Abstract
Sex differences in thrombosis are well described, but their underlying mechanism(s) are not completely understood. Coagulation proteins are synthesized in the liver, and liver gene expression is sex specific and depends on sex differences in growth hormone (GH) secretion — males secrete GH in a pulsatile fashion, while females secrete GH continuously. Accordingly, we tested the hypothesis that sex-specific GH secretion patterns cause sex differences in thrombosis. Male mice were more susceptible to thrombosis than females in the thromboplastin-induced pulmonary embolism model and showed shorter clotting times ex vivo. GH-deficient little (lit) mice were protected from thrombosis, and pulsatile GH given to lit mice restored the male clotting phenotype. Moreover, pulsatile GH administration resulted in a male clotting phenotype in control female mice, while continuous GH caused a female clotting phenotype in control male mice. Expression of the coagulation inhibitors Proc, Serpinc1, Serpind1, and Serpina5 were strongly modulated by sex-specific GH patterns, and GH modulated resistance to activated protein C. These results reveal what we believe to be a novel mechanism whereby sex-specific GH patterns mediate sex differences in thrombosis through coordinated changes in the expression of coagulation inhibitor genes in the liver.
Introduction
Male sex is an independent risk factor for several thrombin-dependent thrombotic processes such as myocardial infarction, venous thromboembolism (VTE), and thrombotic stroke (1–5). Strikingly, males are on average 50% more likely to suffer recurrent VTE than females (2, 5, 6). Given the toll taken by thrombotic diseases, a mechanistic understanding of how sex functions as a disease modifier would be highly desirable. Thrombin is the major effector protease of the coagulation cascade. Through biochemical and genetic studies, we know that exposure of subendothelial tissue factor (TF) triggers thrombin generation through the serial activation of a cascade of proteases and inhibitors (7–10). We and others have found significant sex differences in thrombosis models in mice (11–14). In each case, male mice were more susceptible to thrombus formation than females. Accordingly, we used mouse models to explore the mechanisms underlying sex differences in thrombosis.
Growth hormone (GH) is a pleiotropic hormone synthesized and secreted by the pituitary (15, 16). The pattern of GH secretion is sex specific (17). By convention, male secretion is characterized as pulsatile with episodic bursts occurring every 2–3 hours overlying basal continuous secretion and long interpulse intervals; the female pattern is characterized by more frequent pulses and a short interpulse interval, resulting in continuous presence of GH in the plasma (17–22). There are strong sex differences in liver gene expression, and these are thought to depend on the sex-specific patterns of GH secretion (21–25). Indeed, differences in patterns of gene expression are significantly attenuated by removal of the pituitary and by genetic models of GH deficiency or resistance (26, 27). Replacement of GH in a male or female pattern is sufficient to induce male- or female-specific patterns of gene expression, with the sex-specific effect determined by the length of the interpulse interval (23, 27–30). There are several genetic models of GH deficiency or resistance in mice (31–35). Mice with a spontaneous point mutation in the GH-releasing hormone receptor (Ghrhr) are the best-characterized model of GH deficiency and are termed little (lit) (36–38). Genetic manipulation of GH in mice provides a means of probing the roles of GH-dependent, sex-specific patterns of liver gene expression (34, 39–41).
Because most circulating coagulation factors and inhibitors are synthesized by the liver, we set out to test the hypothesis that sex-specific GH secretion alters hepatic production of these factors to produce differences in susceptibility to thrombosis. Indeed, GH-deficient lit mice were protected from thrombosis. Moreover, sex-specific GH administration patterns strongly modulated the clotting time, presumably through effects on expression of the coagulation inhibitors Proc, Serpinc1, Serpind1, and Serpina5.
Results
Sex differences in hemostasis and thrombosis in WT mice.
All mice were studied between 6 and 8 weeks of age, and all of them were sexually mature. All experiments were conducted by researchers blind to genotype and repeated at least twice, except where otherwise indicated. The background strain of all mice tested was 100% C57BL/6J (B6). In general, littermate controls were used, except where otherwise indicated. The concentration of GH in each treatment group was measured and is reported in Supplemental Table 1 (supplemental material available online with this article; doi: 10.1172/JCI34957DS1).
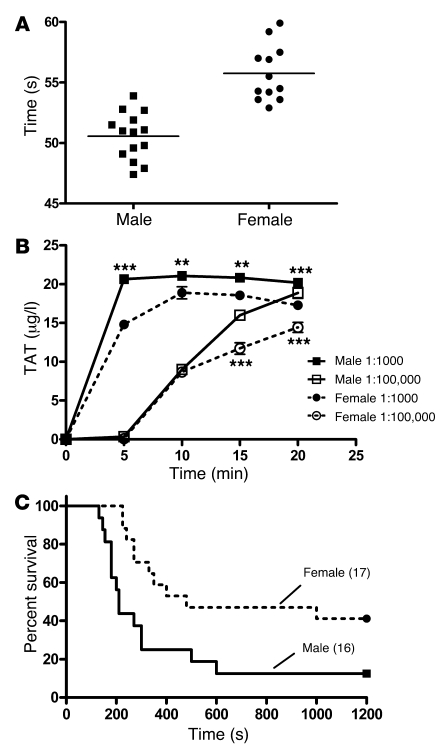
Thrombin generation, and subsequent clot formation is normally triggered by TF. We developed a simple assay whereby clot formation was triggered in whole blood by the addition of dilute TF and found significant sex differences. In a group of 6-week-old WT B6 mice, the mean clotting times were prolonged in female versus male mice (mean clotting times, 50.57 ± 0.5 and 55.76 ± 0.7 seconds in males and females, respectively; P = 0.0001; Figure 1A). The rate and magnitude of thrombin generation were significantly attenuated in females compared with males (Figure 1B). To determine whether the clotting differences were at least partially due to differences in plasma coagulation factors or inhibitors, we measured TF-triggered clotting in platelet-poor plasma (PPP). The sex difference persisted, with mean clotting times of 56.35 ± 0.3 and 63.85 ± 0.5 seconds in males and females, respectively (P = 0.002). This suggested that thrombin generation was attenuated in females as compared with males and that the difference was at least somewhat based on differences in quantity or activity of plasma coagulation factors or inhibitors.
Figure 1. Sex differences in thrombosis in WT mice.
Blood was drawn from 15 male and female WT B6 mice. (A) Mean whole-blood clotting times were significantly shorter in male versus female mice. P < 0.0001, Student’s t test. (B) Whole-blood clotting was triggered with 2 dilutions (indicated) of thromboplastin. The reaction was quenched at 5-minute intervals, and thrombin-antithrombin (TAT) levels were measured. For the 1:1,000 dilution, TAT values (±SEM) were higher in male versus female mice at all time points. For the 1:100,000 dilution, TAT was higher in males at the 15- and 20-minute time points (**P < 0.01, ***P < 0.001; ANOVA with Bonferroni’s post-hoc test). Data represent pooled blood from 5 mice of each sex each run in duplicate and measured in duplicate. (C) The indicated numbers of male and female WT mice were injected with 2 μl/g of a 1:160 dilution of thromboplastin in the in vivo model of PE. Data are presented as percent survival, which was greater in female versus male mice as compared by the log-rank test (P = 0.01).
To assess the in vivo relevance of these findings, we tested mice in the well-characterized thromboplastin-induced pulmonary embolism (PE) model (11, 42). We injected 2 μl/g body weight of a 1:160 dilution of thromboplastin into the inferior vena cava of 8-week-old B6 animals and found that female animals were protected to a significantly greater degree as compared with males, with median survival times of 480 versus 210 seconds and survival percentages of 41% versus 12.5% (Figure 1C). There were no differences in tail bleeding times (data not shown).
