Abstract
Background
Difficulties in defining and characterizing phenotypes has hindered progress in psychiatric genetics and clinical neuroscience. Decreased approach-related behavior and anhedonia (lack of responsiveness to pleasure) are considered cardinal features of depression, but few studies have employed laboratory-based measures to objectively characterize these constructs.
Methods
To assess hedonic capacity in relation to depressive, particularly anhedonic, symptoms, 62 participants completed a signal-detection task based on a differential reinforcement schedule. Anhedonia was operationalized as decreased reward responsiveness.
Results
Unequal frequency of reward between two correct responses produced a response bias, i.e., a systematic preference to identify the stimulus paired with the more frequent reward. Subjects with elevated depressive symptoms (Beck Depression Inventory scores ≥ 16) failed to show a response bias. Impaired reward responsiveness predicted higher anhedonic symptoms one month later after controlling for general negative affectivity.
Conclusions
Impaired tendency to modulate behavior as a function of prior reinforcements may underline diminished hedonic capacity in depression. When applied to a clinical population, objective assessments of participants’ propensity to modulate behavior as a function of reward may provide a powerful tool for improving the phenotypic definition of depression, and thus offer a reliable behavioral screening approach for neuroscience studies of depression.
Keywords: Depression, Anhedonia, Reward Bias, Reinforcers, Appetitive Behavior, Phenotype
Introduction
Recently, it has become increasingly evident that advancement in psychiatric genetics and clinical neuroscience is hindered by the heterogeneity of psychiatric disorders and by difficulties in defining and characterizing the phenotype under investigation. Therefore, failure to obtain reliable results about the etiology and pathophysiology of various psychiatric disorders may partially result from insufficient development of phenotypic characterization (Hasler et al 2004; Hyman 2002; Leboyer et al 1998). Leboyer and colleagues, for example, suggested that identifying more homogeneous forms of diseases through a “candidate symptom approach” might yield better results than relying entirely on classical nosology. Similarly, Hasler et al (2004) proposed that a necessary step toward a better understanding of the genetic and neurobiological basis of depression will involve “dissecting” this complex clinical condition into key components yielding narrow definitions of psychopathological endophenotypes (i.e., phenotypic traits or markers representing more direct expressions of underlying genes; Gottesman and Gould 2003).
Among promising endophenotypes, anhedonia (Hasler et al 2004) - the loss of pleasure or lack of reactivity to pleasurable stimuli - is one of the core symptoms of depression (APA 1994), and has been considered a potential trait marker related to vulnerability to depression (Loas 1996; Meehl 1975). Costello (1972), for example, suggested that the impact of rewarding stimuli is markedly diminished in depression, and Meehl (1975) hypothesized that depressed subjects experience reward-related cues as less reinforcing due to a lowered hedonic capacity.
Various research traditions have provided empirical support for the hypothesis that depression is linked to fundamental deficits in an approach-related system promoting positive affect and motivated behavior. First, factor-analytic studies have generally found that low positive affect, particularly low engagement with the environment, uniquely differentiates depression from anxiety, whereas general negative affectivity represents a nonspecific marker linked to both disorders (Watson et al 1995; Watson and Clark 1984). Second, electroencephalographic (EEG) studies in depressed samples have generally reported dysfunctional brain activation in left and right prefrontal regions previously implicated in approach- and withdrawal-related affect, respectively (Davidson 1998; Gotlib et al 1998; Henriques and Davidson 1991; Pizzagalli et al 2002). Third, clinical observations and empirical findings have highlighted blunted engagement with the environment and diminished emotional response to pleasant cues in depression (Berenbaum and Oltmanns 1992; Buchwald 1977; Gotlib 1982; Henriques et al 1994; Henriques and Davidson 2000; Lewinsohn and Graf 1973; Sloan et al 2001; Suslow et al 2001). This blunted engagement mirrors psychophysiological abnormalities in anhedonic subjects and melancholic patients (e.g. Bruder et al 1991; Fiorito and Simons 1994). In sum, substantial evidence suggests a critical role of an abnormal approach-related system in depression. Of clinical import, these dysfunctions may lead to diminished engagement in pleasurable activities and blunted responsiveness to reinforcing stimuli (reinforcers); this in turn could contribute to the generation, maintenance, or exacerbation of depressive symptoms, particularly symptoms of loss of pleasure, libido, appetite, and interest in the environment.
Considering the importance of anhedonia and abnormal approach-related behavior in depression, it is surprising to note that a paucity of studies has employed laboratory-based measures to objectively characterize these constructs (e.g. Buchwald 1977; Henriques and Davidson 2000). Working toward the ultimate goal of a better phenotypic definition of depression, the present study aimed to assess the relationship between participants’ propensity to modulate behavior as a function of reward and depressive, particularly anhedonic, symptoms. To this end, a signal-detection task using a differential reinforcement schedule with monetary reward was utilized to provide an objective, laboratory-based measure of hedonic capacity, which was operationalized as responsiveness to reinforcing stimuli (“reward responsiveness”).
In signal-detection tasks, participants are typically instructed to choose whether stimulus1 or stimulus2 was presented by making an appropriate response1 or response2 (McCarthy 1991). Participants’ performance can be decomposed into discriminability (sensitivity) and response bias. Discriminability measures the participants’ ability to differentiate between stimulus1 and stimulus2, whereas response bias reflects the participant’s general tendency to define an ambiguous stimulus as a target, i.e., the participant’s tendency to make one or the other response irrespective of which stimulus is presented. Critically, unequal frequency of reward between two types of correct responses typically produces a systematic preference for the response paired with the more frequent (or larger) reward (Macmillan and Creelman 1991; McCarthy 1991). In this study, the degree of response bias toward the more frequently reinforced alternative was used for operationalizing reward responsiveness, which can be understood as the extent to which behavior is modulated by reinforcement history. Note that this operationalization fits with the classical views that (a) a stimulus is rewarding if it positively reinforces behavior, i.e., it reliably increases the likelihood of a behavior (Hull 1943; Rescorla and Wagner 1972); and (b) reinforcers play a critical role in the formation of associations between salient cues and internal rewarding events (Spanagel and Weiss 1999). Within this framework, we reasoned that blunted responses to reinforcers would provide a promising behavioral manifestation of hedonic hypofunctioning. If depression is associated with an impaired approach-related system and blunted responsiveness to reinforcing stimuli, we hypothesized that participants with elevated levels of depressive, particularly anhedonic, symptoms would show an impaired response bias toward the more frequently reinforced stimulus.
Methods and Materials
Participants
Informed written consent was obtained from 62 undergraduate students (23 males, 39 females; age: mean = 20.13, SD = 2.50) recruited from Introductory Psychology courses at Harvard University. All participants were right-handed (Chapman and Chapman 1987), reported to be free of any past or present neurological illness, and received either course credit or $5 for their participation1. They were told they would have the opportunity to win money during the task. The study was approved by the Committee on the Use of Human Subjects at Harvard University.
