Summary
Regulation of synaptic growth is fundamental to the formation and plasticity of neural circuits. Here we demonstrate that Nervous wreck (Nwk), a negative regulator of synaptic growth at Drosophila NMJs, interacts functionally and physically with components of the endocytic machinery, including dynamin and Dap160/Intersectin, and negatively regulates retrograde BMP growth signaling through a direct interaction with BMP receptor, Thickveins. Synaptic overgrowth in nwk is sensitive to BMP signaling levels and loss of Nwk facilitates BMP-induced overgrowth. Conversely, Nwk overexpression suppresses BMP-induced synaptic overgrowth. We observe analogous genetic interactions between dap160 and the BMP pathway, confirming that endocytosis regulates BMP signaling at NMJs. Finally, we demonstrate a correlation between synaptic growth and pMAD levels, and show that Nwk regulates these levels. We propose that Nwk functions at the interface of endocytosis and BMP signaling to ensure proper synaptic growth by negatively regulating Tkv to set limits on this positive growth signal.
Introduction
The formation and plasticity of neural circuits depends on the proper establishment and remodeling of synapses in response to environment and experience. The Drosophila larval neuromuscular junction (NMJ) has become a powerful model system for dissecting the molecular mechanisms that regulate synaptic growth (Collins and DiAntonio, 2007). NMJs are dynamic structures that coordinate their size and strength with muscle growth and undergo changes in morphology and physiology in response to environmental stimuli and altered levels of activity (Budnik et al., 1990; Sigrist et al., 2003; Sigrist et al., 2002).
It is well established that neurons and muscles are in communication to coordinate the development and refinement of pre- and postsynaptic terminals (Haghighi et al., 2003; Marrus and DiAntonio, 2004; Nakayama et al., 2006; Packard et al., 2002; Pielage et al., 2006; Saitoe et al., 2001). Identification of the factors that mediate trans-synaptic coordination of NMJ development has been a key objective for understanding synaptic growth and plasticity. Several recent studies have identified the bone morphogenic protein (BMP) signaling pathway as a critical retrograde (muscle to neuron) growth signal that regulates NMJ development (Aberle et al., 2002; Marques et al., 2002; McCabe et al., 2004; McCabe et al., 2003; Rawson et al., 2003; Sweeney and Davis, 2002). BMPs are members of the transforming growth factor-β (TGF-β) superfamily of signaling proteins (Miyazono et al., 2001). At Drosophila NMJs, the BMP Glass bottom boat (Gbb) is released, presumably by muscles, to activate BMP receptors, Wishful thinking (Wit), Thickveins (Tkv) and Saxophone (Sax) at presynaptic terminals. These receptors represent two classes of transmembrane serine/threonine kinases. Upon ligand binding, Wit, a type II receptor, forms a complex with the type I receptors Tkv and Sax. In turn, phosphorylation of Tkv and Sax by Wit induces them to phosphorylate the receptor-regulated R-Smad Mothers against Dpp (Mad). Mad is a cytoplasmic signal transduction effector that, when phosphorylated, binds the co-Smad Medea (Med) and translocates to the nucleus where the complex activates or represses transcription of target genes (Keshishian and Kim, 2004). Mutations in gbb, wit, tkv, sax, mad and medea all result in pronounced NMJ undergrowth (Aberle et al., 2002; Marques et al., 2002; McCabe et al., 2004; McCabe et al., 2003; Rawson et al., 2003).
An important open question is whether BMP signaling is required simply as a permissive switch to initiate synaptic growth or instead is instructive, modulating synaptic growth in a graded fashion in proportion to signal levels. In the latter case, BMP signaling is itself likely to be subject to positive and negative regulation to fine tune signaling levels in response to external and internal cues. Such regulation could occur at a number of levels including presynaptic regulation of available receptors or trafficking of activated receptor signaling complexes and postsynaptic regulation of Gbb release.
nervous wreck (nwk) was identified as a neuron-specific negative regulator of NMJ growth in a secondary screen of temperature-sensitive paralytic mutants for aberrant synaptic morphology (Coyle et al., 2004). Nwk is the Drosophila representative of a family of proteins, conserved from yeast to humans. A human family member has been identified as the affected gene in a heritable form of mental retardation, highlighting a conserved role for Nwk and Nwk-related proteins in neural development. Nwk-family proteins share a distinctive domain architecture including a newly defined F-BAR domain (Itoh et al., 2005), two SH3 domains (or in some cases one SH3 and one RhoGAP domain), and a C-terminal proline-rich domain (PRD) with numerous PxxP SH3-binding sites. F-BAR domains induce membrane invagination and have been identified in proteins associated with both actin regulation and endocytosis. SH3 domains mediate protein-protein interactions and are commonly found in adaptor proteins in signal transduction pathways. The activity of the Wiscott-Aldrich Syndrome Protein, Wasp, which promotes F-actin assembly by activating the ARP2/3 (actin-related protein) complex, is regulated in part by interacting directly with SH3-domain containing proteins transmitting upstream growth signals (Takenawa and Suetsugu, 2007). Nwk was found to bind Wasp via its first SH3 domain (Coyle et al., 2004). Furthermore, mutations in wasp result in synaptic overgrowth similar to that of nwk and enhance nwk mutants in a dose-dependent manner suggesting that Nwk and Wasp are components of a common pathway regulating NMJ growth. These data suggest Nwk may function by linking growth cues to reorganization of the actin cytoskeleton during synaptic growth.