Effect of GH deficiency on thrombosis in mice.
To determine the effect of GH on sex differences in thrombosis, we made use of lit mice, a genetic model of GH deficiency (36–38). lit mice have a point mutation in the gene encoding GH-releasing hormone receptor (Ghrhr). Animals homozygous for the mutation (litm/m) have essentially no circulating GH and are significantly smaller than WT control animals, while mice heterozygous for the mutation (litm/+) are indistinguishable from WT animals (36). In preliminary work, we compared male and female litm/+ and WT animals in both the whole-blood clotting assay and in the PE model, and there were no differences (Supplemental Figure 1).
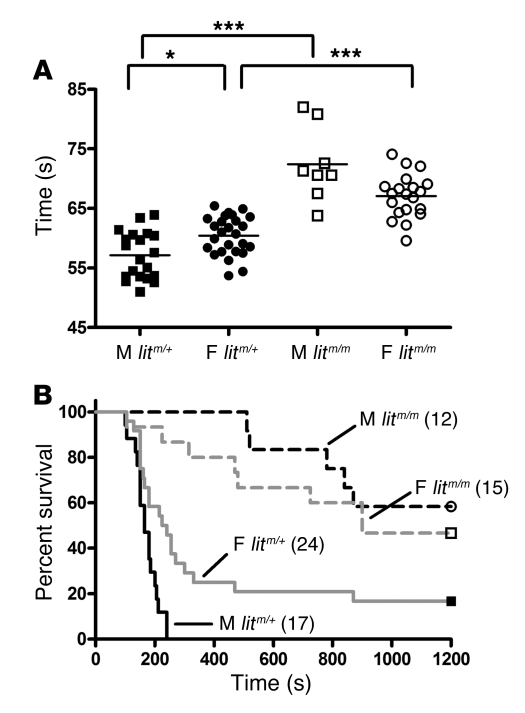
In vitro, male mice homozygous for the lit mutation (litm/m) had significantly prolonged whole-blood clotting times as compared with male litm/+ littermates (mean clotting times, 57.15 ± 0.9 and 60.46 ± 0.5 seconds; and 72.4 ± 2.2 and 67.08 ± 0.8 seconds in male and female litm/+ versus litm/m animals, respectively (P < 0.05, P < 0.001, and P < 0.001 for male versus female litm/+, litm/m versus litm/+ male, and litm/m versus litm/+ female comparisons, respectively; ANOVA with Bonferroni’s post-hoc test) (Figure 2A).
Figure 2. Effect of GH on whole-blood clotting and thrombosis in vivo.
Blood was collected and prepared as in Figure 1. (A) Individual whole-blood clotting times. Mean clotting times (lines) were significantly shorter in male (filled squares) versus female (filled circles) litm/+ mice; *P < 0.01. Mean clotting times were significantly longer in male (open squares) and female (open circles) GH-deficient litm/m mice versus litm/+ mice; ***P < 0.001, ANOVA with Bonferroni’s post-hoc test. (B) The indicated number of animals of each sex were injected with 2 μl/g of a 1:80 dilution of thromboplastin in the PE model as in Figure 1. Median survival was greater in female versus male litm/+ mice (P = 0.001, log-rank test) and in litm/m (dashed lines) animals versus litm/+ (solid lines; P = 0.0001, log-rank test): 165, 232, 1,200, and 900 seconds and 0%, 17%, 58%, and 47% in male and female litm/+ and litm/m mice respectively.
To assess the impact in vivo, we tested male and female litm/+ and litm/m mice in the PE model. In a preliminary dose-finding experiment, we determined that lethality was significantly reduced in litm/m versus control mice. Therefore, we injected 2 μl/g body weight of an increased concentration of thromboplastin (1:80 dilution). We found that both male and female litm/m mice were significantly protected versus litm/+ control animals but the difference between them was not significant (median survival, 165, 232, 1,200, and 900 seconds and survival percentages, 0%, 17%, 58%, and 47% in male and female litm/+ and litm/m mice respectively; P < 0.001, male versus female litm/+, P < 0.0001, male litm/+ versus litm/m, P < 0.0001, female litm/+ versus litm/m, and P = NS, male versus female litm/m; Figure 2B). There was no effect on bleeding times in the lit mice (data not shown). Taken together, these data suggest that the loss of circulating GH caused a female-like attenuation in thrombin generation and prolonged whole-blood clotting times in vitro and protection from thrombosis, in vivo.
Effect of sex-specific GH secretion patterns on clotting.
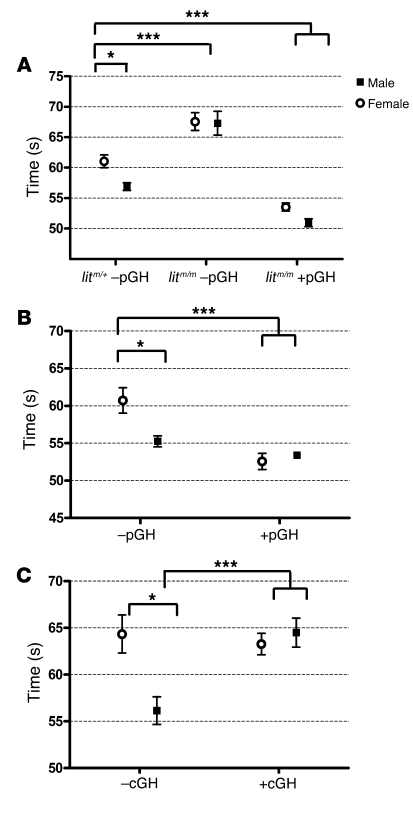
We next asked whether replacement of GH in a pulsatile (male) pattern could restore the male coagulation phenotype in GH-deficient male and female litm/m or female litm/+ mice. To test this, we injected male and female litm/m mice twice daily with 2 μg/g body weight mouse GH for 1 week and compared then with mice injected with vehicle control. We found that pulsatile GH (pGH) replacement restored the whole-blood clotting times of male and female litm/m mice to those seen in control (litm/+) male mice not treated with GH (Figure 3A).
Figure 3. pGH administration rescues the whole-blood clotting defect in lit mice and shortens clotting times in WT female mice, while cGH administration prolongs the clotting time in WT male mice.
Blood was collected and prepared as in Figure 1. (A) Whole-blood clotting times (mean ± SEM) were significantly shorter in male (filled squares) versus female (open circles) litm/+ mice not receiving GH (–pGH); *P < 0.01. Mean clotting times were significantly longer in male and female litm/m –pGH versus litm/+ –pGH mice; ***P < 0.001, ANOVA with Bonferroni’s post-hoc test. Clotting times were shortened to male litm/+ –pGH levels (and were significantly shorter than those in litm/+ –pGH females) in male and female litm/m mice receiving pGH (+pGH); ***P < 0.001. (B and C) WT male and female mice were given an identical dose of GH (28 μg/g body weight) or vehicle either divided into twice-daily doses over 7 days (pGH) (B) or as a continuous infusion (cGH) (C). The clotting times of female animals receiving pGH (WT +pGH) (B) were shortened relative to those of animals not receiving GH (WT –pGH), with no effect on male animals. Whole-blood clotting times (mean ± SEM) were significantly shorter in male (filled squares) than female (open circles) WT –cGH mice in both groups. The clotting times of male animals receiving cGH (WT +cGH) (C) were prolonged relative to those of animals receiving vehicle control (WT –cGH), with no effect on female animals. *P < 0.05, ***P < 0.001, ANOVA with Bonferroni’s post-hoc test.