Task and Procedure
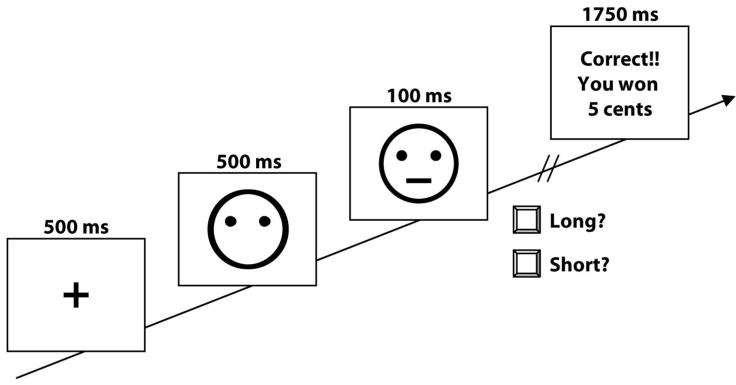
Participants were given verbal instructions about the task and told that the aim of the study was to win as much money as possible (actual money was shown to the participants to increase the credibility of the experiment). Participants then completed the Chapman Handedness Inventory (Chapman and Chapman 1987). Subsequently, written instructions about the task, a short demonstration, and two practice trials were presented. The task, which was adapted from Tripp and Alsop (1999), was presented on a Macintosh PowerMac G4 (866MHz) computer using Psyscope software (Cohen et al 1993), and lasted approximately 30 minutes. The task consisted of 300 trials, divided into 3 blocks of 100 trials, with blocks separated by a 30-sec break. Each trial started with the presentation of an asterisk for 500 ms in the middle of the screen that served as a fixation point (Fig. 1). After 500 ms, a mouth-less cartoon face was presented in the center of the screen. After a delay of 500 ms either a short mouth (11.5 mm) or a long mouth (13 mm) was presented for 100 ms. The face without the mouth remained on the screen until a key response was made. Participants were asked to identify which type of mouth was presented by pressing either the ‘z’ key or the ‘/’ key on the keyboard (counterbalanced across subjects). For each block, the long and short mouths were presented equally often in a pseudo-randomized sequence with the constraint that no more than three instances of the same stimulus were presented consecutively.
Figure 1.
Schematic diagram of the task. After presentation of the mouth stimulus, subjects selected which type of mouth had been presented by pressing either the ‘z’ or the ‘/’ key (counterbalanced across subjects).
An asymmetrical reinforcer ratio (the relative number of reinforcers received after a given correct response vs. another correct response) was used since both animal and human research has shown that this is the critical variable for producing a response bias (Johnstone and Alsop 2000; McCarthy and Davison 1979). In this task, the only kind of feedback provided was reward feedback for correct responses. Subjects were specifically instructed that not all correct responses would be receiving reward feedback. For each block, 40 correct trials were followed by reward feedback (“Correct!! You won 5 cents”), presented for 1750 ms immediately after the correct response. For half of the participants, correct identification of the little mouth was associated with three times more positive feedback (30/40) than correct identification of the big mouth (10/40). For the other half of the participants, the contingencies were reversed. A controlled reinforcer procedure was used so that reward feedback was given according to a pseudo-random schedule that determined which specific trials were to be rewarded for correct identifications (Johnstone and Alsop 2000; McCarthy and Davison 1979). If a subject failed to make a correct identification in a trial for which reward feedback was due according to the schedule, the feedback was delayed until the next correct identification of the same stimulus type. For the entire task, participants “earned” approximately $6.
After the computer task, participants completed several self-report measures of affect and mood. The Trait form of the PANAS (Positive and Negative Affect Scales; Watson et al 1988) was used to assess two fundamental dimensions of dispositional affect, positive and negative affect. The Beck Depression Inventory-II (BDI-II), a reliable and well-validated self-report instrument of depressive symptomatology (Beck et al 1996), was used to assess levels of depressive symptoms. Levels of trait anxiety were assessed by means of the Spielberger Trait Anxiety (Spielberger et al 1970). Finally, anhedonia was assessed by (a) the Revised Physical and Social Anhedonia Scales (Chapman and Chapman 1978; Chapman et al 1976; Chapman et al 1980); and (b) a total score on BDI items associated with anhedonic symptoms (“BDI anhedonic subscore”; Cochran alpha: 0.60): loss of pleasure (item #4), loss of interest (item #12), loss of energy (item #15), and loss of interest in sex (item #21) (e.g. Joiner et al 2003). Finally, in light of our interest in melancholic depression (Pizzagalli et al 2004) -a subtype of major depressive disorder characterized by pervasive anhedonia (Rush and Weissenburger 1994) - a total “melancholic” symptom score was computed by summing scores of BDI items that map onto the DSM-IV criteria for melancholia (“BDI melancholic subscore”; Cochran alpha: 0.45): loss of pleasure (item #4), guilty feelings (item #5), agitation (item #11), loss of interest (item #12), early morning awakening (item #16b), and loss of interest in sex (item #21) (Pizzagalli et al 2004). Table 1 lists the zero-order (Pearson) correlations between the various self-report measures of affect and mood.
Table 1.
Pairwise correlations between self-report measures of affect and mood for the entire sample. Pearson correlations (and n) are reported
| 2. | 3. | 4. | 5. | 6. | 7. | 8. | |
|---|---|---|---|---|---|---|---|
| 1. BDI | 0.59§ | 0.82§ | 0.68§ | 0.03 | 0.03 | -0.23 | 0.46§ |
| (62) | (62) | (33) | (58) | (58) | (60) | (60) | |
| 2. BDI anhedonic scorea | 0.67§ | 0.32 | 0.09 | -0.06 | -0.27* | 0.25 | |
| (62) | (33) | (58) | (58) | (60) | (60) | ||
| 3. BDI melancholic scoreb | 0.44* | 0.05 | -0.04 | -0.21 | 0.38† | ||
| (58) | (58) | (58) | (60) | (60) | |||
| 4. STAI | 0.24 | 0.28 | -0.37* | 0.55† | |||
| (29) | (29) | (33) | (33) | ||||
| 5. Social Anhedonia | 0.37† | -0.39† | -0.05 | ||||
| (58) | (56) | (56) | |||||
| 6. Physical Anhedonia | -0.18 | -0.15 | |||||
| (56) | (56) | ||||||
| 7. PANAS-PA | -0.02 | ||||||
| (60) | |||||||
| 8. PANAS-NA |
Notes: BDI: Beck Depression Inventory-II (Beck et al 1996); STAI: Spielberger Trait Anxiety Inventory (Trait form; Spielberger et al 1970); PANAS: Positive and Negative Affect Scales (PA: positive affect; NA: negative affect; Watson et al 1988). Revised Physical and Social Anhedonia Scales (Chapman and Chapman 1978; Chapman et al 1976; Chapman et al 1980).
Sum of BDI items associated with anhedonic symptoms: loss of pleasure (item #4), loss of interest (item #12), loss of energy (item #15), and loss of interest in sex (item #21).