A number of endocytic proteins, among them dynamin, Dap160/Intersectin, Endophilin, Synaptojanin, Rab11 and Spinster (Spin) have also been identified as negative regulators of NMJ growth (Dickman et al., 2006; Khodosh et al., 2006; Koh et al., 2004; Marie et al., 2004; Sweeney and Davis, 2002). Mutations in the majority of these genes share with nwk the specific morphological defect of supernumerary or satellite bouton formation (Dickman et al., 2006). shibire (shi, Drosophila dynamin) encodes a GTPase that is required for the scission of endocytic vesicles from the plasma membrane. Dap160, originally identified as a binding partner of dynamin, is a scaffolding protein required for the stable formation of a complex of endocytic proteins at presynaptic terminals. In addition to the defined role of these proteins in synaptic vesicle endocytosis at the NMJ, disrupted endocytosis and trafficking of growth signaling receptors may underlie their synaptic overgrowth phenotypes. In fact, spin encodes a late endosomal/lysosomal protein proposed to function, at least in part, in the downregulation of BMP signaling at synapses (Sweeney and Davis, 2002). Recent evidence from a variety of experimental systems has shown that endocytosis is dependent on the precise spatial and temporal regulation of actin assembly (Itoh and De Camilli, 2006; Kim and Chang, 2006; Smythe and Ayscough, 2006). Further, it is becoming increasingly apparent that endocytosis is a key mechanism for the integration and regulation of cell signaling pathways (Conner and Schmid, 2003; Di Fiore and De Camilli, 2001). Together, these findings raise the possibility that nwk mutants disrupt an actin-dependent endocytic mechanism required for the negative regulation of a synaptic growth signal.
In support of this idea, we have found that Nwk interacts functionally and physically with components of both the endocytic machinery and the BMP signaling pathway. Synaptic overgrowth in nwk is sensitive to the dosage of presynaptic BMP receptors and loss of Nwk facilitates BMP-induced synaptic overgrowth. We also found that ectopic BMP signaling results in varying levels of synaptic overgrowth depending on the dose of activated receptor, supporting an instructive role for BMP signaling in synaptic growth, and we demonstrate that overexpression of Nwk suppresses this phenotype. Consistent with a role for Nwk in the regulation of BMP signaling levels, we observe a direct physical interaction between Nwk and Tkv. Finally, we demonstrate a correlation between synaptic growth and pMAD levels, and show that these levels are regulated by Nwk. Based on these results, we propose that Nwk functions at the intersection of actin-dependent endocytosis and BMP signaling to constrain synaptic growth through the endocytic regulation of the presynaptic BMP receptor Tkv.
Results
Synaptic overgrowth in nwk is dominantly enhanced by endocytic mutations
Previous work suggests Nwk negatively regulates synaptic growth at least in part through effects on actin assembly via its direct interactions with Wasp (Coyle et al., 2004). Although actin is involved in a number of mechanisms required for synaptic growth including cell adhesion and process extension, a number of observations led us to hypothesize that Nwk may function at the convergence of actin assembly and endocytosis. First, nwk NMJs display a distinctive phenotype of satellite bouton formation and hyperbudding, in which a given bouton sprouts three or more daughter boutons. A similar phenotype has recently been observed in a number of endocytic mutants, including endo and dap160, suggesting a link between the formation of satellite boutons and defects in endocytosis (Dickman et al., 2006; Khodosh et al., 2006; Koh et al., 2004; Marie et al., 2004). Second, Nwk colocalizes with endocytic proteins at the periactive zones of boutons, including sites of active endocytosis as indicated by dynamin-positive “hot spots” (Coyle et al., 2004; Estes et al., 1996; Roos and Kelly, 1999). Third, Nwk levels are reduced in the absence of endocytic scaffolding protein Dap160 (Koh et al., 2004). Fourth, a large body of recent work has revealed that endocytosis is accompanied by spatially and temporally regulated bursts of actin assembly. Moreover, there is extensive overlap between endocytic proteins and regulators of actin assembly, reflecting the importance of actin-dependent steps in the internalization and trafficking of endocytic vesicles (Itoh and De Camilli, 2006; Kim and Chang, 2006; Smythe and Ayscough, 2006). Finally, recent work in vertebrates has identified a novel domain, named F-BAR, that is able to induce plasma membrane invagination (Itoh et al., 2005). An F-BAR domain is present in Nwk and its vertebrate orthologs.
To explore the possibility that Nwk functions in a common pathway with endocytic proteins during synaptic growth, we assayed genetic interactions between nwk and mutations in a number of known endocytic genes, including dap160, endophilin (endo) and shi. For each of these we observe significant dominant enhancement of both bouton number and satellite bouton formation at all nwk NMJs (Figure 1). To quantify this phenotypic enhancement, we focused on NMJ 4, a simple, stereotypic synapse formed by motor neuron 4-1b on the face of muscle 4 (Hoang and Chiba, 2001). Satellite boutons were defined in accordance with Dickman et al. (2006) as extensions of 5 or fewer boutons emanating from the main branch of the nerve terminal (See arrows in Figure 1B). Mutations in nwk result in a 1.5-fold increase in bouton number and a 3-fold increase in the formation of satellite boutons (Figures 1A–B, I–J). Loss of one copy of endo in a nwk mutant background increases bouton number 1.5-fold and causes a 3.4-fold increase in satellite bouton formation, but has limited effect in a wild-type background (Figures 1C–D, I–J). Similarly, heterozygous mutations in dap160 have no effect on wild-type synapses but cause a 2.5-fold increase in satellite bouton formation and a 1.2-fold increase in bouton number at nwk synapses (Figures 1E–F, I–J). It was previously shown that shits1 mutant larvae raised at the semi-restrictive temperature of 25°C exhibit a 5-fold increase in satellite boutons compared with wild type (Dickman et al., 2006). At this temperature, we did not observe a significant increase in satellite bouton formation in shi heterozygotes alone (Figures 1G and J) but did see significant dominant enhancement of the nwk phenotype (Figures 1H–J). These results place Nwk in a common pathway with endocytic proteins at presynaptic terminals.
Figure 1. Synaptic overgrowth in nwk is dominantly enhanced by endocytic mutations.