Given that pGH restored the male phenotype in lit mice, we next sought to determine the effect of GH administration patterns on WT male and female mice. We gave male and female mice twice-daily injections of GH or vehicle control (WT +pGH or WT –pGH) at 2 μg/g body weight for 1 week. We measured whole-blood clotting, and while there was little effect on the male mice, female mice +pGH had demonstrably shortened clotting times as compared with a matched group of females receiving vehicle control (–pGH) (Figure 3B).
To test the effect of continuous GH (cGH) administration on WT animals we, gave a dose of GH identical to that in the pulsatile injections as a continuous infusion over 7 days (28 μg/g body weight total). Here the results were the exact opposite of those seen after pulsatile treatment. Specifically, there was no significant difference between cGH-treated (WT +cGH) and control (WT –cGH) female mice, but male mice given cGH (WT +cGH) over 1 week had prolonged whole-blood clotting times as compared with those given vehicle control (WT –cGH) (Figure 3C). There was no difference between female mice +cGH or –cGH, and there was no difference between male mice +cGH and either female group.
Effect of sex-specific patterns of GH secretion on coagulation factor activity.
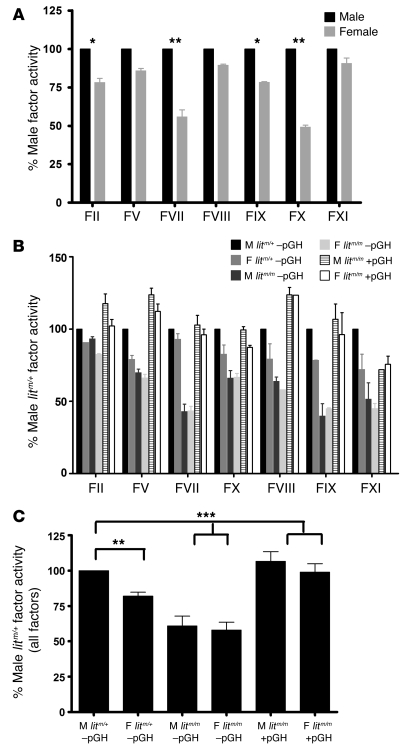
Given the well-described effects of GH on sex differences in liver protein expression, we looked for potential mechanisms underlying the sex-specific coagulation phenotypes by measuring activity of procoagulant coagulation factors. We measured the functional activity of factor II (F2), FV, FVII, FVIII, FIX, FX, and FXI by modifying commercially available human clot–based end-point assays using the relevent human factor–deficient plasmas. To simplify the analysis, we normalized activity to control male levels. In WT animals, we found increases in the activity of each of the procoagulant factors in WT males as compared with females (Figure 4A). These data were consistent with the finding of increased thrombin generation and increased in vitro clotting tendency in male as compared with female mice.
Figure 4. Procoagulant factor activity levels are higher in male than female mice and are GH dependent.
Blood was collected as in Figure 1. Coagulation factor activity assays were adapted for use in mice. (A) Plasma from 4 individual male and female mice was mixed with the indicated human factor–deficient plasma, and factor activity was normalized to that in WT B6 males and expressed as the mean (±SEM) percentage of male activity. Factor activity levels are greater in male versus female plasma for all assays (*P < 0.05, **P < 0.001; ANOVA with Bonferroni’s post-hoc test). (B) Plasma from 4 animals of each indicated group was collected and prepared and mean (±SEM) activity levels expressed as a percentage of male litm/+ –pGH activity. Factor activity levels were generally lower in female litm/+ –pGH and male and female litm/m –pGH mice versus male litm/+ –pGH and male and female litm/m +pGH for all assays. (C) The average of all procoagulant factor activity levels for each group. Factor activity levels were significantly lower in female litm/+ –pGH and male and female litm/m –pGH versus male litm/+ –pGH and male and female litm/m +pGH. **P < 0.01, ***P < 0.0001; ANOVA with Bonferroni’s post-hoc test.
Next, we measured coagulation factor activity in lit mice receiving pGH or vehicle. Again, we normalized activity to that in male litm/+ –pGH mice (100%) and found a consistent trend whereby activity of each factor was increased in male versus female litm/+ –pGH mice; decreased in both male and female litm/m –pGH mice; and increased in both male and female litm/m +pGH mice (Figure 4B). Since the trend was consistent across all factors, to further simplify the analysis, we averaged the relative factor activity of each assay and calculated the mean factor activity of all factors relative to male litm/+ mice –pGH. Here, we found that factor activity levels were 100% and 82% ± 7.4%; 60.85% ± 18.32% and 57.97% ± 14.64%; and 106.60% ± 18.15% and 99.00% ± 15.73% in male and female litm/+ –pGH, male and female litm/m –pGH, and male and female litm/m +pGH mice, respectively (Figure 4C). This provided some rationale for the observed sex differences in thrombin generation and in clotting, with males having increased activity of procoagulant factors as compared with females.
Effect of sex-specific patterns of GH secretion on coagulation-related genes in the liver.
We observed a generalized increase in procoagulant factor activity in male versus female mice. The relative increase in male factor activity was GH dependent. Since GH is known to strongly modulate expression of hepatic genes and given that most coagulation factors and inhibitors are synthesized or modified in the liver, we next measured expression of a panel of coagulation-related genes from the livers of male and female mice by quantitative real-time PCR. We isolated total RNA from the livers of individual mice and synthesized cDNA. From this, we measured gene expression using specific TaqMan primer probe sets individually generated and validated for each gene. It should be noted that the synthesis of FV and FVIII occurs predominantly in platelets and endothelial cells and to a lesser extent in liver. In WT mice, we found that expression of female coagulation factor genes was not significantly different from that in male mice (Figure 5A). Therefore, differences in expression of procoagulant genes did not explain the results of the whole-blood clotting, thrombosis, and coagulation factor activity assays. Previous work by others had shown that increased levels of coagulation inhibitors such as TF pathway inhibitor (TFPI) and antithrombin (AT) could effectively decrease the measurement of procoagulant factor activity in 1-stage assays (43). Since regulation of thrombin generation is generally assumed to occur through TFPI, AT, and the activated protein C (APC) pathway, we hypothesized that the relative differences in procoagulant factor activity were due not to changes in quantity or function of each factor but rather to the changes in concentration or activity of one or more coagulation inhibitors. Therefore, we next measured expression of a panel of coagulation inhibitors.
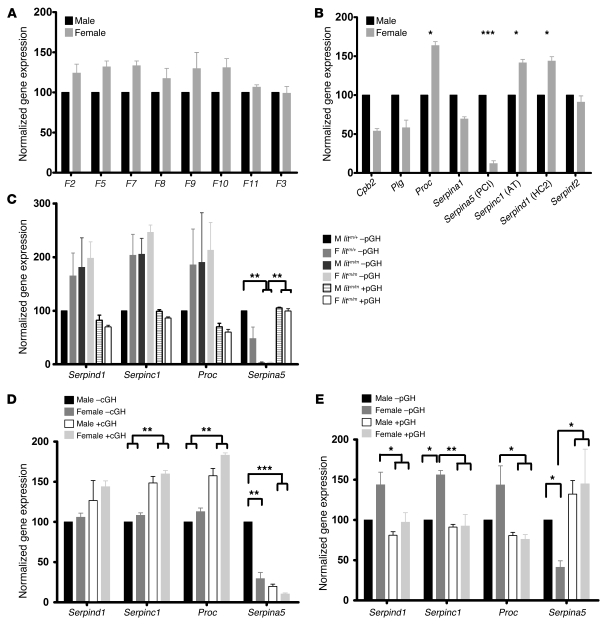
Figure 5. Expression of coagulation factor and inhibitor genes.