Sum of BDI items associated with melancholic symptoms: loss of pleasure (item #4), guilty feelings (item #5), agitation (item #11), loss of interest (item #12), early morning awakening (item #16b), and loss of interest in sex (item #21).
p < 0.0005
p < 0.005
p < 0.05
To assess the test-retest reliability of performance in the signal-detection task and to test whether low response bias at the initial behavioral session (“Time 1”) predicted subsequent self-report measures of affect, a randomly selected sub-sample of the participants was re-contacted at a later point and invited to participate in an identical session (“Time 2”). At Time 2, the BDI and the trait version of the PANAS were administered again, along with the Mood and Anxiety Symptom Questionnaire (MASQ; Watson et al 1995), which assesses anhedonic depression, anxious arousal, and symptoms of general distress, and thus provided an independent assessment of anhedonic symptoms. Twenty-five participants performed the signal-detection task a second time (on average, 38.28 days after the first session, range: 21-120 days).
Data Collection and Reduction
Task performance was analyzed with respect to discriminability, reaction time (RT), and response bias. While discriminability and RT provide information about overall task performance (e.g. attention, response speed), response bias provides an index of the participants’ systematic preference for the response paired with the more frequent reward, and was thus the variable of main interest. Discriminability and response bias were derived from the behavioral model of signal-detection (e.g. McCarthy and Davison 1979; Tripp and Alsop 1999). Specifically, discriminability was computed as:
| (Formula 1) |
where Longcorrect, for example, is the number of correct responses after presentation of the long mouth, Shortincorrect is the number of incorrect responses after presentation of the short mouth, etc. Discriminability is mainly affected by physical differences between the two target stimuli, and thus provides information about task difficulty. Response bias was computed as:
| (Formula 2) |
assuming that correct identification of the long mouth in this example was rewarded more frequently. High response bias scores are produced by high numbers of correct responses to the stimulus associated with more frequent reward (“rich condition”) and high numbers of incorrect response to the stimulus associated with less frequent reward (“lean condition”), which would increase the numerator and decrease the denominator2. Log d is similar to traditional signal-detection measures such as d’ (Johnstone and Alsop 2000). Likewise, log b is functionally equivalent to the criterion location, c, or the choice theory measure, log (b) (Luce 1963; Green and Swets 1966).
For all analyses, trials with RT shorter than 150 ms or longer than 2500 ms were excluded (overall 0.59% of the trials). Further, for each subject, trials with RT (after natural log transformation) falling outside the range of mean ± 3SD were considered outliers. Overall an additional 1.11% of trials were excluded.
Statistical Analyses
Exploratory analyses revealed no differences in behavioral performance between female and male participants; consequently, analyses were performed on the entire sample. For accuracy (percent correct response) and RTs, analyses of variance (ANOVA) with Block (Block 1, 2, 3) and Condition (Rich, Lean) as repeated measures were performed. For response bias and discriminability, the ANOVAs included Block only. Significant ANOVA effects were further explored using post-hoc Newman-Keuls tests. When applicable, the Greenhouse-Geisser correction was used, and epsisol (ε) values are reported. Partial eta squared (η2) values are reported as measures of effect sizes.
To assess whether elevated levels of depressive symptoms modulated the effects, participants with low BDI (range: 0-6, n = 21) and high BDI (range: 16-35, n = 15) scores were compared using Group as an additional between-subject factor. According to the BDI-II manual, a score > 16 reflects mild depression and a study combining the BDI-II and a structured clinical interview in a student sample has shown that a score of 16 is an effective cutoff for determining depressed mood (Sprinkle et al 2002). High BDI (mean BDI and S.D.: 21.33±4.82) and low BDI (3.62±2.27) subjects did not differ with respect to age, or gender ratio (Table 2). The two groups had comparable percentages of trials excluded from the analyses because their RT was too short (< 150 ms), too long (> 2500 ms), or was considered an outlier response (exceeding mean ± 3SD) (high BDI: 0.38%; low BDI: 0.61%).
Table 2.
Mean (and standard deviation) values for demographic and self-report data for high (n = 15) and low (n = 21) BDI subjects
| Low BDI | High BDI | t-value | p-value | |
|---|---|---|---|---|
| N | 21 | 15 | ||
| Female/Male | 14/7 | 10/5 | ||
| Age | 20.76 ± 3.94 | 19.87 ± 1.41 | 0.84 | .40 |
| BDI | 3.62 ± 2.27 | 21.33 ± 4.82 | -14.34 | .001 |
| STAIa | 33.92 ± 4.87 | 48.44 ± 6.69 | -5.77 | .001 |
| PANAS-PA (trait) | 35.15 ± 7.29 | 32.67 ± 5.75 | 1.09 | .28 |
| PANAS-NA (trait) | 13.65 ± 4.39 | 20.47 ± 6.94 | -3.55 | .002 |
| Social Anhedonia | 5.76 ± 4.68 | 7.00 ± 5.64 | -0.72 | .48 |
| Physical Anhedonia | 10.29 ± 7.08 | 12.00 ± 8.04 | -0.68 | .50 |
Notes: BDI: Beck Depression Inventory-II (Beck et al 1996); STAI: Spielberger Trait Anxiety Inventory (Trait form; Spielberger et al 1970); PANAS: Positive and Negative Affect Scales (PA: positive affect; NA: negative affect; Watson et al 1988). Revised Physical and Social Anhedonia Scales (Chapman and Chapman 1978; Chapman et al 1976; Chapman et al 1980).
Low BDI (n =12) and high BDI (n = 10)
For these analyses, ANOVAs on RT and discriminability were performed to assess putative group differences in task-unspecific variables (response speed, attentional processes, and task difficulty); as in prior signal-detection tasks, the main variable of interest was response bias (Henriques et al 1994; Tripp and Alsop 1999).
Finally, to directly assess the development of response bias after initial exposure to the differential reinforcement schedule in Block 1, difference scores between Block 1 and 2 and between Block 1 and 3 were computed. Because the difference score between Block 1 and 3 fully captured the overall development of response bias (“ΔResponse Bias”), this variable was entered within Pearson correlation analyses with self-report measures of mood and affect.
Results
Overall Effect of Task Manipulation (n = 62)
Accuracy (Fig. 2A)
Figure 2.
