Abstract
Smac mimetics (inhibitor of apoptosis [IAP] antagonists) are synthetic reagents that kill susceptible tumor cells by inducing degradation of cellular IAP (cIAP) 1 and cIAP2, nuclear factor κB activation, tumor necrosis factor (TNF) α production, TNF receptor 1 occupancy, and caspase-8 activation. In this issue of The Journal of Cell Biology, Vince et al. (see p. 171) report remarkable similarities in the events leading to tumor cell death triggered by the cytokine TWEAK (TNF-like weak inducer of apoptosis) and IAP antagonists. Although the mechanistic details differ, a common and necessary feature that is also shared by TNF receptor 2 signaling is reduction in the level of cIAP1 and, in some cases, cIAP2 and TNF receptor-associated factor 2. These findings not only extend our appreciation of how cell death pathways are kept in check in tumors, they reinforce the possible utility of induced cIDE (cIAP deficiency) in the selective elimination of neoplastic cells.
The TNF receptor superfamily (TNFRSF) comprises ∼30 genes whose products are Type I transmembrane glycoproteins (Ware, 2003). Receptor occupancy by the corresponding TNF-like ligands affects a host of biological processes, including innate and adaptive immunity, cell death versus survival (homeostasis), and lymphoid development. Receptor engagement results in the assembly of signaling complexes that include enzymes that are also adaptors, one example of which is TNF receptor-associated factor 2 (TRAF2), a RING-containing ubiquitin protein ligase (E3) that recruits cellular inhibitors of apoptosis (cIAP) 1 and 2 to the signaling complex (Rothe et al., 1995). cIAP1 and cIAP2 themselves are RING-containing E3s (Yang et al., 2000), and the interplay between these two protein families and other molecules in the receptor complex is critical in the propagation of downstream signals.
It has recently been appreciated that acute down-regulation of cIAPs is fatal for some tumor cell lines. Smac/DIABLO is a mitochondrial protein that, in stressed cells, is released into the cytosol and binds BIR (Baculovirus IAP repeat) domains of IAPs such as XIAP, displacing active caspases (Shiozaki and Shi, 2004). Based on the notion that the freed caspases would kill the cell, several groups have created IAP antagonists: small peptides or compounds that, like Smac, bind IAPs and interfere with caspase interaction. Although the IAP antagonists indeed did kill some tumor cell lines, the mechanism of action was completely unexpected (Gaither et al., 2007; Petersen et al., 2007; Varfolomeev et al., 2007; Vince et al., 2007). In sensitive cells, the IAP antagonists caused autoubiquitination and proteasomal degradation of cIAP1 and cIAP2 rather than the expected target, XIAP. Acute reduction in these IAPs activated both the canonical and noncanonical NF-κB pathways and caused autocrine TNFα production, which in turn led to TNFR1 occupancy, caspase-8 activation, and cell death. Because most cells lines are insensitive to killing by TNFα in the absence of a sensitizing agent such as cycloheximide, it was a logical inference that the IAP antagonists must also confer sensitization to exogenous TNFα, which proved to be the case.
TNF-like weak inducer of apoptosis (TWEAK; TNFSF12) was described in 1997 (Chicheportiche et al., 1997), and its receptor, the non–death domain–containing TNFRSF member FGF-inducible 14-kD protein (FN14), in 2001 (Wiley et al., 2001). FN14 occupancy leads to TRAF2-dependent activation of MAP kinases and the canonical and noncanonical NF-κB pathways (Saitoh et al., 2003). Like many TNF family members, TWEAK has roles that vary among tissues, including enhancement of proliferation, cell migration, expression and secretion of proinflammatory molecules, and proangiogenic activity (Wiley and Winkles, 2003). As its name suggests, TWEAK was originally found to be a rather poor cytotoxic agent that killed a restricted number of tumor cell lines, usually in conjunction with a sensitizing agent. It has been difficult to pigeonhole a mechanism of action because cell death is mediated by different pathways in different cell lines, with the mechanisms including caspase-dependent apoptosis, cathepsin B–dependent necrosis, and indirect induction of apoptosis by induction of autocrine TNFα production (Schneider et al., 1999; Nakayama et al., 2003).
