Abstract
The controlled differentiation of embryonic stem (ES) cells is of utmost interest to their clinical, biotechnological, and basic science use. Many investigators have combinatorially assessed the role of specific soluble factors and extracellular matrices in guiding ES cell fate, yet the interaction between neighboring cells in these heterogeneous cultures has been poorly defined due to a lack of conventional tools to specifically uncouple these variables. Herein, we explored the role of cell-cell interactions during neuroectodermal specification of ES cells using a microfabricated cell pair array. We tracked differentiation events in situ, using an ES cell line expressing green fluorescent protein (GFP) under the regulation of the Sox1 gene promoter, an early marker of neuroectodermal germ cell commitment in the adult forebrain. We observed that a previously specified Sox1-GFP+ cell could induce the specification of an undifferentiated ES cell. This induction was modulated by the two cells being in contact and was dependent on the age of previously specified cell prior to coculture. A screen of candidate cell adhesion molecules revealed that the expression of connexin (Cx)-43 correlated with the age-dependent effect of cell contact in cell pair experiments. ES cells deficient in Cx-43 showed aberrant neuroectodermal specification and lineage commitment, highlighting the importance of gap junctional signaling in the development of this germ layer. Moreover, this study demonstrates the integration of microscale culture techniques to explore the biology of ES cells and gain insight into relevant developmental processes otherwise undefined due to bulk culture methods.
Keywords: embryonic stem cell, microfabricated cell pairs, neuroectodermal differentiation, connexin-43
The isolation of embryonic stem (ES) cells has galvanized the field of regenerative medicine by identifying a potential renewable source of primary cells that can be differentiated into any cell type in the body [1]. Moreover, ES cells allow for a basic science platform to understand embryonic and fetal development in vitro [2]. To form one distinct cell type, an ES cell makes many lineage-specific decisions that are influenced by cell interactions with its microenvironment, namely surrounding cells, soluble factors and extracellular matrix (ECM) proteins. Many investigators have exploited different combinations of soluble factors and ECMs to drive ES cell fate in vitro [3–8]. Yet, the effect of cell-cell interaction on ES cell differentiation has remained relatively unexplored, in part due to the inability to precisely control cell-cell interactions using conventional culture methods. Nevertheless, intercellular contact in the developing embryo and in cultured ES cells undergoes changes during different stages of neuronal differentiation [13–16] and ES cells in culture differentiate asynchronously into neuroectodermal precursors in a manner that suggests an important role for intercellular interactions [17].
Soluble and insoluble inductive cues (e.g., retinoic acid, sonic hedgehog, and laminin) have been investigated to determine their role in neuroectodermal specification [10], including studies performed with the aid of microfabricated devices [11, 12]. In order to explore the relative importance of diffusible factors versus cell-cell interactions in the developing neuroectoderm, we decided to employ a microfabrication technique to create cell pairs with defined cell contact states in this work. We have also investigated the role of known cell adhesion molecules on neuroectoderm differentiation in a conventional culture system.
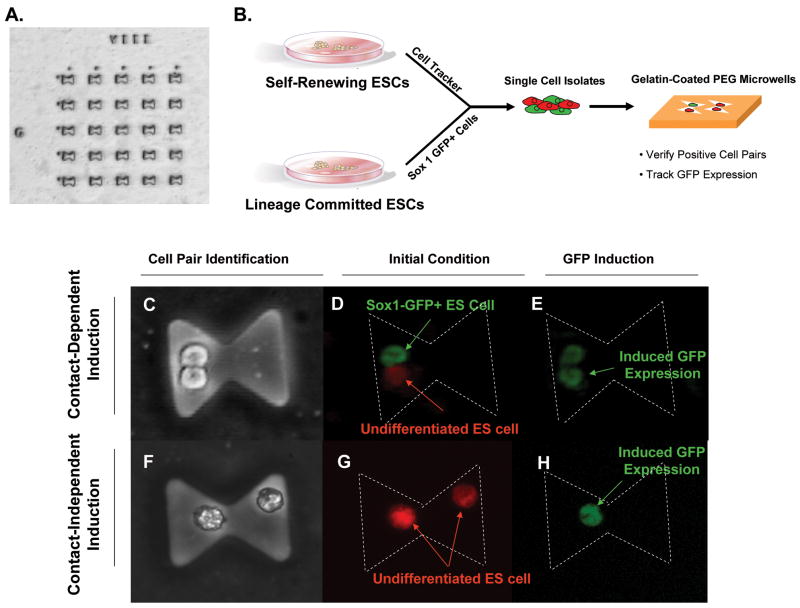
Microwells [9] were fabricated using standard polyethylene glycol (PEG) photolithography techniques as previously described [18, 19] with the following modifications: (1) PEG-DA (MW 575) with 1% DMPA was spin-coated on silane modified wafers at approximately 900 rpm using a spin coater (Machine World Inc., Redding, CA, USA); (2) PEG-coated wafers were aligned with a photomask and pulse exposed (1.2 seconds, 3x) at 15 mW cm−2 to stabilize small feature sizes; and (3) prior to cell seeding, microwells were incubated with 0.1% gelatin for 1 hr. The total surface area for cell attachment was 1250 um2 per well and there was an indexed array of 1 × 104 wells per 2″ borosilicate wafer (Fig. 1A).