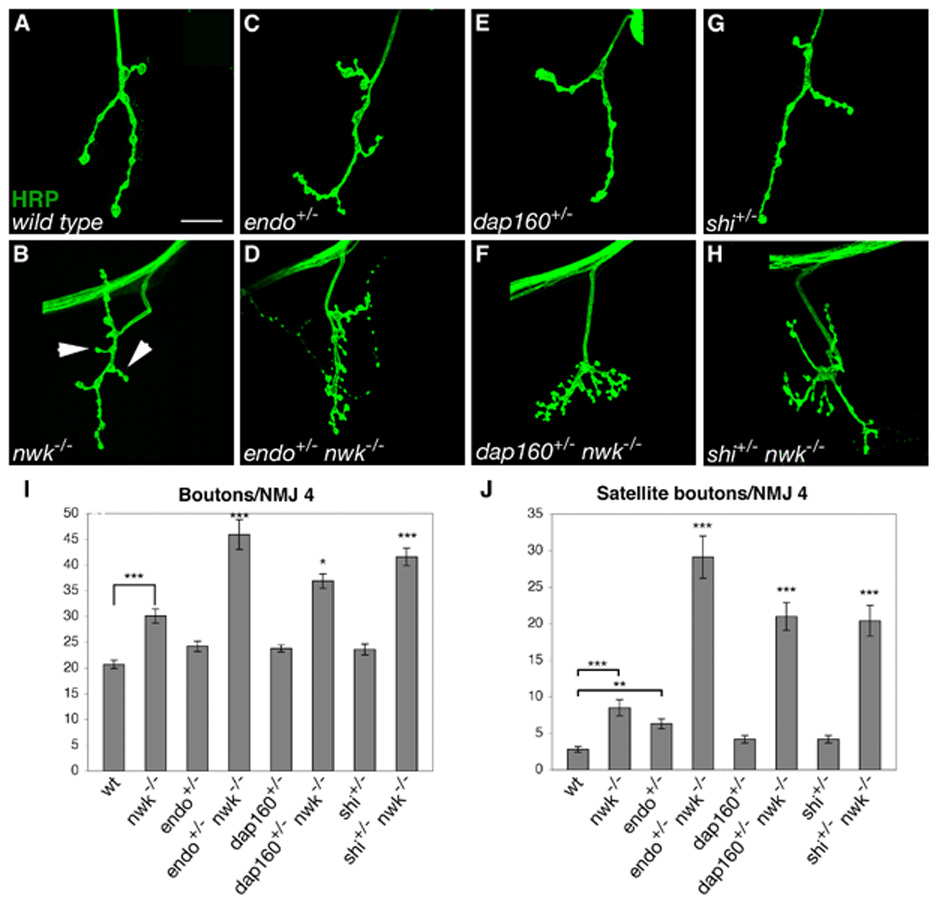
(A–H) Confocal images of NMJ 4 labeled with FITC-conjugated anti-HRP. (A) Wild-type NMJs are relatively unbranched and contain few satellite boutons. (B) nwk NMJs are more branched than wild type and have increased numbers of total boutons and satellite boutons. (C–D) Loss of one copy of endo has no effect on total and a small effect on satellite bouton formation in a wild-type background (C), but results in a large increase in both parameters in a nwk background (D). (E–F) Loss of one copy of dap160 does not affect bouton number or satellite bouton formation in a wild-type background (E), but leads to a significant increase in total and satellite bouton number in nwk mutants (F). (G–H) At the semi-restrictive temperature of 25°C, heterozygosity for shi does not affect total or satellite bouton number at wild-type NMJs (G), but in a nwk background, total bouton number and satellite bouton formation are significantly increased (H). (I–J) Quantification of total (I) and satellite (J) bouton number at NMJ 4 in wild type, nwk1/2, endo2730/+, endo2730 nwk2/+ nwk1, dap160Δ1/+, dap160Δ1/+; nwk1/2, shits1/+, and shits1/+; nwk1/2 backgrounds. * p < 0.05, ** p < 0.01, *** p < 0.001. P-values above individual bars refer to comparisons between nwk and the indicated genotype. Arrows in B point to examples of satellite boutons. Scale bar equals 20 µM.
Nwk physically interacts with the endocytic machinery
Given the strong phenotypic interactions between nwk and mutations affecting known endocytic proteins as well as the colocalization of these proteins at presynaptic periactive zones and endocytic hot spots, we investigated whether Nwk physically associates with the endocytic machinery by GST-pulldown and yeast two-hybrid experiments. Both Dap160 and dynamin GST-fusion proteins precipitate Nwk from fly lysates with high efficiency. Furthermore, pulldown experiments with GST fusions of specific Dap160 and dynamin domains demonstrate that it is the SH3 domains of Dap160 and the proline-rich domain (PRD) of dynamin that bind Nwk (Figures 2A and B).
Figure 2. Nwk physically interacts with dynamin and Dap160.
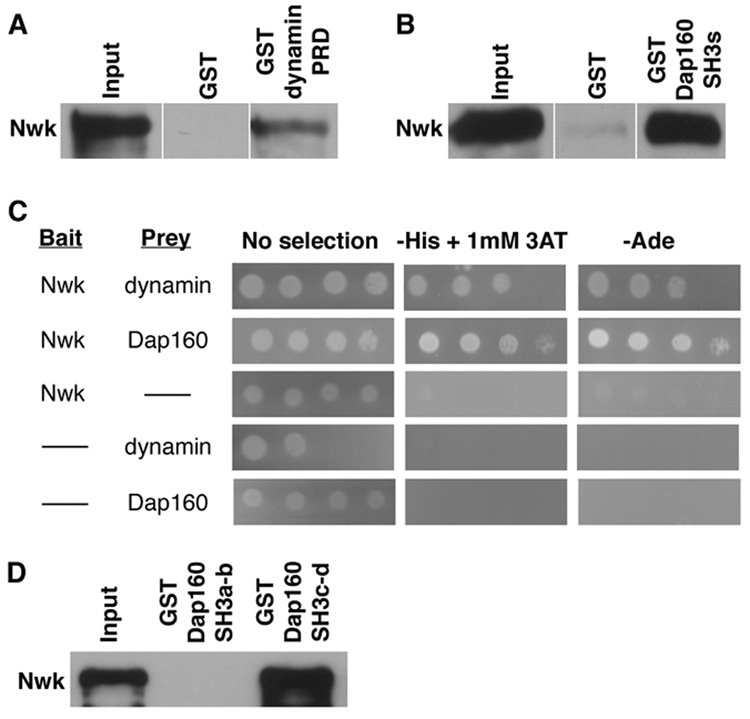
(A) A dynamin-PRD-GST-fusion protein precipitates Nwk-RFP from C155GAL4; UASnwk-RFP fly lysates with high affinity, while GST alone does not. (B) GST-Dap160SH3a-d precipitates Nwk-RFP from cellular lysates. (C) Yeast two-hybrid interactions demonstrate a direct association between full-length Nwk and the proline-rich domain (PRD) of dynamin and between Nwk and the SH3 domains of Dap160. Full-length Nwk is fused to the GAL4 DNA-binding domain (prey) and the dynamin PRD and Dap160 SH3 domains are fused to the GAL4 activation domain (bait). After 3 days at 30°C, yeast transformed with either bait or prey alone grow only in the absence of selection, whereas yeast coexpressing both bait and prey fusion proteins exhibit growth on SD medium lacking either histidine or adenine indicating that transcription of reporter genes has been activated. 3AT inhibits His3p activity and increases the stringency of selection. (D) Dap160-SH3c-d, but not Dap160-SH3a-b, GST-fusion protein precipitates Nwk-RFP from cell lysates. A, B and D are probed with rabbit anti-dsRed; input lanes contain lysate equal to 1/10 of the amount used for the pulldown assays.
