochondrial oxidative functions, which are particularly prominent in skeletal muscle, but occur also in liver and brown adipose tissue [50-52]. The pharmacological activation of Sirt1 with natural or synthetic Sirt1 agonists hence could provide an effective mean to combat diet-induced metabolic disorders by indirectly activating PGC-1α-regulated energy expenditure [51-53]. Finally, Sirt3 can stimulate PGC-1α expression to promote mitochondrial function and adaptive thermogenesis in brown adipose tissue [54].
Concluding remarks
Over the past four years, sirtuins, and especially Sirt1, have emerged as important regulators of mammalian transcription and physiology by targeting and modulating the activity of both histones and additional components of transcriptional complexes. Although the deacetylase activity of sirtuins underlies many of these regulatory actions, future research efforts will undoubtedly reveal novel mechanisms through which the sirtuins integrate complex physiological pathways. To that respect, particular attention should be given to regulations controlled by the often under-looked sirtuins Sirt2 to Sirt7. Given the possibility to pharmacologically target the enzymatic activities of sirtuins and the promise this holds in the treatment of metabolic and age-related diseases [51-53], it will also be of great importance to understand the subtle mechanisms which dictate the selectivity of sirtuin action. Towards that goal, the characterization of the tissue-specific activities of sirtuins as well as of the modulation of their enzymatic activities through their own post-translational modification, such as phosphorylation or SUMOylation [55-57], will provide a way to integrate the action of these transcriptional regulators in broader signaling networks. In addition, the recent identification of a Sirt1 coactivator which enhances its deacetylase activity also opens these networks to additional regulation [58].
Acknowledgments
We apologize to colleagues whose work could not be cited because of space limitations and thank members of the Auwerx lab for stimulating discussions. Work in the authors’ laboratory was supported by grants from CNRS, INSERM, ULP, Hôpital Universitaire de Strasbourg, ARC, FRM, AFM, EU and NIH. JNF is supported by a FEBS fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Mol Endocrinol. 2007;21:1745–1755. doi: 10.1210/me.2007-0079. [DOI] [PubMed] [Google Scholar]
- 3.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 4.Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jing E, Gesta S, Kahn CR. SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metab. 2007;6:105–114. doi: 10.1016/j.cmet.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaquero A, Scher MB, Lee DH, Sutton A, Cheng HL, Alt FW, Serrano L, Sternglanz R, Reinberg D. SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev. 2006;20:1256–1261. doi: 10.1101/gad.1412706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwer B, North BJ, Frye RA, Ott M, Verdin E. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J Cell Biol. 2002;158:647–657. doi: 10.1083/jcb.200205057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci U S A. 2002;99:13653–13658. doi: 10.1073/pnas.222538099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- 11.Scher MB, Vaquero A, Reinberg D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev. 2007;21:920–928. doi: 10.1101/gad.1527307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 13.Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell. 2004;16:93–105. doi: 10.1016/j.molcel.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 15.Vaquero A, Scher M, Erdjument-Bromage H, Tempst P, Serrano L, Reinberg D. SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature. 2007;450:440–444. doi: 10.1038/nature06268. [DOI] [PubMed] [Google Scholar]
- 16.Muth V, Nadaud S, Grummt I, Voit R. Acetylation of TAF(I)68, a subunit of TIF-IB/SL1, activates RNA polymerase I transcription. Embo J. 2001;20:1353–1362. doi: 10.1093/emboj/20.6.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford E, Voit R, Liszt G, Magin C, Grummt I, Guarente L. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 2006;20:1075–1080. doi: 10.1101/gad.1399706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pagans S, Pedal A, North BJ, Kaehlcke K, Marshall BL, Dorr A, Hetzer-Egger C, Henklein P, Frye R, McBurney MW, et al. SIRT1 regulates HIV transcription via Tat deacetylation. PLoS Biol. 2005;3:e41. doi: 10.1371/journal.pbio.0030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha. Mol Cell Biol. 2007;27:4698–4707. doi: 10.1128/MCB.02279-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Qiang L, Farmer SR. Identification of a domain within PPAR{gamma} regulating expression of a group of genes containing FGF21 that are selectively repressed by SIRT1 in adipocytes. Mol Cell Biol. 2008;28:188–200. doi: 10.1128/MCB.00992-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amat R, Solanes G, Giralt M, Villarroya F. SIRT1 Is Involved in Glucocorticoid-mediated Control of Uncoupling Protein-3 Gene Transcription. J Biol Chem. 2007;282:34066–34076. doi: 10.1074/jbc.M707114200. [DOI] [PubMed] [Google Scholar]