Figure 1. PEG microwell array and cell tracking.
(A) Microwells were fabricated using standard photolithography modified for a PEG substrate. The total surface area for cell attachment was 1250 um2 per well and there was an indexed array of 1 × 104 wells per 2″ borosilicate wafer. (B) Depiction of experimental design. Undifferentiated ESCs were labeled with a cell tracker dye and mixed at a 1:1 ratio with Sox1-GFP+ cells that have been differentiated for a certain amount of time. The cell mixture was seeded on the PEG array and cell pairs were verified and tracked using fluorescence microscopy. Phase micrographs of cell-cell contact dependent (C) and independent (F) conditions. Cell pair criteria was determined after initial seeding in microwells as an undifferentiated cell with a differentiated partner (D; denoted U:D) or an undifferentiated partner (G; denoted U:U). Sox1+ cells (green) have differentiated for a certain period of time prior to coculture with an undifferentiated cell loaded with cell tracker dye (red). After two days of coculture each cell pair in the array was analyzed for induction of GFP expression in the undifferentiated cell (E, H).
The ES cells were the ES-D3 mouse embryonic stem cell line (ATCC, Virginia, US). Sox1-GFP+ embryonic stem cells expressing green fluorescent protein (GFP) under the regulation of the Sox1 gene promoter [17] were a gift from Dr. Austin Smith of the Institute for Stem Cell Research at the University of Edinburgh. Cx43−/− cells, a Cx43 knockout ES cell line [21], were a kind gift of Dr. Janet Rossant of the Department of Molecular and Medical Genetics of the University of Toronto.
All cells were propagated on gelatin coated culture flasks in LIF-supplemented medium that was changed daily. Cultures were routinely passaged at 60–75% confluency using 0.25% trypsin/0.1% EDTA and subcultured at a density of approximately 1×103 cells/cm2. Experiments were performed on cells of passage number 6–25. Neuroectodermal differentiation was induced in an adherent, monolayer culture as previously described [17]. Briefly, LIF-supplemented medium was removed from undifferentiated adherent ES cells at 75% confluency, cells were washed with PBS and placed in N2B27 medium, which is a 1:1 mixture of DMEM/F12 medium supplemented with N2 and Neurobasal medium supplemented with B27 (all from Invitrogen, US). The differentiation (N2B27) medium was changed every two days for the duration of the experiment.