In this issue of The Journal of Cell Biology, Vince et al. (see p. 171) explore the consequences of TWEAK-FN14 signaling in human tumor cells and find some unexpected similarities (as well as key differences) between TWEAK and IAP antagonists. Their initial observation was that a high proportion of tumor cell lines express FN14. Consistent with previous yeast two-hybrid and GST-FN14 pulldown studies, pulldown of FN14 with a TWEAK-Fc receptor fusion protein also brought down TRAF2 and cIAP1 from tumor cell lysates, the latter probably brought into the complex by TRAF2. Things began to get interesting with the observation that TWEAK signaling caused a gradual reduction of cIAP1, as previously noted (Varfolomeev et al., 2007), and TRAF2, and although both molecules have E3 activity neither were rescued by proteasome inhibitors. Rather, lysosomal inhibitors such as chloroquine, ammonium chloride, and a selective cathepsin B inhibitor prevented TWEAK-induced TRAF2 and, to a lesser extent, cIAP1 degradation. This was sufficiently reminiscent of the behavior of IAP antagonists to ask if TWEAK-induced NF-κB activation is a consequence of cIAP1–TRAF2 loss. Several lines of evidence suggest that it is. First, both cIAP1- and TRAF2-deficient transformed MEFs had low constitutive levels of cytoplasmic p100 and high levels of nuclear p52 and higher levels of phosphorylated IκB and p65, indicating activation of noncanonical and canonical NF-κB pathways, respectively. In agreement with the IAP antagonist data, the enhanced noncanonical pathway was caused by stabilization of the p100-phosphorylating kinase NIK. How the canonical pathway was activated is unclear, but if the analogy with IAP antagonists holds, it may be caused by recruitment of RIP to preassembled TNF-R1 (TNF receptor 1) trimers (Chan et al., 2000; Vince et al., 2007). Second, enforced expression of TRAF2 and cIAP1, but neither alone, attenuated TWEAK-induced NF-κB activation. Further establishing a link with IAP antagonists, TWEAK stimulation of susceptible tumor cells caused NF-κB–dependent TNFα production and TNF-R1/caspase-8–dependent death, and TWEAK sensitized both long-term tumor lines and early-passage primary human tumors to killing by exogenous TNFα. Primary cells were resistant to TWEAK/TNFα cytotoxicity despite the fact that cIAP1–TRAF2 levels were reduced and NF-κB was activated just as in transformed cells.
Therefore, despite differences in the mechanistic details, there are remarkable similarities between stimulation with TWEAK and with IAP antagonists. One leitmotif is that the activation of NF-κB pathways and TNFα production stems from a reduction in cIAP1 and, in some cases, cIAP2 and TRAF2, a condition that can be termed cIAP deficiency (cIDE). Another is that cIDE sensitizes tumor cells to the cytotoxic effects of TNFα alone. It is interesting in this light to reconsider the observation that TNF-R2 signals for cIAP1 autoubiquitination and ubiquitination of TRAF2, their proteasome-mediated degradation, and sensitization of otherwise resistant cells to TNFα alone (Li et al., 2002). Prevention of autoubiquitination with an E3-defective cIAP1 mutant inhibited TNFα-induced TRAF2 degradation and cell death, demonstrating that in fact self-induced loss of cIAP1 and, perhaps, TRAF2 is proapoptotic. Reinforcing the similarities between these three stimuli, ligation of TNF-R2 (as well as the TNFRSF members CD30 and CD40) caused autocrine TNFα production and TNF-R1–dependent death in a tumor cell line also susceptible to IAP antagonists and TWEAK (Grell et al., 1999).
The following is a simplified framework that deals with elements common to signaling by IAP antagonists, TWEAK/FN14, TNFα/TNF-R2, and perhaps other TNFRSF members (Fig. 1). In this model, cIDE activates the canonical NF-κB pathway by allowing RIP to associate with preassembled TNF-R complexes, and the noncanonical NF-κB pathway by up-regulating NIK, which, as a target of cIAP1 and cIAP2 ubiquitination/degradation (Varfolomeev et al., 2007), is stabilized by their loss. TNFα is produced as a consequence of NF-κB activation (and presumably other signals) and binds TNF-R1. cIDE renders cells sensitive to TNFα alone because it facilitates release of RIP from the liganded TNF-R1 and the formation of an active and c-FLIP–resistant RIP–FADD–caspase-8 complex (Wang et al., 2008). Given that CYLD-mediated deubiquitination of RIP seems to be important for this process, it is possible that cIAPs antagonize RIP release by countering deubiquitination or that they ubiquitinate and target RIP for destruction and, thus, a reduction in cIAP levels would enhance the formation of the death initiator complex. It remains puzzling why primary cells, despite TWEAK-induced loss of cIAP1, are resistant to TNFα. Another question is, how are cIAP1 and TRAF2 marked for lysosomal destruction? The fact that proteasomes are not involved does not rule out ubiquitination as the flag because ubiquitination can target proteins to lysosomes (Urbe, 2005). As with all surprising and provocative observations, there are many more questions than answers, but the emerging theme that down-regulating cIAP levels has profound effects on tumor cell survival may ultimately prove to be profoundly useful.