Total RNA was prepared from the livers of 4 animals from each group. cDNA was reverse transcribed and subjected to quantitative real-time PCR. Abundance of each mRNA relative to internal controls was calculated to generate gene copy number (GCN) (59). GCN was normalized to the relevant male control (black) and expressed as mean (±SEM) percentage normalized expression. (A) Procoagulant gene expression was not different in female versus male liver. (B) Expression of the inhibitors Proc, Serpind1, and Serpinc1 was increased, while expression of Serpina5 was decreased, in female versus male liver. (C) Expression of Proc, Serpind1, and Serpinc1 was increased and expression of Serpina5 was decreased in female litm/+ –pGH and female and male litm/m –pGH mice relative to litm/+ –pGH. Expression was restored to baseline in female and male litm/m +pGH animals. (D) Animals were given cGH as in Figure 3B. Expression of Proc, Serpind1, and Serpinc1 was increased and expression of Serpina5 was decreased in female control mice (WT–cGH) and female and male mice receiving cGH (WT +cGH) relative to male mice –cGH. (E) Animals were given pGH as in Figure 3C. Expression of Proc, Serpind1, and Serpinc1 was decreased and expression of Serpina5 was increased in female control mice (WT –pGH) and female and male mice receiving pGH (WT +pGH) relative to male control WT –pGH mice. *P < 0.01, **P < 0.001, ***P < 0.0001; ANOVA with Bonferroni’s post-hoc test.
Among all the inhibitors tested, we found that expression of the genes encoding protein C (Proc), AT (Serpinc1), and heparin cofactor II (HCII) (Serpind1) was significantly higher in female versus male liver, while expression of protein C inhibitor (PCI) (Serpina5) was significantly lower in female versus male liver (Figure 5B).
To explore the effect of GH secretion on expression and activity of each of these inhibitors, we next measured expression of these genes from the livers of: (a) lit mice ±pGH; (b) WT mice ±cGH; and (c) WT mice ±pGH. To simplify the analysis, we normalized all gene expression levels to those in control male animals (100%). In lit mice, litm/+ gene expression mimicked that in WT. As shown in Figure 5C, expression of Proc, Serpind1, and Serpinc1 was higher in litm/+ –pGH female versus male liver. In male and female litm/m –pGH mice, expression of Proc, Serpind1, and Serpinc1 was increased to female litm/+ –pGH levels and was greater than that in litm/+ –pGH males, while expression was decreased to male litm/+ –pGH levels in male and female litm/m +pGH livers. Interestingly, expression of the purported inhibitor of APC, Serpina5, was higher in litm/+ –pGH males versus females. Expression was significantly reduced to undetectable levels in male and female litm/m –pGH mice versus litm/+ –pGH controls and increased to male control levels in male and female litm/m +pGH mice.
As shown in Figure 5D, these results were mirrored in WT mice given cGH (28 μg/g body weight) for 1 week. Here there was an increase in expression of Proc, Serpind1, and Serpinc1 in the livers of male animals receiving cGH (WT +cGH) versus control (WT –cGH). Serpina5 expression also mirrored that seen in the lit mice, with a significant reduction in Serpina5 gene expression in male mice given cGH (WT +cGH) as compared with male mice given vehicle control (WT –cGH) (Figure 5D).
Remarkably, WT mice given pGH for 1 week (2 μg/g body weight twice daily) (WT +pGH) showed the exact opposite effect, with decreased expression of Proc, Serpind1, and Serpinc1 in female liver (Figure 5E) and a substantial increase in Serpina5 gene expression in males and females as compared with control mice injected with vehicle (WT –pGH) (Figure 5E).
Effect of GH on resistance to APC.
The gene expression data provided circumstantial evidence that sex differences in coagulation factor activity, whole-blood clotting, and thrombosis in vivo were not due to changes in expression of procoagulant factors; rather, the effects appeared to be secondary to coordinated changes in the synthesis of multiple regulators of thrombin generation or action. Serpind1 and Serpinc1 encode the well-known thrombin inhibitors HCII and AT. Proc encodes the zymogen precursor to APC, the predominant plasma-based regulator of thrombin generation, while Serpina5, also known as PCI, is a potent inhibitor of APC and a host of other serine proteases.
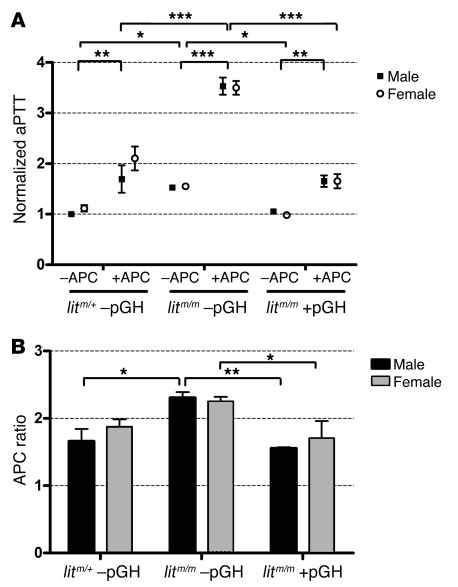
Increased or decreased concentrations of AT, HCII, and protein C are known to significantly affect thrombosis in humans in mice, but the effect of PCI on thrombosis remains elusive. This led us to question whether GH might modulate APC resistance through changes in concentration of PCI or perhaps another APC cofactor or inhibitor. To test this, we measured APC resistance using the activated partial thromboplastin time (aPTT) method. We measured aPTT in the presence and absence of a low concentration of APC (2.5 nM) in male and female litm/+ –pGH, litm/m –pGH, and litm/m +pGH (2 μg/g body weight) animals, and normalized the results to those in control males (litm/+ –pGH). We found that the baseline aPTT was nonsignificantly higher in litm/+ –pGH females versus males but was significantly prolonged in both male and female litm/m –pGH mice and significantly lower in litm/m +pGH (Figure 6A). As expected, APC significantly prolonged the aPTT in all mice; however, the degree of prolongation was greater in litm/m –pGH than in litm/+ –pGH or litm/m +pGH animals. Indeed, with APC, the aPTT was significantly higher in plasma from litm/m –pGH versus litm/+ –pGH or litm/m +pGH animals. As shown in Figure 6B, we divided the level the raw aPTT with APC by that without APC to determine the APC ratio — a measure of APC resistance — and found that the ratio was significantly higher in litm/m –pGH than litm/+ –pGH or litm/m +pGH animals, suggesting that deficiency of GH caused a relative decrease in APC resistance that was restored in animals receiving pGH for 1 week. Additionally, we measured APC resistance in WT animals receiving 1 week of pGH or cGH and found that the APC ratio was significantly lower in male versus female WT –pGH. Treatment with cGH increased the APC ratio in male WT animals to the level in WT females, while treatment with pGH decreased the APC ratio in WT female mice to the level in WT males (Supplemental Figure 2). This strongly suggests that GH modulates the expression of one or more molecules that regulate the actions of APC and mediate changes in APC resistance.
Figure 6. Effect of GH on resistance to APC.
Blood was collected and plasma prepared as in Figure 1. Plasma from individual littermate animals was diluted 1:5 in 0.9% saline containing 2 mg/ml BSA. (A) The aPTT was measured in 3 animals from each indicated group (in duplicate) both with and without APC (final concentration, 2.5 nM) and normalized to control (male litm/+ –pGH). The APC was mixed with CaCl2, which was used to trigger the reaction. There was a small but nonsignificant increase in the normalized aPTT in female (open circles) versus male (filled squares) control animals. The aPTT was increased in male and female litm/m –pGH animals relative to litm/+ –pGH or litm/m +pGH animals. The aPTT was increased significantly in all groups by the addition of APC; however, the effect or APC was especially pronounced in litm/m –pGH animals relative to litm/+ –pGH or litm/m +pGH (*P < 0.05, **P < 0.01, ***P < 0.001; ANOVA with Bonferroni’s post-hoc test). (B) The raw aPTT plus APC was divided by the aPTT minus APC to generate an APC ratio for each group. The APC ratio is inversely proportional to APC resistance and was nonsignificantly higher in female litm/+ –pGH than male control animals. The APC ratio was significantly increased in male litm/m –pGH animals relative to litm/+ –pGH or litm/m +pGH animals and female litm/m –pGH animals relative to litm/m +pGH animals, suggesting that APC resistance is mediated by circulating GH concentration (*P < 0.05, **P < 0.01; ANOVA with Bonferroni’s post-hoc test).