ES cells at different stages of differentiation were plated into poly(ethylene glycol) (PEG) microwells [18, 19]. The microwells were fabricated in the shape of an hourglass (Figure 1A) so as to force the cell pairs into either interaction by physical contact (Figure 1C) or by diffusion of secreted molecules (Figure 1F). For seeding into microwells, undifferentiated Sox1-GFP+ cells were incubated with CyberRed (Molecular Probes, Eugene, OR) for 5 minutes prior to harvesting. Undifferentiated and differentiated Sox1-GFP+ cells of 3, 7, 11, 14 and 17 day in N2B27 were trypsinized, made into single cell isolates, and were mixed to a total of 2×106 cells that were seeded on the device at a 1:1 ratio. After 6 hours of incubation, non-adherent cells were removed and fresh medium was added. Fluorescence microscopy was used to determine the initial location (based on index array format) and identity of cell pairs with respect to cell contact and differentiation (Fig. 1). The GFP expression of the undifferentiated cells was monitored after two days of coculture using fluorescence microscopy. After two days of culture, the yield of the experiment was scored as the number of pairs where the undifferentiated cell became specified to a neuroectodermal cell and expressed Sox1-GFP divided by the total cell pairs analyzed. For cell viability experiments, cells were seeded in microwells or standard 25 cm2 tissue culture flasks and cultured for 0–72 hours. At t = 24, 48, and 72 hours, a live/dead assay using calcein AM/ethidium homodimer (Molecular Probes, Eugene, OR) was performed on cultures using the vendor’s protocol. Experiments were performed in triplicate and data was normalized to initial seeding viability.
Endpoint and kinetic PCR were used to determine gene expression of cell adhesion molecules known to be involved in neuroectodermal differentiation. RNA was extracted from ES cells using the Nucleospin RNA purification kit (BD Biosciences, Palo Alto, CA) as previously described [20]. Approximately 100ng-1 μg of total mRNA was reverse transcribed to cDNA using the TwoStep RT-PCR Kit (Qiagen, Valencia, CA) per manufacturer’s instructions and amplified in a Perkin Etus Thermal Cycler 480. Primers used for amplification were designed using the public software algorithm Primer3 or developed within the ATRC and are listed in Supplementary Table 1.
Results from microscale experiments were statistically analyzed using a Wilcoxon Ranked Sum Test. Experiments in bulk cultured were analyzed using unpaired student’s t-tests. Data is presented as the mean ± s.e.m.
Studies on cell viability demonstrated cellular integrity within the microwells during the two day duration of experiments (Supplementary Fig. 1). Initial experiments sought to determine if a neighboring, lineage-committed cell could alter the fate of an undifferentiated cell by contact dependent and/or independent mechanisms. Thus, two cell co-cultures consisted of a Sox1-GFP+ cell (differentiated for 3 days) and an undifferentiated cell with (Fig. 1C–E) or without cell contact (Fig. 1F–H).
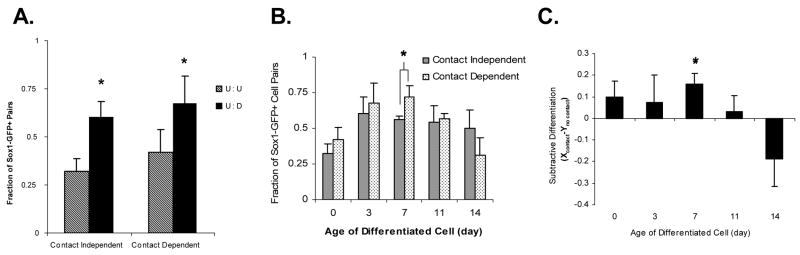
A co-culture of two undifferentiated cells, with or without cell contact, served as negative controls and showed a baseline yield of 32 +/− 6% and 42 +/−8%, respectively (Fig. 2A). This yield can most likely be attributed to the differentiation medium, substratum and potentially stochastic mechanisms. In contrast, when an undifferentiated cell was co-cultured with an early Sox1+ committed cell, neuroectodermal specification was induced in the undifferentiated cell, independent of cell contact. These data suggest that early interactions with a differentiated cell can increase the degree of specification of uncommitted cells (Fig. 2A).
Figure 2. Neuroectoderm-committed cells induce specification of undifferentiated ES cells in a dynamic, cell-contact interaction.