To determine if the interactions of Nwk with Dap160 and dynamin are direct, we performed yeast two-hybrid experiments. Growth of yeast cells co-transformed with Nwk and either Dap160 or dynamin constructs and grown on multiple selective media reveals direct binding between Nwk and both endocytic proteins (Figure 2C). Finally, we investigated the potential for Nwk, dynamin and Dap160 to interact as a complex. Because Nwk binds dynamin through its SH3 domains and binds Dap160 through its PRD, it has the capacity to bind both proteins simultaneously. On the other hand, both Nwk and dynamin bind the SH3 domains of Dap160 and might compete for the same binding site. The interaction between Dap160 and dynamin is mediated exclusively by the SH3a and b domains of Dap160 (Roos and Kelly, 1998). We performed pulldown experiments with GST constructs containing either the SH3a and b or the SH3c and d domains of Dap160. The SH3c and d domains of Dap160 precipitate Nwk with high affinity, while the SH3a and b domains do not (Figure 2D). Thus, Dap160 has the capacity to form a multi-protein complex containing Nwk and dynamin. These findings are consistent with data from Drosophila and vertebrates indicating that Dap160/Intersectin is a scaffolding protein responsible for coordinating the activities of a number of proteins that bind sequentially as endocytosis progresses (Hussain et al., 2001; Koh et al., 2004; Marie et al., 2004; Roos and Kelly, 1998; Zamanian and Kelly, 2003).
Synaptic overgrowth in nwk depends on the level of retrograde BMP signaling
Nwk’s role as a negative regulator of synaptic growth together with the phenotypic, genetic and biochemical evidence indicating a functional association between Nwk and the endocytic machinery suggested Nwk might be required for downregulation of a positive growth signal at the NMJ. A number of recent studies demonstrate that the BMP signaling pathway constitutes the primary positive retrograde growth signal at developing NMJs (Aberle et al., 2002; Marques et al., 2002; McCabe et al., 2004; McCabe et al., 2003; Rawson et al., 2003; Sweeney and Davis, 2002), so we examined genetic interactions between nwk and BMP pathway components.
The BMP pathway is required for synaptic overgrowth in nwk mutants as loss of wit fully suppresses the synaptic overgrowth observed in nwk mutants (Figures 3A–H, K). At NMJ 4, wild-type synapses have an average of 21 boutons (Figures 3B and K), compared with 30 in nwk (Figures 3D and 3K). Conversely, wit mutants are severely undergrown, with an average of 7.9 boutons per NMJ 4 (Figures 3F and K). In nwk wit double mutants, the average number of boutons at NMJ 4 is essentially the same as in wit single mutants (Figures 3H and K). Thus, synaptic overgrowth in nwk requires BMP signaling.
Figure 3. Synaptic overgrowth in nwk is sensitive to BMP signaling levels.
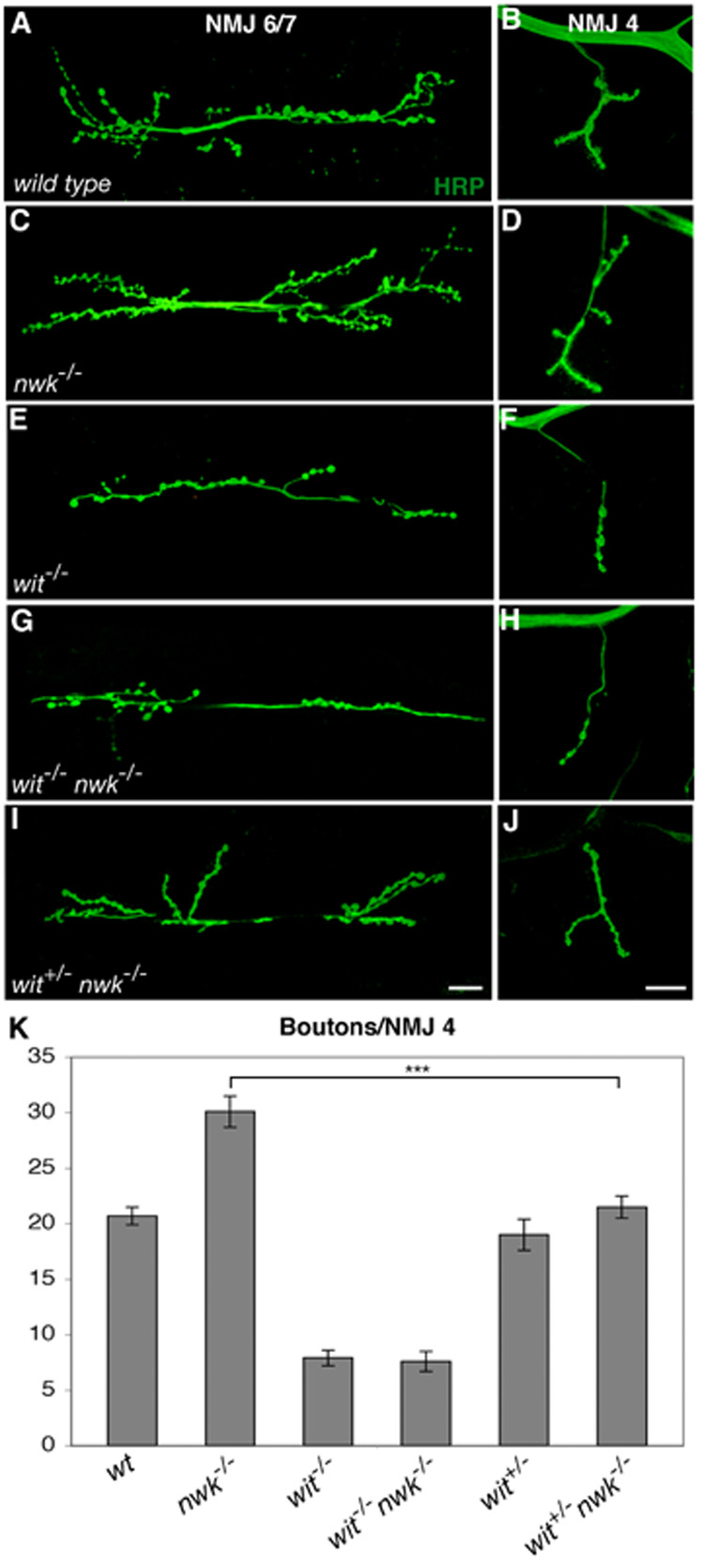