Discussion
In this study, we found significant sex differences in thrombosis in mice; these differences were abrogated in mice deficient in GH. Specific GH administration patterns modulated changes in expression of the genes encoding the principal regulators of thrombin generation in plasma, protein C (Proc), AT (Serpinc1), and HCII (Serpind1) and the purported procoagulant inhibitor PCI (Serpina5) in the liver. Thus, the changes in whole-blood clotting and PE susceptibility appear to be mediated by the effects of GH and resultant changes in expression of several plasma-based modulators of thrombin generation.
To our knowledge, this represents the first description of the effects of GH on sex differences in coagulation or thrombosis in humans or rodents. We believe this is an important step in understanding the biological mechanism underlying important sex differences in disease susceptibility. While there are potential confounding effects of age, supplemental sex hormones, or pregnancy, and while there is no evidence of sex differences in the rate of incident VTE (44), there is substantial evidence for strong sex differences in the rate of recurrent VTE (1, 2, 5, 6). Thus, there is great value in understanding the mechanisms underlying such differences, as they may lead to novel paradigms of diagnosis or management of thrombotic disorders.
There is also substantial evidence of sex-specific effects in thrombosis in mouse models. Such models are of fundamental importance to the field of hemostasis and thrombosis. We and others have found that male mice are more susceptible to thrombosis in vivo, yet there has been, until now, no explanation for such differences (11–14). We hope that this work will help to elucidate the very real sex differences in thrombosis models in mice and may explain why effects of drugs or gene knockout studies have been sex dependent.
To our knowledge, our study represents the most detailed analysis of the effects of GH on coagulation yet published and is the first to examine the effects of GH on thrombosis in vivo. Previous studies have evaluated the effect of GH on coagulation in rats (45, 46). However, these studies demonstrated changes exclusively in either male or female rats and found conflicting results, and none measured effects on thrombosis in vivo. The data regarding the effects on GH on human thrombosis are sparse. A current review of the literature reveals 1 recent article examining the effect of GH replacement on coagulation parameters in male and female patients with GH deficiency. There were no differences between control and GH-deficient patients, but replacement of GH caused prolongation of aPTT only in males and of the prothrombin time only in females (47).
The effects of GH on thrombosis in this study were modulated at least to some degree by changes in the concentration of anticoagulant inhibitors. Each of these inhibitors is known to be a principal regulator of thrombin generation in plasma. The models used in this study are clearly thrombin dependent. We previously demonstrated that mice with abnormal platelet thrombin signaling are protected in the well-characterized PE model (11, 12). Here, we showed that the model is thrombosis dependent, with dose-dependent changes in intravascular thrombus formation and subsequent decreases in postmortem lung perfusion (11, 12). APC has also been shown to protect mice in a similar model of thrombin-induced PE (48), and in prior work, we have found that mice with heterozygous deficiency of AT are more susceptible in the PE model, with median survival times of 210 versus 280 seconds in AT+/– versus AT+/+ male mice (R.E. Levy and E.J. Weiss, unpublished observations). Indeed, while a direct comparison across experiments is limited by such caveats as background strain and differences in experimental conditions, the magnitude of protection afforded by GH deficiency was at least a great as previously observed in Par3- and Par4-deficient animals. Triggering thrombin generation and thrombosis with dilute TF as we have done in our models incorporates both extrinsic and intrinsic pathway processes. This model is therefore sensitive to alterations in concentration or activity of primary inhibitors of thrombin generation or thrombin’s actions; or those of inhibitors of thrombin-mediated amplification. Alterations in concentrations of any of these molecules would alter the dynamics of thrombin generation or the activity of thrombin and would certainly change the rate of thrombus formation in vitro and in vivo (49).
It is important to note 2 important caveats about the PE model: (a) it is weight based; therefore, male mice do receive a higher dose of thromboplastin than females. Furthermore, the male-female weight difference is largely abrogated in lit mice. While we cannot rule out an effect of weight on mortality in this specific line, we have previously characterized several other mouse knockout lines and inbred strains and have observed no relationship between weight and thromboplastin susceptibility (R.E. Levy and E.J. Weiss, unpublished observations). (b) As described above, both the PE model and the whole-blood clotting model could be influenced by changes in platelet number or function. While we cannot rule out a platelet contribution, we feel that the differences in clotting in PPP, in the coagulation factor assays, and in the APC assays (all performed on plasma) argue for a significant effect of plasma-based proteins.
The effects on the protein C pathway appear to be dual. The gene expression data suggest that there are GH-mediated sex differences in the synthesis of Proc. In addition, the observation of acquired APC resistance strongly suggests that there are also changes in concentration or activity of one or more of the known APC cofactors such as protein S (50) or growth arrest–specific gene 6 (51) or known APC antagonists such as PCI (Serpina5), α1-proteinase inhibitor, or α2-macroglobulin (52). Among the genes we tested, both the baseline sex differences and the effects of GH were greatest on Serpina5. Serpina5 is expressed abundantly in human liver but is not expressed at significant levels outside of the reproductive tract in rodents (53, 54). The mouse knockout of Serpina5 offered no insight into its role in thrombosis because of the low baseline expression (53–55). To our knowledge, mice with a targeted disruption in Serpina5 have not been examined for changes in APC resistance, thrombin generation, or thrombosis in vivo. Given that GH caused a dose-dependent increase in APC resistance while causing a concomitant increase in the expression of Serpina5, it is tempting to implicate Serpina5 in the GH-mediated changes in thrombosis. However, the true effect of Serpina5 on thrombosis in vivo remains to be determined.
Work in the past 20 years has helped uncover the molecular basis for how sex-specific patterns of GH secretion lead to sex differences in hepatic gene expression and effects on growth and metabolism. The seminal work of Norstedt and Palmiter established that sex-specific patterns of GH release can mediate sex-specific patterns of liver protein expression (23). They hypothesized that male-specific liver proteins were induced by the longer male GH interpulse interval and subsequent discontinuous IGF-1 secretion. Female mice — by virtue of the continuous presence of GH — have continuous IGF-1 production and therefore downregulation of IGF-1 receptors, which induce female-specific proteins. lit mice — with near absent GH — have markedly decreased IGF-1 production and therefore a female-like pattern of liver gene expression (23). Since then, there has been tremendous progress in understanding the signaling pathways involved in mediating sex-specific liver gene expression. Much of this work has focused on mice deficient in STAT5 factors. STAT5-knockout mice are GH resistant and have demonstrated significant changes in sex-specific gene expression patterns (29, 34). The IGF-1 hypothesis is bolstered by work in STAT5-deficient animals, as the loss of STAT5 generally feminizes liver gene expression, and STAT5 is necessary for normal IGF-1 production in the liver (56). However, by virtue of the loss of IGF-1–mediated feedback inhibition, STAT5-deficient animals have supraphysiologic GH levels (29, 57). Therefore, the specific effects of decreased IGF-1 versus increased GH itself remain to be determined.