(A) Undifferentiated ES cells were cocultured with either another undifferentiated ES cell (U:U, striped bar) or a Sox1+ cell, differentiated for 3 days prior (U:D, solid bar). Induction of Sox1-mediated GFP expression in the undifferentiated cell was monitored after two days. The ordinate is cell-cell contact. The abscissa is the number of cell pairs, in which Sox1-GFP expression was induced in the undifferentiated cell, divided by the total number of cells pairs monitored. GFP expression was enhanced in U:D compared to U:U and was independent of cell-cell interaction (contact-independent: P = 0.05; contact-dependent: P = 0.02). The ordinate in panels B-C is the duration of differentiation culture (0–14 days) experienced by the Sox1-GFP+ cell prior to coculture with the undifferentiated cell. The abscissa is the same yield term previously used. Neuroectoderm specification was increased in undifferentiated ES cells in contact with a 7-day committed cell relative to no cell contact (B; P = 0.06). (C) The subtractive differentiation variable is defined as the difference between the yields of cell contact dependent and independent data. Data represent the mean ± s.e.m. of three separate experiments analyzing between 5–25 cell pairs/experiment.
We explored the effect of cell maturity (measured as time in differentiation culture) of Sox1+ neuroectodermal cells on the specification of ES cells in the presence or absence of cell contact by microwell co-cultures of undifferentiated ES cells and cells differentiated in N2B27 medium for up to 14 days.
Contact-independent experiments followed a saturating exponential trajectory with a threshold yield of 56 +/− 2% on Day 7 (Fig. 2B). On the contrary, contact-dependent yields followed a sigmoidal trajectory, increasing to a maximal yield of 72 +/− 7% on Day 7 and to a minimal yield of 31 +/− 12% on Day 14 (Fig. 2B). The differential effect of cell contact is shown in Fig. 2C. These time points (Day 7) may be indicative of lineage committed states where cell-cell interaction may have an impact on the fate of a primitive cell.
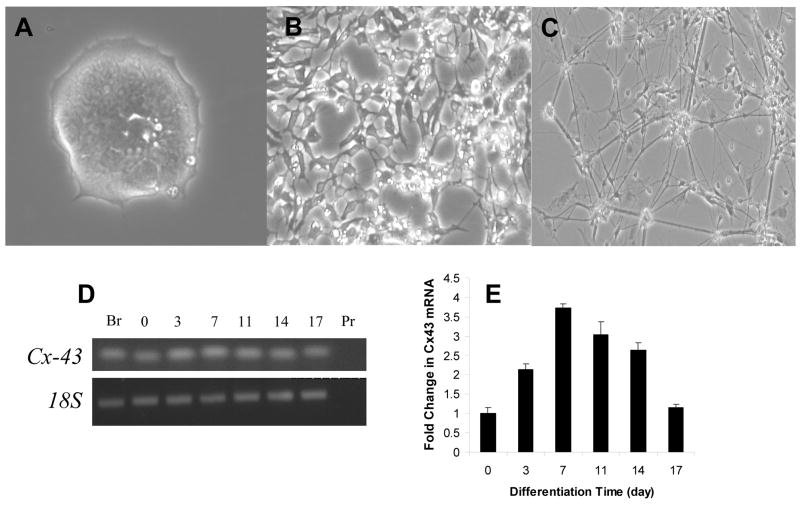
Based on the insight into the effects of cell contact on neuroectodermal specification gained using microscale culture, we next studied whether cell contact affected ES cell differentiation in bulk culture. Indeed, when observing cell morphology in differentiating ES cells over time, distinct changes in cell contact were associated with differentiation time (Fig. 3A–C). Over time, ES cells adopted a more network-like configuration with qualitatively less reliance on cell junctional complexes. These morphological changes may correlate to the cooperative effect of cell contact stated previously.
Figure 3. Morphological maturation and temporal expression of cell adhesion molecules during neuroectodermal differentiation.
Phase contrast images ES cells differentiated in N2B27 medium for (A) 5, (B) 11 or (C) 17 days. (D) Detection of mRNA transcripts by endpoint RT-PCR in ES cells after 0, 3, 7, 11, 14 and 17 days of differentiation in N2B27 medium. Mouse brain tissue (Br) served as an external control and 18S and primers only served as internal controls. Quantitative RT-PCR of Cx-43 (E) expression, relative to an internal housekeeping gene, normalized to expression of Day 0 ES cells. Error bars represent s.e.m. of three independent experiments.