(A–J) Confocal images of NMJ 6/7 (A, C, E, G and I) and NMJ 4 (B, D, F, H and J) labeled with FITC-conjugated anti-HRP. (A, B) wild type. (C, D) nwk1/2 mutants exhibit synaptic overgrowth. (E, F) witA12/B11 mutants have dramatically undergrown synapses. (G,H) witA12 nwk2 double mutants exhibit synaptic undergrowth that is essentially identical to that observed in wit single mutants. (I, J) Loss of one copy of wit dominantly suppresses synaptic overgrowth of nwk (witA12/+ nwk1/2). (K) Quantification of bouton number at NMJ 4 in wild type, nwk1/2, witA12/B11, witA12 nwk2, witA12/+ and witA12 nwk2/+ nwk1 backgrounds. *** p < 0.001. Scale bars equal 20 µM.
Furthermore, we have found that the nwk phenotype depends on wild-type levels of Wit, supporting the idea of a direct link between the level of BMP signaling and synaptic overgrowth in nwk. Loss of one copy of wit, which has no effect in a wild-type background, significantly suppresses synaptic overgrowth in nwk mutants (Figures 3J and K). We observe similar dosage-sensitive interactions between nwk and all other components of the BMP signaling pathway analyzed, including gbb, tkv, med and mad (data not shown). These findings are consistent with a model in which Nwk restrains synaptic growth via the endocytic downregulation of BMP signaling.
Nwk constrains BMP-induced synaptic overgrowth
Overexpression of a constitutively active Tkv receptor (TkvACT) causes striking gain-of-function phenotypes in a number of tissues, notably the developing wing (Hoodless et al., 1996). At the NMJ, neuronal expression of TkvACT does not affect synaptic growth (McCabe et al., 2004) (Figures 4A, C and E). One possibility is that BMP signaling is constrained at the NMJ by mechanisms that set upper limits on the pathway. If Nwk were acting to set a limit on BMP signaling, we would expect its removal to permit TkvACT-induced synaptic overgrowth. In analogous experiments, expression of TkvACT in highwire (hiw) mutants resulted in an increase in bouton formation beyond that observed in the absence of Hiw alone (McCabe et al., 2004). We found that neuronal expression of TkvACT in nwk mutants does not cause a significant increase in bouton number compared with nwk alone (data not shown). However, TkvACT expression in a nwk background results in a 1.7-fold increase in satellite bouton formation over that observed in nwk alone (Figures 4B–E). These results indicate that indeed Nwk does play a role in downregulating BMP signaling at the NMJ and that this regulation is particularly important for controlling the emergence of satellite boutons.
Figure 4. Nwk constrains BMP-induced synaptic overgrowth.
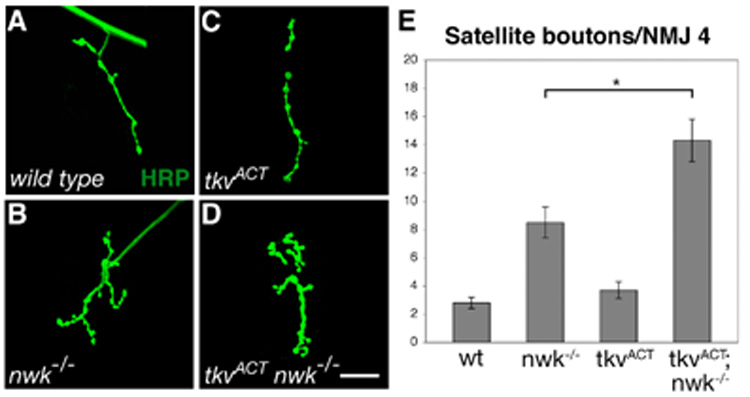
(A–D) Confocal images of NMJ 4 labeled with FITC-conjugated anti-HRP. (A) wild type. (B) nwk1/2. (C) Neuronal expression of TkvACT in a wild-type background does not result in an increase in satellite bouton number. (D) TkvACT expression in the absence of nwk results in an increase in satellite boutons over that resulting from loss of nwk alone. (E) Quantification of satellite bouton number at NMJ 4 in wild type, nwk1/2, elavGAL4/UAStkvACT and elavGAL4 nwk2/UAStvkACT nwk2 backgrounds. * p < 0.05. Scale bar equals 20 µM.