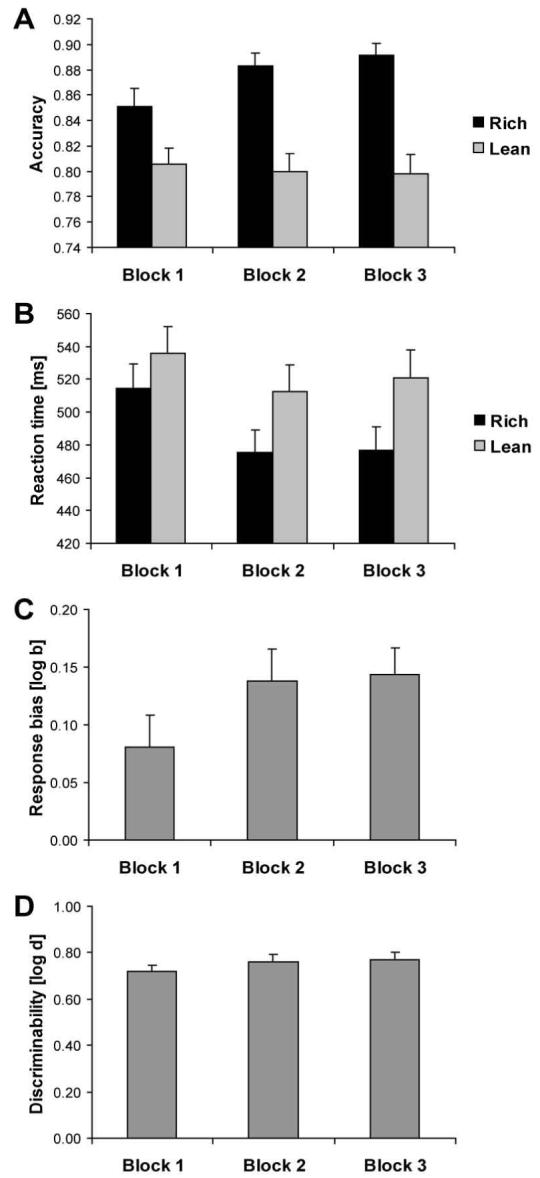
Overall effect of task manipulation. Mean accuracy (A), reaction time (B), response bias (C), and discriminability (D) for the entire sample (n = 62). Error bars represent standard errors. For accuracy and RT, the rich condition (black bars) refers to the stimulus associated with more frequent reward, whereas the lean condition (light gray bars) refers to the stimulus associated with less frequent reward.
The two-way ANOVA with Block and Condition as factors revealed a main effect of Condition, F(1,61) = 29.50, p < .001 (partial η2: 0.33) and a significant Block × Condition interaction, F(2,122) = 4.43, p < .020 (ε: 0.87; partial η2: 0.07). As expected, accuracy for the rich condition was higher than for the lean condition (Fig. 2A), and this difference increased over time [linear contrast of the interaction: F(1,61) = 5.98, p < .020]. Post-hoc Newman-Keuls tests clarified that accuracy for the rich condition was higher than for the lean condition in all three blocks (all ps < .001). Also, the accuracy for the rich condition increased between block 1 and block 2 (p < .01), and between block 1 and block 3 (p < .005), but not between block 2 and block 3 (p > .25). Conversely, accuracy for the lean condition did not change over the blocks (all ps > .60).
Reaction Time (Fig. 2B)
The two-way ANOVA revealed significant effects of Block, F(2,122) = 7.98, p < .003 (partial η2: 0.12), Condition, F(1,61) = 51.51, p < .001 (partial η2: 0.46), and Block × Condition, F(2,122) = 5.94, p < .005 (partial η2: 0.09). As shown in Fig. 2B, the rich condition led to significantly shorter RTs than the lean condition in all three blocks (Newman-Keuls: all ps < .001). For both conditions, RTs were significantly shorter for Blocks 2 and 3 than Block 1 (all ps < .005), with no differences between Blocks 2 and 3.
Response bias (Fig. 2C)
The one-way ANOVA on response bias revealed a trend for Block, F(2,122) = 3.03, p = .060 (ε: 0.86), due to a significant increase from block 1 (0.08±0.22) to block 2 (0.14±0.22; Newman-Keuls p < .050) and no further increase from block 2 to block 3 (0.14±0.19).
Discriminability (Fig. 2D)
The one-way ANOVA revealed no significant effects (ps > .10).
High (n = 15) vs. Low (n = 21) BDI Subjects
Self-report measures of mood and affect
High BDI subjects reported significantly higher dispositional anxiety (STAI) and negative affect (PANAS-NA) than low BDI subjects (Table 2).
Response Bias
The only effect emerging from the 3 (Block) × 2 (Group) ANOVA was the interaction3, F(2,68) = 3.27, p < .045 (ε: 0.99; partial η2: 0.09). As shown in Fig. 3A, the interaction was due to increases in response bias for low BDI subjects from Block 1 (0.09±0.16) to Block 3 (0.21±0.14; Newman-Keuls p < .05), with no differences between Blocks 1 and 2 (p > .60) or between Block 2 and 3 (p > .20). Contrary to these findings, high BDI subjects failed to show any changes in response bias between the blocks (all ps > .60; Fig. 3A).
Figure 3.
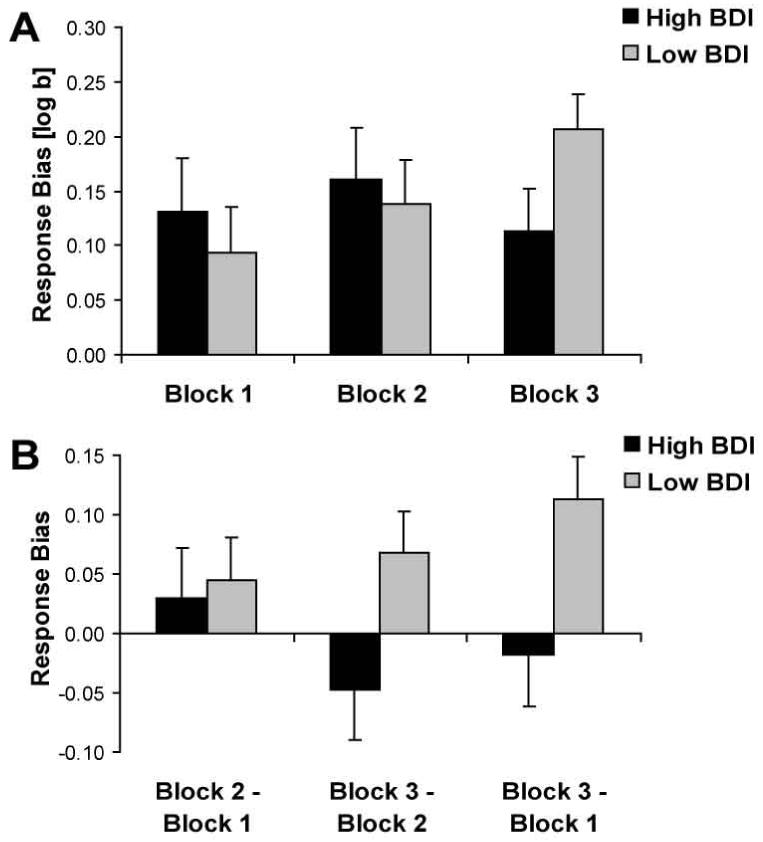
Mean response bias (A) and (B) changes in response bias for high (black bars; n = 15) and low (light gray bars; n = 21) BDI subjects. Error bars represent standard errors.
To directly assess whether high and low BDI subjects differed in their development of response bias, difference scores between Block 1 and 2 and between Block 1 and 3 were entered in a Group × Phase (Block 2 - Block 1; Block 3 - Block 1) ANOVA. The only significant effect was the interaction [F(1,34) = 4.45, p < .045; partial η2: 0.12] (Fig. 3B). Compared to high BDI subjects, low BDI subjects had significantly higher increases in response bias from block 1 to 3 (Newman-Keuls p < .001), with no group differences between blocks 1 and 2 (p > .7).
Discriminability
The two-way ANOVA revealed no significant effects.
Reaction Time
The three-way ANOVA revealed no significant effects involving Group.