- 23.Fu M, Liu M, Sauve AA, Jiao X, Zhang X, Wu X, Powell MJ, Yang T, Gu W, Avantaggiati ML, et al. Hormonal control of androgen receptor function through SIRT1. Mol Cell Biol. 2006;26:8122–8135. doi: 10.1128/MCB.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai Y, Ngo D, Forman LW, Qin DC, Jacob J, Faller DV. Sirtuin 1 is required for antagonist-induced transcriptional repression of androgen-responsive genes by the androgen receptor. Mol Endocrinol. 2007;21:1807–1821. doi: 10.1210/me.2006-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MY, Woo EM, Chong YT, Homenko DR, Kraus WL. Acetylation of estrogen receptor alpha by p300 at lysines 266 and 268 enhances the deoxyribonucleic acid binding and transactivation activities of the receptor. Mol Endocrinol. 2006;20:1479–1493. doi: 10.1210/me.2005-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 27.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 29.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 30.Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci U S A. 2004;101:10042–10047. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1) J Biol Chem. 2004;279:28873–28879. doi: 10.1074/jbc.M401138200. [DOI] [PubMed] [Google Scholar]
- 32.Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280:20589–20595. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
- 33.Nakae J, Cao Y, Daitoku H, Fukamizu A, Ogawa W, Yano Y, Hayashi Y. The LXXLL motif of murine forkhead transcription factor FoxO1 mediates Sirt1-dependent transcriptional activity. J Clin Invest. 2006;116:2473–2483. doi: 10.1172/JCI25518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Hou H, Haller EM, Nicosia SV, Bai W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. Embo J. 2005;24:1021–1032. doi: 10.1038/sj.emboj.7600570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem. 2006;281:39915–39924. doi: 10.1074/jbc.M607215200. [DOI] [PubMed] [Google Scholar]
- 37.Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 39.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 40.Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, Minucci S, Pelicci PG, Kouzarides T. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. Embo J. 2002;21:2383–2396. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bensaad K, Vousden KH. p53: new roles in metabolism. Trends Cell Biol. 2007;17:286–291. doi: 10.1016/j.tcb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Kume S, Haneda M, Kanasaki K, Sugimoto T, Araki S, Isshiki K, Isono M, Uzu T, Guarente L, Kashiwagi A, et al. SIRT1 inhibits transforming growth factor beta-induced apoptosis in glomerular mesangial cells via Smad7 deacetylation. J Biol Chem. 2007;282:151–158. doi: 10.1074/jbc.M605904200. [DOI] [PubMed] [Google Scholar]
- 43.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. Embo J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 45.Senawong T, Peterson VJ, Avram D, Shepherd DM, Frye RA, Minucci S, Leid M. Involvement of the histone deacetylase SIRT1 in chicken ovalbumin upstream promoter transcription factor (COUP-TF)-interacting protein 2-mediated transcriptional repression. J Biol Chem. 2003;278:43041–43050. doi: 10.1074/jbc.M307477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- 47.Bouras T, Fu M, Sauve AA, Wang F, Quong AA, Perkins ND, Hay RT, Gu W, Pestell RG. SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J Biol Chem. 2005;280:10264–10276. doi: 10.1074/jbc.M408748200. [DOI] [PubMed] [Google Scholar]
- 48.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1alpha and SIRT1 pathways. FEBS Lett. 2007 doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 50.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. Embo J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 52.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005;280:13560–13567. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- 55.Dryden SC, Nahhas FA, Nowak JE, Goustin AS, Tainsky MA. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol. 2003;23:3173–3185. doi: 10.1128/MCB.23.9.3173-3185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.North BJ, Verdin E. Mitotic regulation of SIRT2 by cyclin-dependent kinase 1-dependent phosphorylation. J Biol Chem. 2007;282:19546–19555. doi: 10.1074/jbc.M702990200. [DOI] [PubMed] [Google Scholar]
- 57.Yang Y, Fu W, Chen J, Olashaw N, Zhang X, Nicosia SV, Bhalla K, Bai W. SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol. 2007;9:1253–1262. doi: 10.1038/ncb1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007;28:277–290. doi: 10.1016/j.molcel.2007.08.030. [DOI] [PubMed] [Google Scholar]