BMP-induced synaptic overgrowth is suppressed by overexpression of Nwk
If the level of BMP signaling at the NMJ plays an instructive role in determining the extent of synaptic growth, further manipulation of TkvACT expression might result in synaptic overgrowth. In fact, we found that neuronal expression of two copies of UAStkvACT via C155GAL4 results in a 2-fold increase in bouton number and 4-fold increase in satellite bouton formation (Figures 5A–D, K–L). We do not observe overgrowth in the absence of GAL4 indicating that the phenotype is dependent on overexpression of tkvACT and not the result of a second-site mutation (Figures 5K and L). Consistent with previous studies in the embryonic CNS, we also found that co-overexpression of one copy each of TkvACT and SaxACT results in synaptic overgrowth (Allan et al., 2003)(data not shown). Hence, increased levels of BMP signaling cause corresponding increases in synaptic growth in general and the formation of satellite boutons, in particular. The similarity of this phenotype to that associated with defects in endocytosis is consistent with the idea that a common mechanism is disrupted in both cases.
Figure 5. BMP-induced synaptic overgrowth is suppressed by neuronal overexpression of Nwk.
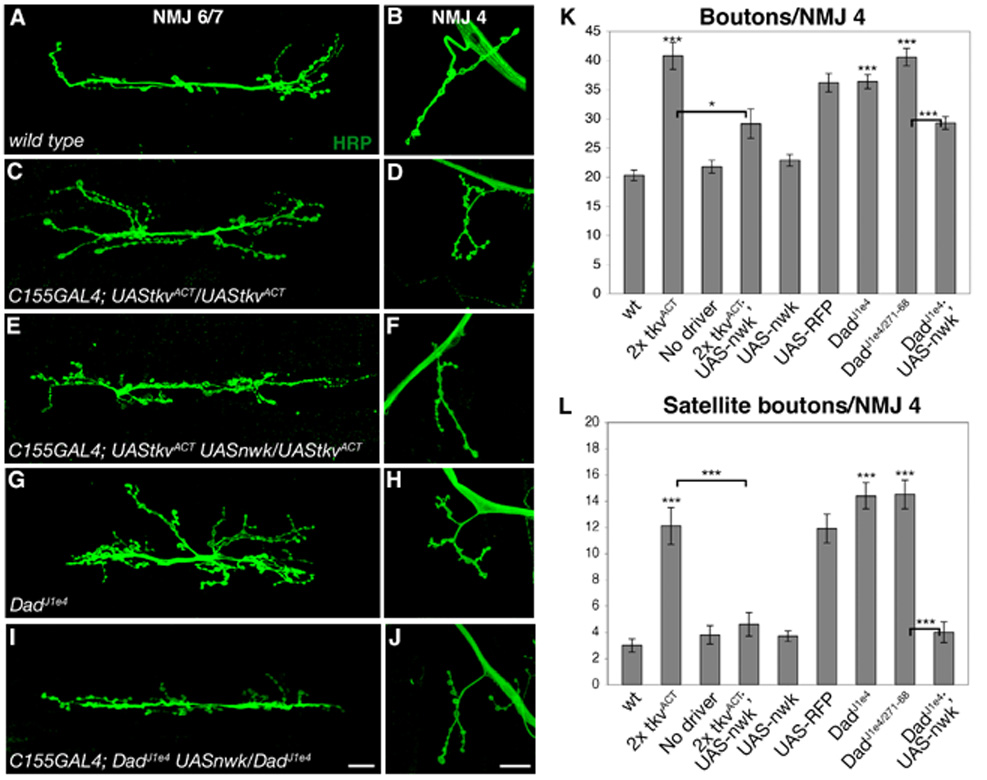
(A–J) Confocal images of NMJ 6/7 (A, C, E, G and I) and NMJ 4 (B, D, F, H and I) labeled with FITC-conjugated anti-HRP. (A, B) wild type. (C, D) C155GAL4; UAStkvACT/UAStkvACT synapses display an increase in bouton number and satellite bouton formation demonstrating that sufficiently high levels of BMP signaling can induce synaptic overgrowth. (E, F) Neuronal overexpression of Nwk suppresses TkvACT-induced synaptic overgrowth. (G, H) Increased bouton number and satellite bouton formation are observed in DadJ1e4 mutants, demonstrating that loss of negative regulation of BMP signaling results in synaptic overgrowth. (I, J) Synaptic overgrowth in DadJ1e4 is suppressed by neuronal overexpression of Nwk. (K, L) Quantification of total (K) and satellite (L) bouton number at NMJ 4 in wild type, C155GAL4; UAStkvACT/UAStkvACT, UAStkvACT/UAStkvACT, C155GAL4; UAStkvACT UASnwk/UAStkvACT, C155GAL4; UASnwk/+, C155GAL4; UAStkvACT/UASmyrRFP/UAStkvACT, DadJ1e4, DadJ1e4/Dad271-68 and C155GAL4; DadJ1e4 UASnwk/DadJ1e4 backgrounds. * p < 0.05, *** p < 0.001. P-values above individual bars refer to comparisons between wild type and the indicated genotype. Scale bars equal 20 µM.
If Nwk acts to downregulate BMP signaling, then simultaneous overexpression of wild-type nwk in neurons should counteract the effect of two doses of UAStkvACT. We found that coexpression of a single copy of UASnwk suppresses the increase in both bouton number and satellite boutons (Figures 5E–F, K–L), while Nwk expression in a wild-type background does not affect synaptic growth (Figures 5K and L). The suppression of synaptic overgrowth by UASnwk is not simply an indirect consequence of the dilution of GAL4 by the presence of a third UAS element because coexpression of UASmyrRFP does not suppress the overgrowth phenotypes (Figures 5K and L). These results demonstrate the ability of Nwk to limit BMP signaling levels at NMJs.