Control analyses
Since reinforcer ratio (the relative number of reinforcers received after a given correct response vs. another correct response) is the critical variable for producing a response bias (McCarthy and Davison 1979), it was important to rule out the possibility that the groups differed in the number of feedbacks received during the experiment. Separate t tests revealed that high and low BDI subjects received virtually identical reward feedback [rich: 88.40±1.77 vs. 88.10±2.88; lean: 29.40±0.63 vs. 29.52±0.68; rich/lean ratio: 3.01±0.09 vs. 2.99±0.13; all t(34) < 0.56, all ps > .50]. Finally, the two groups did not differ in the number of participants allocated to the sessions where the short (high BDI: 8/15; low BDI: 11/21) or the long mouth was the condition more frequently rewarded.
Correlations Between Self-report Measures of Affect and Changes in Response Bias (n = 61)
One participant had a ΔResponse Bias that was more than 4 SDs from the mean, and thus was excluded from these analyses4. Change in response bias from block 1 to 3 (block 3 - block 1) was negatively correlated with BDI “melancholic” subscores (r = -0.28, p < .035, n = 61) assessed at Time 1. This change in Response Bias was not correlated with the Time 1 assessment of the Social Anhedonia (r = -0.06, ns), Physical Anhedonia (r = -0.06, ns), or general positive affectivity (PANAS-NA trait; r = -0.09, ns) scores. Notably, ΔResponse Bias at Time 1 was negatively correlated with the total BDI score (r = -0.46, p < .025, n = 25) and BDI “melancholic” subscore (r = -0.41, p < .05, n = 25; Fig. 4) at Time 2. (These correlations remained significant when using Spearman Rank coefficients).
Figure 4.
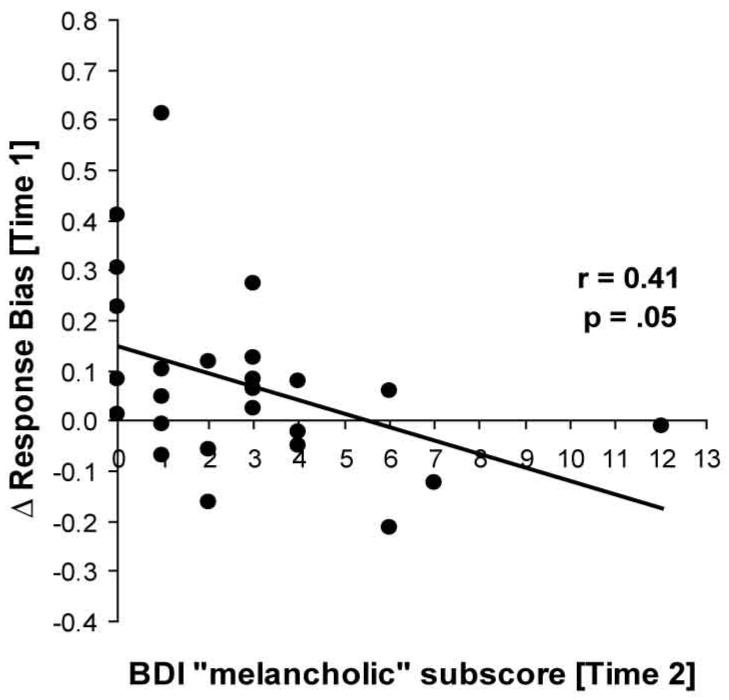
Scatterplot and Pearson’s correlation between ΔResponse Bias (block 3 - block1) at Time 1 and BDI “melancholic” subscore at Time 2.
To test the specificity of these findings and the predictive value of response bias, two hierarchical regression analyses were run. In the first, we tested whether ΔResponse Bias at Time 1 predicted BDI “melancholic” subscores at Time 2 after controlling for BDI “melancholic” subscores at Time 1 (entered in the first step) and general negative affectivity (PANAS-NA trait) (entered in the second step). Findings revealed that ΔResponse Bias at Time 1 continued to predict BDI “melancholic” subscores at Time 2 even after controlling for these variables, ΔR2 = 0.19, ΔF(1,20) = 4.83, p = .040. When considering the total BDI score, an identical hierarchical regression analysis showed only a statistical trend, ΔR2 = 0.13, ΔF(1,20) = 3.79, p = .066.
Self-Report Measures of Participants with Positive (n = 39) vs. Negative (n = 22) ΔResponse Bias
When considering ΔResponse Bias at Time 1, 36.07% of the subjects had a negative change from block 1 to 3, indicating that these participants were not responsive to the differential reinforcement schedule. As listed in Table 3, participants with negative ΔResponse Bias at Time 1, reported higher scores on various anhedonic measures at both Time 1 (BDI melancholic subscore, Chapman’s Physical Anhedonia scale) and Time 2 (BDI anhedonic subscore, BDI melancholic subscore, Anhedonic Depression subscale of the MASQ), and continued to have significantly lower ΔResponse Bias at Time 2.
Table 3.
Mean (and standard deviation) values for self-report data for participants with positive (n = 39) and negative (n = 22) ΔResponse Bias at Time 1
| Δ Response Biasa < 0 | Δ Response Bias > 0 | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | t-value | p-value | |
| Time 1 | ||||||||
| BDI | 12.86 | 8.49 | 22 | 9.52 | 6.76 | 39 | 1.69 | .096 |
| BDI anhedonic scoreb | 1.95 | 1.84 | 22 | 1.38 | 1.25 | 39 | 1.44 | .155 |
| BDI melancholic scorec | 4.09 | 2.74 | 22 | 2.87 | 1.47 | 39 | 2.27 | .027 |
| Social Anhedoniad | 6.70 | 7.04 | 20 | 5.78 | 4.12 | 37 | 0.62 | .537 |
| Physical Anhedoniad | 12.95 | 6.98 | 20 | 9.59 | 6.17 | 37 | 1.87 | .067 |
| Time 2 | ||||||||
| BDI | 13.17 | 7.35 | 9 | 6.31 | 4.92 | 16 | 2.80 | .010 |
| BDI anhedonic scoreb | 2.22 | 2.11 | 9 | 0.75 | 1.06 | 16 | 2.34 | .028 |
| BDI melancholic scorec | 4.33 | 3.57 | 9 | 1.88 | 1.78 | 16 | 2.31 | .030 |
| MASQ AD | 70.22 | 12.65 | 9 | 52.31 | 16.15 | 13 | 2.78 | .012 |
| ΔResponse Biasa | -0.01 | 0.17 | 8 | 0.21 | 0.24 | 16 | -2.26 | .034 |
Notes: BDI: Beck Depression Inventory-II (Beck et al 1996); MASQ: Mood and Anxiety Symptom Questionnaire (Watson et al 1995), AD: Anhedonic Depression.
Difference score between response bias in block 1 and 3 (ΔResponse Bias: block 3 - block 1).
Sum of BDI items associated with anhedonic symptoms: loss of pleasure (item #4), loss of interest (item #12), loss of energy (item #15), and loss of interest in sex (item #21).