We think that this work raises interesting and unresolved questions. Most notably, Why are there sex differences in thrombosis or thrombosis-related gene expression? Recently, it was shown that Par4 deficiency rescues fetal loss in a genetic model exploring the interactions of fetal and maternal thrombophilia (58). This and other work suggest that increases in maternal thrombin generation are deleterious to fetal development and may partially explain the relative decrease in baseline clotting in females. While this and other questions remain unanswered, with this work, we believe that we have described a novel and important mechanism driving sex differences in clotting in mice that ultimately may help to explain the strong sex differences in susceptibility to human clotting-related diseases. We hope that this work and future work designed to define the mechanism of these important sex differences might someday lead to advances in sex-specific risk assessment, diagnosis, or management of thrombosis-related diseases.
Methods
Mice.
Mouse care and use for these studies was approved by the UCSF Institutional Animal Care and Use Committee. Male and female WT B6 mice and lit mice carrying the Ghrhrlit mutation (referred to as litm) were purchased from The Jackson Laboratory. The lit mice were on a 100% B6 background. Offspring used in these experiments were generated from female litm/m mice mated to male litm/+ intercrosses. No breeder pair was more than 3 generations removed from the original founders purchased from The Jackson Laboratory. Mouse recombinant GH was purchased from A.F. Parlow at the National Hormone and Pituitary Program, UCLA Medical Center, Torrance, California, USA. GH was resuspended in 0.03 M NaCO3, 0.15 M NaCl, pH 9.5. For pulsatile injections, mice were given a dose of either 0 (vehicle) or 2 μg/g body weight intraperitoneally at 7 am and 7 pm for 7 days. The volume of each injection was kept constant. For continuous infusions, ALZET mini-osmotic pumps were loaded with 100 μl of either vehicle or GH at a concentration of 28 μg/g body weight. The pumps were implanted under anesthesia and remained in place for the duration of the life of the mouse. Serum GH levels were measured by RIA carried out by the laboratory of A.F. Parlow.
Ex vivo coagulation assays.
In general, mice were bled at 6 weeks of age, except the GH-treated animals, which were bled at 7 weeks. Blood was drawn via retro-orbital puncture with a 1.5-cm segment of uncoated glass microcapillary tube into 3.2% sodium citrate. Plasma and whole blood were diluted 1:4 in saline (whole blood) or imidizole buffer (Trinity Biotech) (plasma) and tested on an Amelung KC4 Delta micro coagulation analyzer. The blood or plasma was warmed to 37°C for 100 seconds and the reaction triggered with the addition of 100 μl of warmed dilute thromboplastin reagent containing a 1:1,000 dilution of thromboplastin (Trinity Biotech) in saline containing 15 mM calcium chloride. The end point was the time to clot formation as measured automatically. We measured the functional activity of FII, FV, FVII, FVIII, FIX, FX, and FXI. The assays were modified from human clinical assays by mixing mouse plasma with factor-deficient human plasma. Otherwise, the experiments were performed exactly as per the manufacturer’s protocol (Trinity Biotech). Standard curves for each assay were generated using male WT B6 plasma, and percent activity of each factor (relative to normal mouse reference) was calculated. All activities were then normalized to a reference of 100%. aPTTs were measured on mouse plasma diluted in 0.9% saline containing 2 mg/ml BSA and incubated at room temperature for at least 5 minutes. Plasma (50 μl) was aliquoted into coagulation cuvettes and incubated for 60 seconds at 37°C in the coagulometer. Warmed aPTT reagent (50 μl; Alexin HS; Trinity Biotech) was then added to each sample and incubated for an additional 180 seconds. The reaction was then triggered by the addition of warmed calcium chloride reagent (0.02 mol/l; Trinity Biotech) with or without human APC (final concentration, 2.5 nM; Haematologic Technologies Inc.), and clot times were recorded up to a maximum of 300 seconds.
Thrombin-antithrombin complex formation in whole blood.
Ex vivo thrombin generation was measured using a method adapted from Rand et al. to measure the rate and magnitude of thrombin generation in whole mouse blood after initiation of clotting with dilute TF (9). Blood was drawn from the inferior vena cavae of 5 male and female 8-week-old WT B6 mice into 3.2% sodium citrate (1:9). The blood from each sex was pooled, and 50 μl of citrated whole blood was aliquoted into 1.5-ml Eppendorf tubes. Each time point for both sexes and both triggers was tested in duplicate and averaged. Clotting was triggered with 100 μl of 2 separate dilutions (1:1,000 and 1:100,000) of thromboplastin (Trinity Biotech) in saline containing 15 mM calcium chloride. For the 0 time point, quenching solution was added before triggering clotting. The clotting reactions were quenched at 0, 5, 10, and 20 minutes by the addition of 150 μl of 50 mmol/l EDTA, 10 mmol/l benzamidine in HEPES-buffered saline followed by 1 μl of 5 mmol/l d-phenylalanylprolyarginyl-chloromethyl ketone (FPR-ck; Haematologic Technologies Inc.), in 0.01 N HCI. Samples were stored on ice for a maximum of 30 minutes until they were centrifuged. The supernatant was collected and stored at –80°C for thrombin-antithrombin (TAT) analysis. Before analysis, each sample was diluted 1:6 in saline. TAT measurement was performed in duplicate for each sample using a commercially available ELISA (Dade-Behring; Siemens) as per the manufacturer’s protocol on a 96-well SpectraMax384 microplate spectrophotometer (Molecular Devices).
Thromboplastin-mediated PE model.
Mice were tested in vivo at 8 weeks of age, allowing for 2 weeks of recovery after phlebotomy at 6 weeks. Mice were anesthetized with a mixture of ketamine and xylazine. Thromboplastin (2 μl/g body weight; ThromboMAX with Calcium; Trinity Biotech) was injected into the inferior vena cava essentially as previously described (11, 12). For the experiments on WT animals, a thromboplastin dilution of 1:160 was used to achieve approximately 40% lethality in females. For the experiments on lit animals, a thromboplastin dilution of 1:80 was used to allow for the accurate detection of decreased lethality. The end point for this model was death as defined by no spontaneous respirations for more than 30 seconds. At the conclusion of the experiment (death or 20 minutes), liver, heart, and lung tissue was harvested, snap-frozen in liquid nitrogen, and stored at –80°C for later analysis.
Gene expression.
We generated and characterized TaqMan primer/probe sets (5'FAM/3'BHQ; Biosearch Technologies) for real-time PCR for mouse coagulation factor, inhibitor, modifying enzyme, or GH-related genes using Primer Express (Applied Biosystems). Total RNA was isolated and purified from individual mouse organs by TRIzol (Invitrogen) extraction, followed by purification with RNeasy Mini Column (with on-column DNaseI treatment; QIAGEN). First-strand cDNA synthesis was performed using the Superscript First-Strand Synthesis System (Invitrogen) and oligo-dT primers. Quantitative real-time PCR reactions were performed in a 384-well format using Platinum qPCR mix (Invitrogen) and total reaction volumes of 10 μl on an ABI 7900HT (Applied Biosystems). Absolute gene expression was quantified using the method of Dolganov et al. using GAPDH, β-actin, and cyclophilin as control genes (59).
Statistics.
All experiments were performed on individual animals in groups of at least 4, except where indicated. The animals tested were all 100% inbred B6 mice. In all cases, a P value of less than 0.05 was taken to indicate statistical significance. Differences in mean values were analyzed by either 2-tailed Student’s t test or ANOVA with a Bonferroni’s post-hoc test modification. Kaplan-Meier survival plots were analyzed using the log-rank test to determine the effect of genotype or sex.