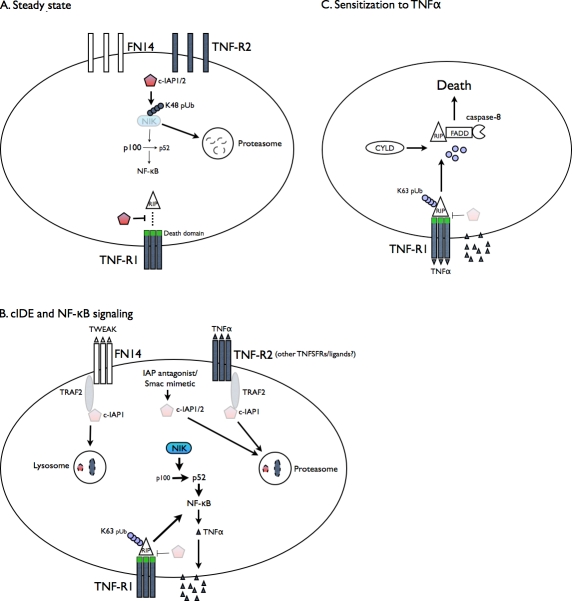
Figure 1.
cIDE signaling: pathways leading to and emanating from cIAP reduction in sensitive tumor cells. (A) Under steady-state conditions, cIAP1/2 ubiquitinates NIK with K48-linked chains, maintaining it and noncanonical NF-κB signaling at low levels. cIAP1/2 also prevents spontaneous association of RIP with preexisting TNF-R1–assembled trimers. The ability of death domainless TNFRSF members like CD30 and CD40 to kill by cIDE has not been directly examined but is indirectly supported by the finding that CD30 signaling can cause TRAF2 degradation (Duckett and Thompson, 1997). (B) Stimulation results in cIAP autoubiquitination and degradation in either proteasomes (TNFα/TNF-R2 or IAP antagonists) or lysosomes (TWEAK/FN14). TRAF2 is also degraded after receptor-mediated but not IAP antagonist stimulation. This results in NIK stabilization and increases in noncanonical NF-κB activation and recruitment of RIP to TNF-R1 leading to canonical NF-κB activation. TNFα is produced and exported to the membrane and secreted. (C) TNFα binds to and signals via TNF-R1. In the absence of cIAP, receptor-associated RIP is deubiquitinated by CYLD and does not gain K48-linked polyubiquitin, leaves the receptor, and assembles a death-initiating complex with FADD and caspase-8.
Abbreviations used in this paper: cIAP, cellular inhibitors of apoptosis; cIDE, cIAP deficiency; TNFRSF, TNF receptor superfamily; TRAF2, TNF receptor-associated factor 2; TWEAK, TNF-like weak inducer of apoptosis.