Synaptic overgrowth in Dad mutants is suppressed by overexpression of Nwk
The NMJ phenotypes of larvae ectopically expressing TkvACT indicates that sufficiently high levels of BMP signaling cause synaptic overgrowth. To investigate this further, we examined NMJ morphology in Dad mutants, in which loss of the inhibitory Smad should result in a substantial increase in endogenous BMP signaling (Sweeney and Davis, 2002). We observed significant synaptic overgrowth in all Dad mutant combinations examined. For example, bouton number in DadJ1e4 mutants is 1.8 fold greater than wild type and satellite bouton formation is increased 4.8-fold (Figures 5G–H, K–L). At DadJ1e4/Dad271-68 NMJs, bouton number is doubled with a 4.8-fold increase in satellite boutons (Figures 5K–L). We observed similar levels of overgrowth in hemizigous backgrounds, confirming a role for Dad in the negative regulation of BMP-dependent synaptic growth at larval NMJs.
If Nwk downregulates BMP signaling then increased levels of Nwk should also mitigate the phenotypic effects of loss of negative regulation of endogenous BMP signaling by Dad. To test this, we expressed Nwk pan-neuronally in Dad mutants, and found that Nwk overexpression does, in fact, suppress Dad. Specifically, we found that bouton number is significantly reduced by Nwk expression in a Dad mutant, as is the incidence of satellite boutons (Figures 5I–L). These data provide further evidence that Nwk normally acts to limit BMP signaling at NMJs.
dap160 interacts with BMP signaling pathway components
Our genetic and biochemical data suggest that synaptic overgrowth and satellite bouton formation in nwk and other endocytic mutants may occur through a common mechanism, specifically the failure to appropriately downregulate BMP signaling. This model predicts that satellite bouton formation and synaptic overgrowth in other endocytic mutants are also sensitive to BMP signaling levels. We directly tested this by investigating genetic interactions between dap160 and components of the BMP signaling pathway. First, we analyzed dap160; wit double mutants and the effect of reducing wit dosage on synaptic overgrowth at dap160 NMJs. dap160; wit double mutants exhibit synaptic undergrowth similar to that observed in wit mutants, and loss of one copy of wit suppresses synaptic overgrowth at dap160 synapses (Figures 6A–C, G). These results demonstrate that synaptic overgrowth in dap160 mutants, as in nwk, is dependent upon and sensitive to the dosage of BMP signaling. Next, we tested whether Dap160 also constrains BMP signaling levels by assaying the effect of removing dap160 on the ability of one copy of TkvACT to induce synaptic overgrowth. We found that loss of one copy of dap160 is sufficient to enable one copy of TkvACT to induced synaptic overgrowth (Figures 6D, G–H). Finally, we investigated the ability of Dap160 overexpression to suppress BMP-induced synaptic overgrowth. At NMJs expressing two copies of TkvACT or mutant for Dad, we found that neuronal overexpression of Dap160 suppressed both total and satellite bouton number (Figures 6E–I). These results demonstrate that Dap160 regulates BMP signaling and provide strong support for our model that BMP-dependent growth at presynaptic terminals is attenuated via endocytic regulation by proteins such as Nwk, Dap160, and their interacting partners.
Figure 6. dap160 interacts with BMP signaling pathway components.
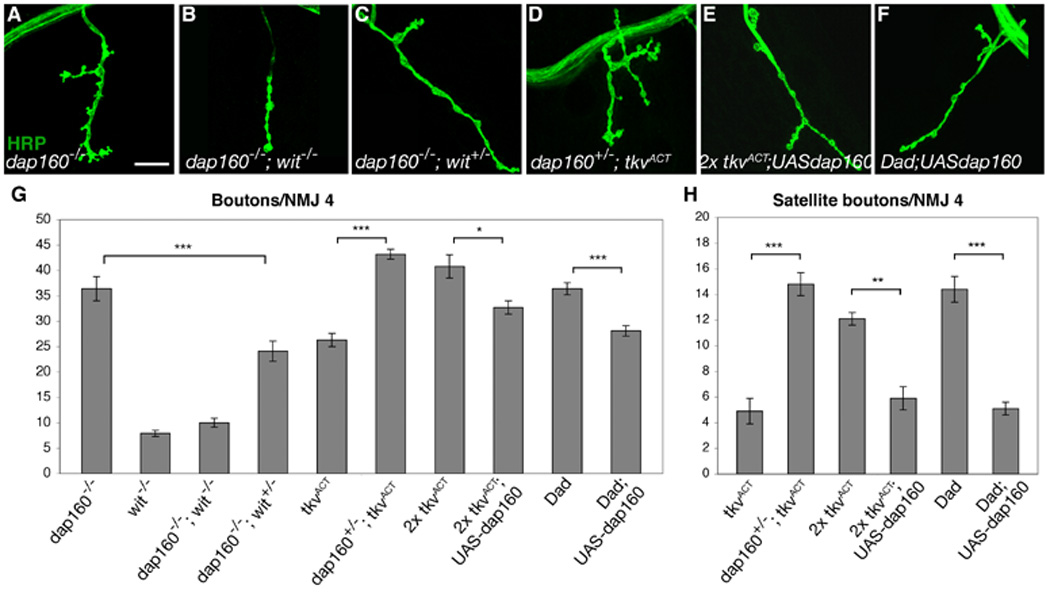
(A–F) Confocal images of NMJ 4 labeled with FITC-conjugated anti-HRP. (A) Synaptic overgrowth in dap160Δ1/EMS. (B) dap160Δ1; witA12/B11 double mutants exhibit the wit synaptic undergrowth phenotype. (C) Loss of one copy of wit suppresses synaptic overgrowth in dap160 mutants. (D) Reducing the dose of dap160 by 50% facilitates TkvACT-induced synaptic overgrowth. (E–F) Neuronal overexpression of Dap160 suppresses synaptic overgrowth in neurons expressing two copies of TkvACT (E) and lacking Dad (F). (G) Quantification of total bouton number at NMJ 4 in dap160Δ1/EMS, witA12/B11, dap160Δ1/Δ1; witA12/B11, dap160Δ1/Δ1; witA12/+, C155GAL4; UAStkvACT/+, C155GAL4; dap160Δ1/+; UAStkvACT/+, C155GAL4; UAStkvACT/UAStkvACT, C155GAL4; UAStkvACT UASdap160/UAStkvACT, DadJ1e4/J1e4 and C155GAL4; DadJ1e4 UASdap160/DadJ1e4 backgrounds. (G) Quantification of satellite boutons at NMJ 4 in C155GAL4; UAStkvACT/+, C155GAL4; dap160Δ1/+; UAStkvACT/+, C155GAL4; UAStkvACT/UAStkvACT, C155GAL4; UAStkvACT UASdap160/UAStkvACT, DadJ1e4/J1e4 and C155GAL4; DadJ1e4 UASdap160/DadJ1e4. * p < 0.05, ** p < 0.01, *** p < 0.001. Scale bar equals 20 µM.