Supplementary Material
Acknowledgments
We thank Shaun Coughlin for vital discussions and critical review of the manuscript; Jean Regard for assistance with the gene expression experiments; and Ivo Cornelissen, Eric Camerer, David Sulciner, Neil Chi, Robin Shaw, and O.K. Gilman for important discussions of the work. We also thank A.F. Parlow for help with the GH measurements. This work was supported in part by NIH grant K08 HL74922-01 and an American Society of Hematology Junior Faculty Scholar Award in Basic Science (to E.J. Weiss). J.H. Wong was supported by an American Heart Association Summer Research Fellowship for Medical Students. J. Dukes was supported by a UCSF Office of Student Research Quarterly Research Fellowship.
Footnotes
Nonstandard abbreviations used: APC, activated protein C; aPTT, activated partial thromboplastin time; AT, antithrombin; B6, C57BL/6J; cGH, continuous GH; GH, growth hormone; HCII, heparin cofactor II; litm, Ghrhrlit mutation; PCI, protein C inhibitor; PE, pulmonary embolism; pGH, pulsatile GH; TF, tissue factor; VTE, venous thromboembolism.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:2969–2978 (2008). doi:10.1172/JCI34957
References
- 1.Rothwell P.M., et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 2.Kyrle P.A., et al. The risk of recurrent venous thromboembolism in men and women. N. Engl. J. Med. 2004;350:2558–2563. doi: 10.1056/NEJMoa032959. [DOI] [PubMed] [Google Scholar]
- 3.Evans A., et al. The genetics of coronary heart disease: the contribution of twin studies. Twin Res. 2003;6:432–441. doi: 10.1375/136905203770326439. [DOI] [PubMed] [Google Scholar]
- 4.Marenberg M.E., Risch N., Berkman L.F., Floderus B., de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N. Engl. J. Med. 1994;330:1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- 5.McRae S., Tran H., Schulman S., Ginsberg J., Kearon C. Effect of patient’s sex on risk of recurrent venous thromboembolism: a meta-analysis. Lancet. 2006;368:371–378. doi: 10.1016/S0140-6736(06)69110-1. [DOI] [PubMed] [Google Scholar]
- 6.Baglin T., Luddington R., Brown K., Baglin C. High risk of recurrent venous thromboembolism in men. J Thromb Haemost. 2004;2:2152–2155. doi: 10.1111/j.1538-7836.2004.01050.x. [DOI] [PubMed] [Google Scholar]
- 7.Mann K.G. Thrombin formation. Chest. 2003;124:4S–10S. doi: 10.1378/chest.124.3_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 8.Jones K., Mann K. A model for the tissue factor pathway to thrombin. II. A mathematical simulation [erratum 1995, 270:9026]. J. Biol. Chem. 1994;269:23367–23373. [PubMed] [Google Scholar]
- 9.Rand M., Lock J., van’t Veer C., Gaffney D., Mann K. Blood clotting in minimally altered whole blood. Blood. 1996;88:3432–3445. [PubMed] [Google Scholar]
- 10.Woodward M., et al. Epidemiology of coagulation factors, inhibitors and activation markers: The Third Glasgow MONICA Survey. II. Relationships to cardiovascular risk factors and prevalent cardiovascular disease. Br. J. Haematol. 1997;97:785–797. doi: 10.1046/j.1365-2141.1997.1232935.x. [DOI] [PubMed] [Google Scholar]
- 11.Weiss E.J., Hamilton J.R., Lease K.E., Coughlin S.R. Protection against thrombosis in mice lacking PAR3. Blood. 2002;100:3240–3244. doi: 10.1182/blood-2002-05-1470. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton J.R., Cornelissen I., Coughlin S.R. Impaired hemostasis and protection against thrombosis in protease-activated receptor 4-deficient mice is due to lack of thrombin signaling in platelets. J. Thromb. Haemost. 2004;2:1429–1435. doi: 10.1111/j.1538-7836.2004.00783.x. [DOI] [PubMed] [Google Scholar]
- 13.Vandendries E.R., Hamilton J.A., Coughlin S.R., Furie B.C., Furie B. Protease-activated receptor 4 is required for maximal thrombus growth but not for fibrin generation in thrombi after laser injury [abstract]. Blood. 2004;104:181a. [Google Scholar]
- 14.Nanda N., et al. Platelet aggregation induces platelet aggregate stability via SLAM family receptor signaling. Blood. 2005;106:3028–3034. doi: 10.1182/blood-2005-01-0333. [DOI] [PubMed] [Google Scholar]
- 15.Jansson J.O., Eden S., Isaksson O. Sexual dimorphism in the control of growth hormone secretion. Endocr. Rev. 1985;6:128–150. doi: 10.1210/edrv-6-2-128. [DOI] [PubMed] [Google Scholar]
- 16.Isaksson O.G., Eden S., Jansson J.O. Mode of action of pituitary growth hormone on target cells. Annu. Rev. Physiol. 1985;47:483–499. doi: 10.1146/annurev.ph.47.030185.002411. [DOI] [PubMed] [Google Scholar]
- 17.MacLeod J.N., Pampori N.A., Shapiro B.H. Sex differences in the ultradian pattern of plasma growth hormone concentrations in mice. J. Endocrinol. 1991;131:395–399. doi: 10.1677/joe.0.1310395. [DOI] [PubMed] [Google Scholar]
- 18.Giustina A., Veldhuis J.D. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr. Rev. 1998;19:717–797. doi: 10.1210/er.19.6.717. [DOI] [PubMed] [Google Scholar]
- 19.Tannenbaum G.S., Choi H.K., Gurd W., Waxman D.J. Temporal relationship between the sexually dimorphic spontaneous GH secretory profiles and hepatic STAT5 activity. Endocrinology. 2001;142:4599–4606. doi: 10.1210/en.142.11.4599. [DOI] [PubMed] [Google Scholar]
- 20.Eden S. Age- and sex-related differences in episodic growth hormone secretion in the rat. Endocrinology. 1979;105:555–560. doi: 10.1210/endo-105-2-555. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal A.K., Shapiro B.H. Intrinsic signals in the sexually dimorphic circulating growth hormone profiles of the rat. Mol. Cell. Endocrinol. 2001;173:167–181. doi: 10.1016/S0303-7207(00)00401-9. [DOI] [PubMed] [Google Scholar]
- 22.Waxman D.J., Pampori N.A., Ram P.A., Agrawal A.K., Shapiro B.H. Interpulse interval in circulating growth hormone patterns regulates sexually dimorphic expression of hepatic cytochrome P450. Proc. Natl. Acad. Sci. U. S. A. 1991;88:6868–6872. doi: 10.1073/pnas.88.15.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norstedt G., Palmiter R. Secretory rhythm of growth hormone regulates sexual differentiation of mouse liver. Cell. 1984;36:805–812. doi: 10.1016/0092-8674(84)90030-8. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro B.H., Agrawal A.K., Pampori N.A. Gender differences in drug metabolism regulated by growth hormone. Int. J. Biochem. Cell Biol. 1995;27:9–20. doi: 10.1016/1357-2725(94)00056-5. [DOI] [PubMed] [Google Scholar]
- 25.Flores-Morales A., et al. Microarray analysis of the in vivo effects of hypophysectomy and growth hormone treatment on gene expression in the rat. Endocrinology. 2001;142:3163–3176. doi: 10.1210/en.142.7.3163. [DOI] [PubMed] [Google Scholar]
- 26.Noshiro M., Negishi M. Pretranslational regulation of sex-dependent testosterone hydroxylases by growth hormone in mouse liver. . J. Biol. Chem. 1986;261:15923–15927. [PubMed] [Google Scholar]
- 27.Ahluwalia A., Clodfelter K.H., Waxman D.J. Sexual dimorphism of rat liver gene expression: regulatory role of growth hormone revealed by deoxyribonucleic acid microarray analysis. Mol. Endocrinol. 2004;18:747–760. doi: 10.1210/me.2003-0138. [DOI] [PubMed] [Google Scholar]