To evaluate these findings in depth, we elected to take a candidate approach based on previous reports to identify molecular mediators involved in cell contact modulation of neuroectodermal specification. For a molecule to be implicated in the cooperative effect of cell contact, we qualitatively would expect dynamic changes in expression over time that would have accentuated patterns on Days 7 when significant differences were observed. We examined the gene expression of cell adhesion molecules known to be involved in neuroectodermal specification at days 0–14 of differentiation of ES cells in N2B27 medium. Of the three gene products (Cx-43, N-cadherin, and neuronal cell adhesion molecule (NCAM)-1) analyzed with endpoint PCR, Cx-43 expression was correlated with the temporal effects of cell contact based on our microwell experiments (Fig. 3D). When quantified by qPCR, only Cx-43 (Fig. 3E) had dynamics that were consistent with our previous observation of induction at Day 7. Specifically, Cx-43 had a “two-tailed” expression with a maximum at Day 7. These data suggest that Cx-43-mediated pathways may be involved in the inductive effects of cell contact on neuroectodermal specification and link ES cell differentiation contact dependence found at the microscale with gene expression findings in bulk culture.
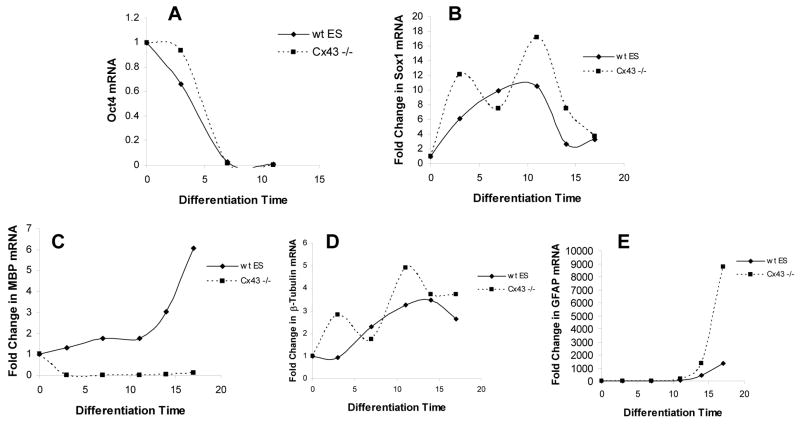
We tested our hypothesis that Cx43 is integral in the stabilization of a neuroectodermal germ layer by differentiating Cx43−/− ES cells in N2B27 medium and comparing the expression of genes involved in self-renewal, germ layer specification and lineage commitment to wild-type (wt) ES cells.
The transcription factor, Oct-4, is one of the core transcriptional elements that maintain an ES cell in a self-renewal state. During differentiation we saw an expected decline in Oct-4 expression over time in both wild-type and Cx43−/− ES cells indicating that lineage commitment in general was unaffected by the mutation in Cx43 (Fig. 4A). We then studied Sox1 to determine if there was any effect on neuroectodermal specification. Prior studies have shown that Sox1 rises to a maximal level at approximately E11.5 and is subsequently downregulated [17], which we reproduced in wild-type ES cells (Fig. 4B). In contrast, Cx-43 −/− ES cells had a bimodal expression pattern of Sox1 mRNA suggesting an alteration in germ layer populations within the neuroectoderm. As a surrogate for individual lineage commitment, we chose cell-specific mRNA indicative of neurons, oligodendrocytes, and astrocytes. Expression of neuronal marker, β-III tubulin, tracked precisely with the dynamics of Sox1, which shows that Sox1 most likely is restricted in expression to differentiated neurons (Fig. 4D). These data also suggest a punctuated, rather than gradual, development of neurons without Cx-43 signaling. Neuronal development is intimately coordinated with the maturation of glial cells in vivo [22]. Cx-43 −/− ES cells exhibited a failure in oligodendrocyte differentiation and a presumably compensatory rise in astrocyte differentiation as assessed by expression of myelin basic protein (MBP) and glial fibrillar actin protein (GFAP), respectively (Fig. 4C,E). In total, these results demonstrate the Cx-43 is necessary for the appropriate development of neuroectodermal cells.
Figure 4. Cx-43 −/− ES cells have altered expression kinetics of Sox1 and neuronal lineage-specific genes.