Sum of BDI items associated with melancholic symptoms: loss of pleasure (item #4), guilty feelings (item #5), agitation (item #11), loss of interest (item #12), early morning awakening (item #16b), and loss of interest in sex (item #21).
Revised Physical and Social Anhedonia Scales (Chapman and Chapman 1978; Chapman et al 1976; Chapman et al 1980).
Test-retest Reliability
Twenty-five participants performed the signal-detection task a second time (38.28±20.72 days later). As shown in Fig. 5, ΔResponse Bias at Time 1 and Time 2 were significantly correlated (r = 0.57, p < .004).
Figure 5.
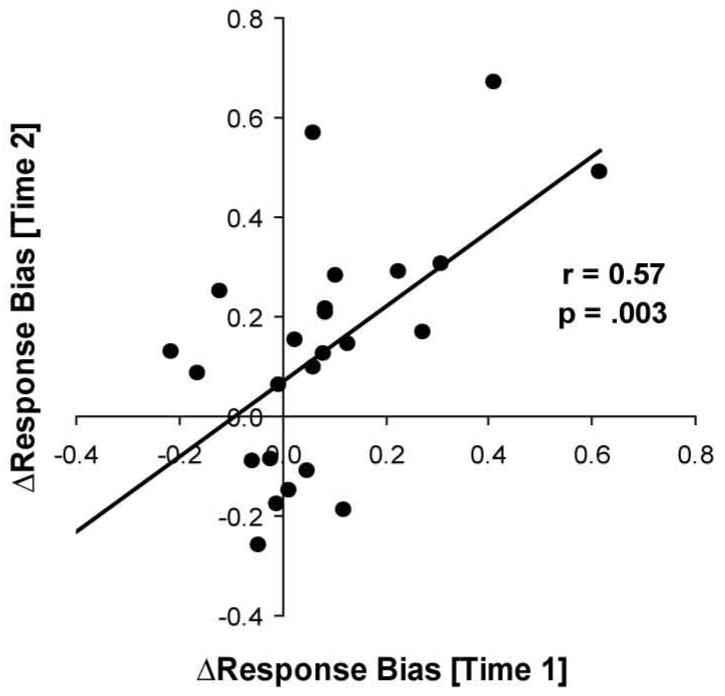
Scatterplot and Pearson’s correlation between ΔResponse Bias (block 3 - block1) at Time 1 and ΔResponse Bias (block 3 - block1) at Time 2.
Discussion
Rooted within a classical view that a stimulus is rewarding if it positively reinforces behavior, i.e., it reliably increases the likelihood of behavior (Hull 1943; Rescorla and Wagner 1972), the goal of the present study was (1) to test the feasibility, convergent validity, and predictive validity of a laboratory-based measure of hedonic capacity based on a signal-detection approach; and (2) to test the hypothesis that elevated levels of depressive, particularly anhedonic, symptoms were associated with impaired reward responsiveness. Reward responsiveness was objectively operationalized as the participants’ propensity to modulate behavior as a function of prior exposure to reinforced stimuli. The following findings emerged. First, the task revealed robust behavioral differences, including a response bias, in favor of the stimulus reinforced more frequently. Second, unlike participants with low BDI scores, participants with elevated depressive symptoms failed to show increases in response bias toward the reinforcing stimulus, indicating reduced reward responsiveness. Importantly, high and low BDI participants did not differ in their discriminability and RT scores, or in the number of reward feedbacks received during the experiment, indicating that group differences in reward responsiveness were not due to differences in task difficulty, differential exposure to positive feedback, or different stimulus-outcome contingencies. Third, participants failing to develop a response bias throughout the blocks (i.e., subjects with negative ΔResponse Bias) reported significantly higher scores on various self-report assessments of anhedonic and melancholic symptoms (e.g. loss of pleasure, energy, interest, and libido) in their daily activities, providing important convergence between subjective and objective measures of “anhedonia”. These findings are intriguing, as BDI items assessing “loss of social interest”, “appetite”, “sexual interest and satisfaction”, and “work inhibition” best characterized depressed inpatients with extreme anhedonic features in an earlier clinical study (Fawcett et al 1983). Of note, participants failing to develop a response bias at Time 1 reported higher anhedonic symptoms at Time 2, and continued to have significantly lower Response Bias at Time 2 compared to participants developing a response bias. Further, highlighting the specificity of these findings, hierarchical regression analyses clarified that ΔResponse Bias at Time 1 predicted “melancholic” symptoms at Time 2 even after controlling for initial “melancholic” symptoms and general negative affectivity. Therefore, the lower the response bias development in the signal-detection task, the higher the degree of melancholic, including anhedonic, symptoms at the day of the experiment as well as one month later. Finally, a test-retest analysis suggested a moderately strong stability, within a month period (r = 0.57), of the propensity to develop a response bias.
Approach-Related System Abnormalities in Depression: Replication and Extension
Collectively, the present findings of blunted responsiveness to reinforcing stimuli in subjects with elevated depressive symptoms agree with prior empirical evidence and theoretical propositions that have suggested a critical role of an abnormal approach-related system in depression (Costello 1972; Davidson 1992; Depue and Iacono 1989; Loas 1996; Watson and Clark 1984). Particularly, our findings replicate and extend earlier reports in depression of blunted hedonic responses, which, as in the present study, were assessed using laboratory-based measures. Using a progressive ratio schedule with increasing reinforcement, Hughes et al (1985) showed in a small study with six melancholic depressed participants that the number of responses to obtain money increased in the three treatment responders but not in the three nonresponders. Using a task assessing the effect of incentives on performance during a verbal memory task, Henriques and coworkers reported that dysphoric and clinically depressed subjects displayed a more conservative bias during the reward condition; depressed subjects showed a weaker tendency to report that a word had been presented before, a strategy that in controls led to maximization of earning (Henriques et al 1994; Henriques and Davidson 2000). Together with reports of low levels of self-reinforcement (Gotlib 1982), underestimation of frequency of positive reinforcements (Buchwald 1977), diminished response to pleasant cues (Berenbaum and Oltmanns 1992; Sloan et al 2001), and decreased attention to positive facial expression (Suslow et al 2001), these findings suggest that the use of laboratory-based measures may offer a powerful tool for objectively assessing hedonic capacity in depression. The present findings specifically clarify that impairments in the extent to which behavior can be modulated by reinforcement history may be a potential mechanism underlying an abnormal approach-related system in depression. Because reinforcers are stimuli that increase the frequency of specific behavioral responses (Hull 1943; Rescorla and Wagner 1972) and play a critical role in the formation of associations between salient cues and internal rewarding events (Spanagel and Weiss 1999), lowered responsiveness to reinforcers may lead to diminished engagement in pleasurable activities and decreased motivational drive to pursue future rewards (Depue and Iacono 1989; Kasch et al 2002). Although longitudinal studies will be required to determine whether impaired reward responsiveness may represent a vulnerability factor to depression, we speculate that subtle impairments in hedonic responsiveness may predispose individuals to lower engagement with the environment. In line with this conjecture, studies using self-report measures of affect have reported that low BAS levels (particularly, reward responsiveness) negatively predicted outcome eight months later (Kasch et al 2002). Similarly, a deficit in positive affect represented a risk factor for the development and/or maintenance of depressive symptoms in both community (Lonigan et al 1999) and inpatient (Joiner and Lonigan 2000) samples of children and adolescents. Moreover, in a large population-based study, anhedonia at study entry was associated with poor outcome 12 months later (Spijker et al 2001). The present finding that low reward responsiveness predicted anhedonic symptoms one month later is consistent with and extends these prior findings, which were based on self-report measures.