- 28.Davey H.W., Wilkins R.J., Waxman D.J. STAT5 signaling in sexually dimorphic gene expression and growth patterns. Am. J. Hum. Genet. 1999;65:959–965. doi: 10.1086/302599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holloway M.G., et al. Loss of sexually dimorphic liver gene expression upon hepatocyte-specific deletion of Stat5a-Stat5b locus. Endocrinology. 2007;148:1977–1986. doi: 10.1210/en.2006-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waxman D.J., Celenza J.L. Sexual dimorphism of hepatic gene expression: novel biological role of KRAB zinc finger repressors revealed. Genes Dev. 2003;17:2607–2613. doi: 10.1101/gad.1154603. [DOI] [PubMed] [Google Scholar]
- 31.Alba M., Salvatori R. A mouse with targeted ablation of the growth hormone-releasing hormone gene: a new model of isolated growth hormone deficiency. Endocrinology. 2004;145:4134–4143. doi: 10.1210/en.2004-0119. [DOI] [PubMed] [Google Scholar]
- 32.Andersen B., et al. The Ames dwarf gene is required for Pit-1 gene activation. Dev. Biol. 1995;172:495–503. doi: 10.1006/dbio.1995.8040. [DOI] [PubMed] [Google Scholar]
- 33.Lupu F., Terwilliger J.D., Lee K., Segre G.V., Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev. Biol. 2001;229:141–162. doi: 10.1006/dbio.2000.9975. [DOI] [PubMed] [Google Scholar]
- 34.Udy G.B., et al. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc. Natl. Acad. Sci. U. S. A. 1997;94:7239–7244. doi: 10.1073/pnas.94.14.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y., et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc. Natl. Acad. Sci. U. S. A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin S.C., et al. Molecular basis of the little mouse phenotype and implications for cell type-specific growth. Nature. 1993;364:208–213. doi: 10.1038/364208a0. [DOI] [PubMed] [Google Scholar]
- 37.Jansson J.O., Downs T.R., Beamer W.G., Frohman L.A. Receptor-associated resistance to growth hormone-releasing factor in dwarf “little” mice. Science. 1986;232:511–512. doi: 10.1126/science.3008329. [DOI] [PubMed] [Google Scholar]
- 38.Eicher E.M., Beamer W.G. Inherited ateliotic dwarfism in mice. Characteristics of the mutation, little, on chromosome 6. J. Hered. 1976;67:87–91. doi: 10.1093/oxfordjournals.jhered.a108682. [DOI] [PubMed] [Google Scholar]
- 39.Davey H.W., Park S.H., Grattan D.R., McLachlan M.J., Waxman D.J. STAT5b-deficient mice are growth hormone pulse-resistant. Role of STAT5b in sex-specific liver p450 expression. J. Biol. Chem. 1999;274:35331–35336. doi: 10.1074/jbc.274.50.35331. [DOI] [PubMed] [Google Scholar]
- 40.Clodfelter K.H., et al. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol. Endocrinol. 2006;20:1333–1351. doi: 10.1210/me.2005-0489. [DOI] [PubMed] [Google Scholar]
- 41.Teglund S., et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/S0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 42.Leon C., et al. Platelet ADP receptors contribute to the initiation of intravascular coagulation. Blood. 2004;103:594–600. doi: 10.1182/blood-2003-05-1385. [DOI] [PubMed] [Google Scholar]
- 43.Nordfang O., Kristensen H.I., Valentin S., Ostergaard P., Wadt J. The significance of TFPI in clotting assays — comparison and combination with other anticoagulants. Thromb. Haemost. 1993;70:448–453. [PubMed] [Google Scholar]
- 44.White R.H. The epidemiology of venous thromboembolism. Circulation. 2003;107:I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 45.Negrev N., Nyagolov Y., Stanchewa E. Somatotropin and somatostatin effects on vitamin K-dependent plasma coagulation factors. Eur. J. Pharmacol. 1995;277:145–149. doi: 10.1016/0014-2999(95)00056-Q. [DOI] [PubMed] [Google Scholar]
- 46.Savendahl L.S., Grankvist K., Engstrom K.G. Growth hormone deficiency impairs blood clotting and reduces factor VII coagulant activity in rat. Thromb. Haemost. 1995;73:626–629. [PubMed] [Google Scholar]
- 47.Miljic D., et al. Changes in prothrombin and activated partial thromboplastin time during replacement therapy with human recombinant growth hormone in growth hormone deficient adults. Hormones (Athens). 2006;5:187–191. doi: 10.14310/horm.2002.11183. [DOI] [PubMed] [Google Scholar]
- 48.Gresele P., et al. Activated human protein C prevents thrombin-induced thromboembolism in mice. Evidence that activated protein C reduces intravascular fibrin accumulation through the inhibition of additional thrombin generation. . J. Clin. Invest. 1998;101:667–676. doi: 10.1172/JCI575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mann K.G., Butenas S., Brummel K. The dynamics of thrombin formation. Arterioscler. Thromb. Vasc. Biol. 2003;23:17–25. doi: 10.1161/01.ATV.0000046238.23903.FC. [DOI] [PubMed] [Google Scholar]
- 50.Walker F.J., Fay P.J. Regulation of blood coagulation by the protein C system. FASEB J. 1992;6:2561–2567. doi: 10.1096/fasebj.6.8.1317308. [DOI] [PubMed] [Google Scholar]
- 51.Manfioletti G., Brancolini C., Avanzi G., Schneider C. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol. Cell. Biol. 1993;13:4976–4985. doi: 10.1128/mcb.13.8.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Esmon C.T. Inflammation and the activated protein C anticoagulant pathway. Semin. Thromb. Hemost. 2006;32(Suppl. 1):49–60. doi: 10.1055/s-2006-939554. [DOI] [PubMed] [Google Scholar]
- 53.Zechmeister-Machhart M., et al. Molecular cloning and sequence analysis of the mouse protein C inhibitor gene. Gene. 1997;186:61–66. doi: 10.1016/S0378-1119(96)00681-6. [DOI] [PubMed] [Google Scholar]
- 54.Geiger M., et al. Protein C inhibitor (PCI). Immunopharmacology. 1996;32:53–56. doi: 10.1016/0162-3109(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 55.Uhrin P., et al. Disruption of the protein C inhibitor gene results in impaired spermatogenesis and male infertility. J. Clin. Invest. 2000;106:1531–1539. doi: 10.1172/JCI10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davey H.W., et al. STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology. 2001;142:3836–3841. doi: 10.1210/en.142.9.3836. [DOI] [PubMed] [Google Scholar]
- 57.Liu J.L., Yakar S., LeRoith D. Conditional knockout of mouse insulin-like growth factor-1 gene using the Cre/loxP system. Proc. Soc. Exp. Biol. Med. 2000;223:344–351. doi: 10.1046/j.1525-1373.2000.22349.x. [DOI] [PubMed] [Google Scholar]
- 58.Sood R., et al. Fetal gene defects precipitate platelet-mediated pregnancy failure in factor V Leiden mothers. J. Exp. Med. 2007;204:1049–1056. doi: 10.1084/jem.20062566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dolganov G.M., et al. A novel method of gene transcript profiling in airway biopsy homogenates reveals increased expression of a Na+-K+-Cl- cotransporter (NKCC1) in asthmatic subjects. Genome Res. 2001;11:1473–1483. doi: 10.1101/gr.191301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.