Quantitative RT-PCR of (A) Oct-4, (B) Sox1, (C) β-III tubulin, (D) MBP, and (E) GFAP expression in wt-ES and Cx-43 −/− ES cells, relative to an internal housekeeping gene, normalized to expression of Day 0 ES cells. Results are representative of two independent experiments.
Embryonic development is a highly orchestrated process that involves the precise spatiotemporal expression of appropriate cues. ES cells have been used as a platform to study the regulation of lineage-specific differentiation in order to understand normal development and pathogenesis as well as the therapeutic potential of adult cell derivation from ES cells. The role of cell contact in the specification and commitment of ES cells has not been well defined primarily due to a lack of conventional, experimental conditions to precisely study cell-cell interactions. We used a microfabricated approach to study the age-dependent effects of cell contact in ES cells using cell pair experiments. We monitored lineage specification via a GFP reporter ES cell line that allowed for in situ visualization of the expression of Sox1, an early and specific marker of neuroectoderm [23], within the device. Microscale studies were designed to measure the specification of an undifferentiated ES cell when paired in culture with or without cell contact to a neuroectodermal-specified ES cell.
We observed that an early neuroectodermal-specified ES cell can induce the specification of an uncommitted cell independent of cell contact. However, the role of cell contact was revealed as the specified cell had matured further in age prior to co-culture. After seven days of neuroectodermal differentiation, a specified cell in contact with an unspecified cell could induce a significant increase in the number of cell pairs where an undifferentiated cell began expressing Sox1. This observation motivated the study of cell adhesion molecules in conventional ES cell cultures to determine whether these results translated to an ensemble of cells. Morphological changes, particularly with respect to cell adhesion, during neuroectodermal differentiation were evident in vitro. Screening of a number of candidate cell adhesion molecules known to be expressed in the developing neuroectoderm identified Cx-43, whose gene expression temporally correlated with the effects of cell contact in microscale studies, as a possible mediator of the inductive effects of cell contact during certain periods of neuroectodermal development. We then studied ES cells deficient in Cx-43 and observed three interesting phenomena: (1) Sox1 expression tracked with neuronal differentiation and was bimodal in Cx-43 −/− ES cells compared to a uni-modal profile in wt-ES cells; (2) a failure of oligodendrocyte development without Cx43; and (3) an amplification of astrocytic cells in Cx-43 −/− ES cells. These results demonstrate that Cx43-mediated signaling is an essential component of neuroectodermal germ layer formation.
We hypothesize that shuttling of intracellular molecules, such as retinoic acid, that have size constraints allowable for passage through Cx43 may be another viable route to deliver inductive cues for the differentiation of a neighboring cell, although further investigation is needed to definitively test this theory. Furthermore, the unanticipated alterations in glial cell differentiation indicate an overall impairment in germ layer development that is ultimately correlated with Cx-43 deficiency. It remains unclear whether these alterations in glia are a direct or indirect consequence of Cx-43 deficiency. Homologous mutation of Cx-43 in mice leads to a neonatal lethal phenotype, primarily due to cardiac defects, thus making in vivo studies of the developing forebrain in Cx-43 −/− mice potentially confounded without more elaborate genetic manipulations [21].
In conclusion, we describe the modulatory effect of cell contact in the development of ES-derived neuroectodermal cells that was systematically motivated by the use of microfabricated cell pairs. These findings are a proof-of-principle that microfabrication technology can enable the study of cell-cell contact in stem cell differentiation and potentially discover novel pathways that cannot be precisely explored using conventional culture methods.
Supplementary Material
Acknowledgments
The authors would like to thank Octovio Hurtado and Dr. Alexander Revzin for technical support and assistance. This work was funded by grants from the NIH (P41 EB-002503, K18 DK076819 and R01 DK43371) and the Shriners Hospitals for Children. B.P. was supported by a National Science Foundation predoctoral fellowship.