References
- Chan, F.K., H.J. Chun, L. Zheng, R.M. Siegel, K.L. Bui, and M.J. Lenardo. 2000. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 288:2351–2354. [DOI] [PubMed] [Google Scholar]
- Chicheportiche, Y., P.R. Bourdon, H. Xu, Y.M. Hsu, H. Scott, C. Hession, I. Garcia, and J.L. Browning. 1997. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J. Biol. Chem. 272:32401–32410. [DOI] [PubMed] [Google Scholar]
- Duckett, C.S., and C.B. Thompson. 1997. CD30-dependent degradation of TRAF2: implications for negative regulation of TRAF signaling and the control of cell survival. Genes Dev. 11:2810–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaither, A., D. Porter, Y. Yao, J. Borawski, G. Yang, J. Donovan, D. Sage, J. Slisz, M. Tran, C. Straub, et al. 2007. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res. 67:11493–11498. [DOI] [PubMed] [Google Scholar]
- Grell, M., G. Zimmermann, E. Gottfried, C.M. Chen, U. Grunwald, D.C. Huang, Y.H. Wu Lee, H. Durkop, H. Engelmann, P. Scheurich, et al. 1999. Induction of cell death by tumour necrosis factor (TNF) receptor 2, CD40 and CD30: a role for TNF-R1 activation by endogenous membrane-anchored TNF. EMBO J. 18:3034–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Y. Yang, and J.D. Ashwell. 2002. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 416:345–347. [DOI] [PubMed] [Google Scholar]
- Nakayama, M., K. Ishidoh, Y. Kojima, N. Harada, E. Kominami, K. Okumura, and H. Yagita. 2003. Fibroblast growth factor-inducible 14 mediates multiple pathways of TWEAK-induced cell death. J. Immunol. 170:341–348. [DOI] [PubMed] [Google Scholar]
- Petersen, S.L., L. Wang, A. Yalcin-Chin, L. Li, M. Peyton, J. Minna, P. Harran, and X. Wang. 2007. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 12:445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe, M., M.G. Pan, W.J. Henzel, T.M. Ayres, and D.V. Goeddel. 1995. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 83:1243–1252. [DOI] [PubMed] [Google Scholar]
- Saitoh, T., M. Nakayama, H. Nakano, H. Yagita, N. Yamamoto, and S. Yamaoka. 2003. TWEAK induces NF-kappaB2 p100 processing and long lasting NF-kappaB activation. J. Biol. Chem. 278:36005–36012. [DOI] [PubMed] [Google Scholar]
- Schneider, P., R. Schwenzer, E. Haas, F. Muhlenbeck, G. Schubert, P. Scheurich, J. Tschopp, and H. Wajant. 1999. TWEAK can induce cell death via endogenous TNF and TNF receptor 1. Eur. J. Immunol. 29:1785–1792. [DOI] [PubMed] [Google Scholar]
- Shiozaki, E.N., and Y. Shi. 2004. Caspases, IAPs and Smac/DIABLO: mechanisms from structural biology. Trends Biochem. Sci. 29:486–494. [DOI] [PubMed] [Google Scholar]
- Urbe, S. 2005. Ubiquitin and endocytic protein sorting. Essays Biochem. 41:81–98. [DOI] [PubMed] [Google Scholar]
- Varfolomeev, E., J.W. Blankenship, S.M. Wayson, A.V. Fedorova, N. Kayagaki, P. Garg, K. Zobel, J.N. Dynek, L.O. Elliott, H.J. Wallweber, et al. 2007. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 131:669–681. [DOI] [PubMed] [Google Scholar]
- Vince, J.E., D. Chau, B. Callus, W.W.L. Wong, C.J. Hawkins, P. Schneider, M. McKinlay, C.A. Benetatos, S.M. Condon, S.K. Chunduru, et al. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1/TRAF2 complex to sensitize tumor cells to TNFα. J. Cell Biol. 182:171–184. [DOI] [PMC free article] [PubMed]
- Vince, J.E., W.W. Wong, N. Khan, R. Feltham, D. Chau, A.U. Ahmed, C.A. Benetatos, S.K. Chunduru, S.M. Condon, M. McKinlay, et al. 2007. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 131:682–693. [DOI] [PubMed] [Google Scholar]
- Wang, L., F. Du, and X. Wang. 2008. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 133:693–703. [DOI] [PubMed] [Google Scholar]
- Ware, C.F. 2003. The TNF superfamily. Cytokine Growth Factor Rev. 14:181–184. [DOI] [PubMed] [Google Scholar]
- Wiley, S.R., L. Cassiano, T. Lofton, T. Davis-Smith, J.A. Winkles, V. Lindner, H. Liu, T.O. Daniel, C.A. Smith, and W.C. Fanslow. 2001. A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity. 15:837–846. [DOI] [PubMed] [Google Scholar]
- Wiley, S.R., and J.A. Winkles. 2003. TWEAK, a member of the TNF superfamily, is a multifunctional cytokine that binds the TweakR/Fn14 receptor. Cytokine Growth Factor Rev. 14:241–249. [DOI] [PubMed] [Google Scholar]
- Yang, Y., S. Fang, J.P. Jensen, A.M. Weissman, and J.D. Ashwell. 2000. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science. 288:874–877. [DOI] [PubMed] [Google Scholar]