Limitations
The limitations of this study should be acknowledged. First, although the BDI is a reliable and well-established measure of depressive symptomatology (e.g. Beck et al 1988), this self-report measure is not indicative of the full clinical range of depression. Consequently, while the present report indicates that elevated levels of depressive, particularly anhedonic, symptoms were associated with decreased reward responsiveness, it is unknown whether these findings will extend to a clinical sample. Moreover, some of the statistically significant results emerging from the present study were relatively weak. For example, the correlation between change in response bias and BDI melancholic subscore was r = -0.28. Accordingly, future studies will be required to evaluate the reliability, specificity, and predictive validity of the present signal-detection approach to assess hedonic capacity in clinically depressed patients. Additional studies will also be needed to test the specificity of the present findings with respect to clinical nosology, and to assess whether the proposed laboratory-based approach may be useful for distinguishing among different psychopathologies. One could speculate that schizophrenic patients, particularly those with a deficit syndrome, may show impaired hedonic capacity and reduced ability to discriminate between stimuli (reflected in abnormal discriminability scores). Furthermore, individuals with anxiety disorders may show normative hedonic capacity but increased response bias toward punishment-related cues, in line with data indicating attentional biases toward threat-related cues in anxiety disorders (Mineka et al 2003).
Conclusions and Implication for Neuroscience Research on Depression
In conclusion, a signal-detection task was utilized to provide an objective assessment of participants’ propensity to modulate behavior as a function of reward-related cues. Participants reporting elevated depressive symptoms failed to modulate their behavior despite receiving identical monetary rewards, indicating that they were less sensitive or reactive to pleasurable stimuli, i.e., more anhedonic. Critically, participants with diminished response bias reported relatively lower pleasure/interest in their daily activities even one month later, providing important convergent and predictive validity to the “anhedonia” construct used in the present study. These findings confirm that deficits in an approach-related system may be a cardinal feature of depression, and warrant further development of laboratory-based measures of symptoms in clinical populations.
As recently reviewed by Hasler et al (2004), one of the main challenges in biological psychiatry will be to improve the phenotypic definition of depression in order to achieve a better understanding of the genetic and neurobiological underpinnings of this debilitating disease. Dissecting the depressive phenotypes into key components yielding narrow definitions of psychopathological endophenotypes is of outmost priority toward this goal (Hasler et al 2004; Hyman 2002; Leboyer et al 1998); endophenotypes are indeed expected to provide a “means for identifying the ‘downstream’ traits or facets of clinical phenotypes, as well as the ‘upstream’ consequences of genes” (p. 637; Gottesman and Gould 2003). It was in this spirit that the present study was designed, and research is currently underway in our laboratory adopting this approach for identifying more homogenous subtypes of depression and assessing neural underpinnings underlying impaired hedonic capacity in depression.
Acknowledgments
This work was supported by grants from NIMH (R01MH68376) and the William F. Milton Fund, Harvard University to DAP. The authors are grateful for the constructive criticisms provided by three anonymous reviewers.
Footnotes
Exploratory analyses revealed no differences in task performance, including response bias, between participants receiving course credit or $5 for their participation.
Formula 2 can be generalized as:
A similar ANOVA considering only Blocks 2 and 3 confirmed that this effect was independent from response biases in Block 1, in which participants may have not been fully exposed to the differential reinforcement schedule; Group × Block interaction: F(1,34) = 4.45, p < .05. Further, since high and low BDI subjects differed in trait anxiety and general negative affectivity, analyses were repeated entering separately the STAI and PANAS scores as covariates. The Group x Block interaction remained significant [STAI: F(1,18) = 6.71, p < .025; trait negative affect: F(1,31) = 3.58, p = .068].
This subject had an intermediate BDI score (BDI = 14), and did not fulfill the inclusion criteria for the ANOVAs with the low and high BDI groups. Because the subject’s accuracy, RT, and response bias were within the mean ± 2SD, she was included in the ANOVAs with the entire sample (n = 62).
No reliable correlations emerged when considering the Chapman’s anhedonia scales. One possible explanation for this null finding is that these scales may have predictive validity primarily for schizophrenia spectrum disorders (Kwapil 1998), and thus may be less sensitive for assessing dysphoria or depression. In line with this view, a recent study found that a state measure of anhedonia that was correlated with depressive symptoms was unrelated to the Physical Anhedonia scores (Herbener and Harrow 2002). An alternative, not mutually exclusive explanation could be that the anhedonic items included in the BDI (e.g. loss of libido, loss of pleasure) may be more closely related to our operationalization of hedonic capacity. More work is needed to clarify this issue.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Press; Washington, D.C.: 1994. [Google Scholar]
- Beck AT, Steer RA, Garbin M. Psychometric proprieties of the Beck Depression Inventory; 25 years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2nd ed. The Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Berenbaum H, Oltmanns TF. Emotional experience and expression in schizophrenia and depression. J Abnorm Psychol. 1992;101:37–44. doi: 10.1037//0021-843x.101.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Towey JP, Stewart JW, Friedman D, Tenke C, Quitkin FM. Event-related potentials in depression: Influence of task, stimulus hemifield and clinical features on P3 latency. Biol Psychiatry. 1991;30:233–246. doi: 10.1016/0006-3223(91)90108-x. [DOI] [PubMed] [Google Scholar]
- Buchwald AM. Depressive mood and estimates of reinforcement frequency. J Abnorm Psychol. 1977;86:443–446. doi: 10.1037//0021-843x.86.4.443. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Revised physical anhedonia scale. 1978 Unpublished manuscript. [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of handedness. Brain Cogn. 1987;6:175–183. doi: 10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Edell WS, Chapman JP. Physical anhedonia, perceptual aberration and psychosis proneness. Schizophr Bull. 1980;6:639–653. doi: 10.1093/schbul/6.4.639. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: A new graphic interactive environment for designing psychology experiments. Behav Res Methods Instrum Comput. 1993;25:257–271. [Google Scholar]
- Costello CG. Depression: Loss of reinforcers or loss of reinforcer effectiveness? Behavior Therapy. 1972;3:240–247. doi: 10.1016/j.beth.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 1992;20:125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior electrophysiological asymmetries, emotion, and depression: conceptual and methodological conundrums. Psychophysiology. 1998;35:607–614. doi: 10.1017/s0048577298000134. [DOI] [PubMed] [Google Scholar]
- Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Annu Rev Psychol. 1989;40:57–492. doi: 10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Clark DC, Scheftner WA, Hedeker D. Differences between anhedonic and normally hedonic depressive states. Am J Psychiatry. 1983;140:1027–1030. doi: 10.1176/ajp.140.8.1027. [DOI] [PubMed] [Google Scholar]
- Fiorito ER, Simons RF. Emotional imagery and physical anhedonia. Psychophysiology. 1994;31:513–521. doi: 10.1111/j.1469-8986.1994.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Gotlib IH. Self-reinforcement and depression in interpersonal interaction: the role of performance level. J Abnorm Psychol. 1982;91:3–13. doi: 10.1037//0021-843x.91.1.3. [DOI] [PubMed] [Google Scholar]
- Gotlib IA, Ranganath C, Rosenfeld JP. Frontal EEG alpha asymmetry, depression, and cognitive functioning. Cognition and Emotion. 1998;12:449–478. [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Green D, Swets JA. Signal Detection Theory and Psychophysics. John Wiley; New York: 1966. [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. J Abnorm Psychol. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cognition and Emotion. 2000;14:711–714. [Google Scholar]
- Henriques JB, Glowacki JM, Davidson RJ. Reward fails to alter response bias in depression. J Abnorm Psychol. 1994;103:460–466. doi: 10.1037//0021-843x.103.3.460. [DOI] [PubMed] [Google Scholar]
- Herbener ES, Harrow M. The course of anhedonia during 10 years of schizophrenic illness. J Abn Psychology. 2002;111:237–248. [PubMed] [Google Scholar]
- Hughes JR, Pleasants CN, Pickens RW. Measurement of reinforcement in depression: a pilot study. J Behav Ther Exp Psychiatry. 1985;16:231–236. doi: 10.1016/0005-7916(85)90068-0. [DOI] [PubMed] [Google Scholar]
- Hull CL. Principles of Behavior. An Introduction to Behavior Theory. Appleton-Century-Crofts; New York: 1943. [Google Scholar]
- Hyman SE. Neuroscience, genetics, and the future of psychiatric diagnosis. Psychopathology. 2002;35:139–144. doi: 10.1159/000065134. [DOI] [PubMed] [Google Scholar]
- Johnstone V, Alsop B. Reinforcer control and human signal-detection performance. J Exp Anal Behav. 2000;73:275–290. doi: 10.1901/jeab.2000.73-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner TE, Brown JS, Metalsky GI. A test of the tripartite model’s prediction of anhedonia’s specificity to depression: patients with major depression versus patients with schizophrenia. Psychiatry Res. 2003;119:243–250. doi: 10.1016/s0165-1781(03)00131-8. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Lonigan CJ. Tripartite model of depression and anxiety in youth psychiatric inpatients: relations with diagnostic status and future symptoms. J Clin Child Psychol. 2000;29:372–382. doi: 10.1207/S15374424JCCP2903_8. [DOI] [PubMed] [Google Scholar]
- Kwapil TR. Social anhedonia as a predictor of the development of schizophrenia-spectrum disorders. J Abn Psychology. 1998;107:558–565. doi: 10.1037//0021-843x.107.4.558. [DOI] [PubMed] [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. J Abnorm Psychol. 2002;111:589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- Leboyer M, Bellivier F, Nosten-Bertrand M, Jouvent R, Pauls D, Mallet J. Psychiatric genetics: search for phenotypes. Trends Neurosci. 1998;21:102–105. doi: 10.1016/s0166-2236(97)01187-9. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Graf M. Pleasant activities and depression. J Consult Clin Psychol. 1973;41:261–268. doi: 10.1037/h0035142. [DOI] [PubMed] [Google Scholar]
- Loas G. Vulnerability to depression: a model centered on anhedonia. J Affect Disord. 1996;41:39–53. doi: 10.1016/0165-0327(96)00065-1. [DOI] [PubMed] [Google Scholar]
- Lonigan CJ, Hooe ES, David CF, Kistner JA. Positive and negative affectivity in children: confirmatory factor analysis of a two-factor model and its relation to symptoms of anxiety and depression. J Consult Clin Psychol. 1999;67:374–386. doi: 10.1037//0022-006x.67.3.374. [DOI] [PubMed] [Google Scholar]
- Luce RD. A threshold theory for simple detection experiments. Psychol Review. 1963;70:61–79. doi: 10.1037/h0039723. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman DC. Detection Theory: A User’s Guide. Cambridge University Press; New York: 1991. [Google Scholar]
- McCarthy DC. Behavioral detection theory: Some implications for applied human research. In: Nevin JA, Davison MC, Commons ML, editors. Signal Detection: Mechanisms, Models, and Applications. Erlbaum; Hillsdale, NJ: 1991. pp. 239–255. [Google Scholar]
- McCarthy D, Davison M. Signal probability, reinforcement, and signal detection. J Exp Anal Behav. 1979;32:373–382. doi: 10.1901/jeab.1979.32-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl PE. Hedonic capacity: some conjectures. Bull Menninger Clin. 1975;39:295–307. [PubMed] [Google Scholar]
- Mineka S, Rafaeli E, Yovel I. Cognitive bias in emotional disorders: Information processing and social-cognitive perspective. In: Davidson RJ, Scherer KS, Goldsmith HH, editors. Handbook of Affective Sciences. Oxford University Press; New York: 2003. pp. 976–1009. [Google Scholar]
- Pizzagalli DA, Nitschke JB, Pascual-Marqui RD, Larson CL, Abercrombie HC, Schaefer SM, et al. Brain electrical tomography abnormalities in depression: The importance of melancholic features and anxiety. Biol Psychiatry. 2002;52:73–85. doi: 10.1016/s0006-3223(02)01313-6. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes TR, Fox AS, Chung MK, Larson CL, Abercrombie HC, et al. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;9:393–405. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning and the effectiveness of reinforcement and non-reinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning. 2. Current Research and Theory. Appleton-Century-Crofts; New York: 1972. pp. 64–69. [Google Scholar]
- Rush AJ, Weissenburger JE. Melancholic symptom features and DSM-IV. Am J Psychiatry. 1994;151:489–498. doi: 10.1176/ajp.151.4.489. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Strauss ME, Wisner KL. Diminished response to pleasant stimuli by depressed women. J Abnorm Psychol. 2001;110:488–493. doi: 10.1037//0021-843x.110.3.488. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushere RE. Manual of the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- Spijker J, Bijl RV, de Graaf R, Nolen WA. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Acta Psychiatr Scand. 2001;103:122–130. doi: 10.1034/j.1600-0447.2001.103002122.x. [DOI] [PubMed] [Google Scholar]
- Sprinkle SD, Lurie D, Insko SL, Atkinson G, Jones GL, Logan AR, et al. Criterion validity, severity cut scores, and test-retest reliability of the Beck Depression Inventory-II in a university counseling center sample. J Counsel Psychol. 2002;49:381–385. [Google Scholar]
- Suslow T, Junghanns K, Arolt V. Detection of facial expressions of emotions in depression. Percept Mot Skills. 2001;92:857–868. doi: 10.2466/pms.2001.92.3.857. [DOI] [PubMed] [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. J Clin Child Psychol. 1999;28:366–375. doi: 10.1207/S15374424jccp280309. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. Negative affectivity: the disposition to experience aversive emotional states. Psychol Bull. 1984;96:465–490. [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]