ABBREVIATIONS
- ES
embryonic stem cell
- ECM
extracellular matrix
- PEG
poly(ethylene)-glycol
- NCAM
neural cell adhesion molecule
- MBP
myelin basic protein
- GFAP
glial fibrillary acidic protein
- RT-PCR
reverse transcriptase-polymerase chain reaction
- GFP
green fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lerou PH, Daley GQ. Therapeutic potential of embryonic stem cells. Blood Rev. 2005;19:321–331. doi: 10.1016/j.blre.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 3.Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- 5.Barberi T, Willis LM, Socci ND, Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:e161. doi: 10.1371/journal.pmed.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat Biotechnol. 2004;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 7.D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 8.Flaim CJ, Chien S, Bhatia SN. An extracellular matrix microarray for probing cellular differentiation. Nat Methods. 2005;2:119–125. doi: 10.1038/nmeth736. [DOI] [PubMed] [Google Scholar]
- 9.Nelson CM, Pirone DM, Tan JL, Chen CS. Vascular endothelial-cadherin regulates cytoskeletal tension, cell spreading, and focal adhesions by stimulating RhoA. Mol Biol Cell. 2004;15:2943–2953. doi: 10.1091/mbc.E03-10-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang KJ, Rathjen J, Vassilieva S, Rathjen PD. Differentiation of embryonic stem cells to a neural fate: a route to re-building the nervous system? J Neurosci Res. 2004;76:184–192. doi: 10.1002/jnr.20036. [DOI] [PubMed] [Google Scholar]
- 11.Chin VI, Taupin P, Sanga S, Scheel J, Gage FH, Bhatia SN. Microfabricated platform for studying stem cell fates. Biotechnol Bioeng. 2004;88:399–415. doi: 10.1002/bit.20254. [DOI] [PubMed] [Google Scholar]
- 12.Chung BG, Flanagan LA, Rhee SW, Schwartz PH, Lee AP, Monuki ES, Jeon NL. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip. 2005;5:401–406. doi: 10.1039/b417651k. [DOI] [PubMed] [Google Scholar]
- 13.Laplante I, Beliveau R, Paquin J. RhoA/ROCK and Cdc42 regulate cell-cell contact and N-cadherin protein level during neurodetermination of P19 embryonal stem cells. J Neurobiol. 2004;60:289–307. doi: 10.1002/neu.20036. [DOI] [PubMed] [Google Scholar]
- 14.Nadarajah B, Makarenkova H, Becker DL, Evans WH, Parnavelas JG. Basic FGF increases communication between cells of the developing neocortex. J Neurosci. 1998;18:7881–7890. doi: 10.1523/JNEUROSCI.18-19-07881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okado H, Takahashi K. A simple “neural induction” model with two interacting cleavage-arrested ascidian blastomeres. Proc Natl Acad Sci U S A. 1988;85:6197–6201. doi: 10.1073/pnas.85.16.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka-Kunishima M, Takahashi K. Cleavage-arrested cell triplets from ascidian embryo differentiate into three cell types depending on cell combination and contact timing. J Physiol. 2002;540:153–176. doi: 10.1113/jphysiol.2001.013293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 18.Revzin A, Sekine K, Sin A, Tompkins RG, Toner M. Development of a microfabricated cytometry platform for characterization and sorting of individual leukocytes. Lab Chip. 2005;5:30–37. doi: 10.1039/b405557h. [DOI] [PubMed] [Google Scholar]
- 19.Revzin A, Rajagopalan P, Tilles AW, Berthiaume F, Yarmush ML, Toner M. Designing a hepatocellular microenvironment with protein microarraying and poly(ethylene glycol) photolithography. Langmuir. 2004;20:2999–3005. doi: 10.1021/la035827w. [DOI] [PubMed] [Google Scholar]
- 20.Levine JB, Leeder AD, Parekkadan B, Berdichevsky Y, Rauch SL, Smoller JW, Konradi C, et al. Isolation rearing impairs wound healing and is associated with increased locomotion and decreased immediate early gene expression in the medial prefrontal cortex of juvenile rats. Neuroscience. 2008;151:589–603. doi: 10.1016/j.neuroscience.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, et al. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 22.Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood HB, Episkopou V. Comparative expression of the mouse Sox1, Sox2 and Sox3 genes from pre-gastrulation to early somite stages. Mech Dev. 1999;86:197–201. doi: 10.1016/s0925-4773(99)00116-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.