Nwk physically associates with the BMP receptor Tkv
The model that Nwk functions with the presynaptic endocytic machinery to downregulate retrograde BMP growth signaling implies Nwk is a component of an endocytic mechanism that acts to limit the abundance or signaling capacity of membrane-associated BMP receptors. Thus, Nwk may physically interact with one or more of the BMP receptors, providing a link between BMP receptors and the endocytic machinery, to regulate their trafficking or degradation via receptor-mediated endocytosis. Consistent with the possibility of physical association in vivo, we found that Nwk and neuronally expressed Tkv-GFP both localize to presynaptic periactive zones where they colocalize to puncta consistent with endocytic vesicles (Figures 7A and 7B) (Hsiung et al., 2005). Moreover, the intracellular domain of Tkv (Tkv-IC) expressed as a GST-fusion construct efficiently precipitates Nwk from fly lysates (Figure 7C). We also conducted the reciprocal pulldown experiment and found that GST-Nwk precipitates full-length Tkv from fly lysates (Figure 7D). Thus, either full-length Tkv or its intracellular domain alone can physically associate with Nwk. The presence of multiple PxxP SH3-binding domains in Tkv-IC, raised the possibility of a direct interaction, and yeast two-hybrid experiments confirm that the association between Nwk and Tkv-IC is direct (Figure 7E). Together, these findings support the mechanistic interpretation that Nwk attenuates BMP signaling via endocytic regulation of Tkv.
Figure 7. Nwk physically associates with Tkv.
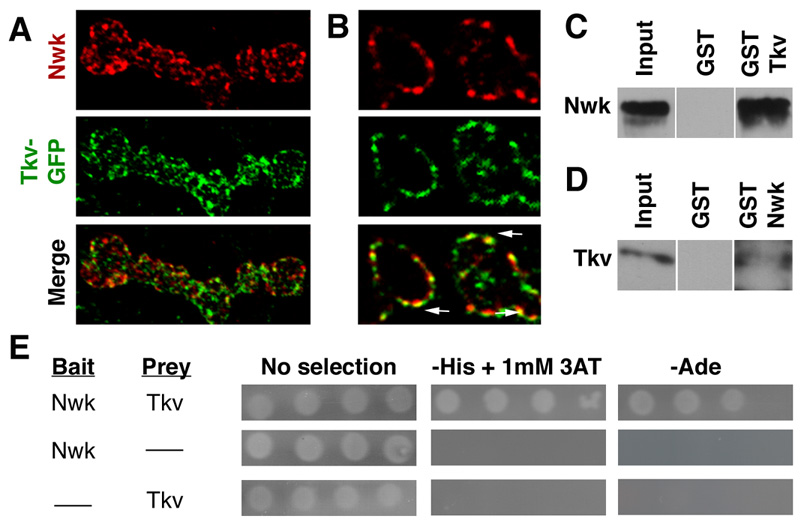
(A) Z-series projection of C155GAL4; UAStkv-GFP boutons co-labeled with antibodies to Nwk. Note that while both proteins exhibit periactive zone localization, both also localize to discrete puncta. (B) Thin (0.1µm) optical cross section of C155GAL4; UAStkv-GFP boutons co-labeled with antibodies to Nwk. Arrows indicate several examples of colocalization of Nwk and Tkv-GFP to membrane-associated puncta. (C) GST-Tkv-IC precipitates Nwk-RFP from C155GAL4; UASnwk-RFP fly lysates with high affinity. Nwk-RFP is detected with rabbit anti-dsRed. We obtained similar results in analogous experiments using lysate from wild-type flies expressing only endogenous Nwk, excluding potential artifacts due to overexpression of Nwk or the RFP tag (data not shown). (D) GST-Nwk precipitates full-length tagged-Tkv from C155GAL4; UAStkv-GFP lysates as detected by rabbit anti-GFP. Input lanes in C and D contain lysate equal to approximately 1/10 of the amount used for the pulldown assays. (E) Yeast two-hybrid interactions demonstrate direct binding of full-length Nwk (prey) and Tkv-IC (bait). After 3 days at 30°C, only yeast coexpressing both bait and prey fusion proteins exhibit growth on SD medium lacking either histidine or adenine.
Presynaptic pMAD levels correlate with synaptic growth and are regulated by Nwk
Activation of BMP receptors leads to the phosphorylation of Mad. Thus, pMAD levels serve as a molecular read out of BMP signaling levels at presynaptic terminals. Our model that Nwk negatively regulates BMP signaling at presynaptic terminals predicts an increase in pMAD levels in nwk mutants. To test this, we quantified pMAD levels at wild- type and nwk synapses and found that pMAD levels are substantially higher (3.3-fold) in nwk mutants (Figures 8A–B, I). A recent study posited an anterograde BMP signal at the NMJ based on the observation of postsynaptically localized pMAD (Dudu et al., 2006). To confirm that the ectopic pMAD we observe is presynaptic, we co-labeled nwk synapses with pMAD and pre- and postsynaptic markers, including Bruchpilot (Brp), which labels presynaptic active zones, and Discs large (Dlg), which labels postsynaptic membrane. We found significant overlap between pMAD and Brp, but essentially no overlap between pMAD and Dlg at nwk NMJs, indicating that the ectopic pMAD is primarily or entirely presynaptic (Figures 8B and C).
Figure 8. pMAD levels vary with Nwk expression and correlate with satellite bouton formation.
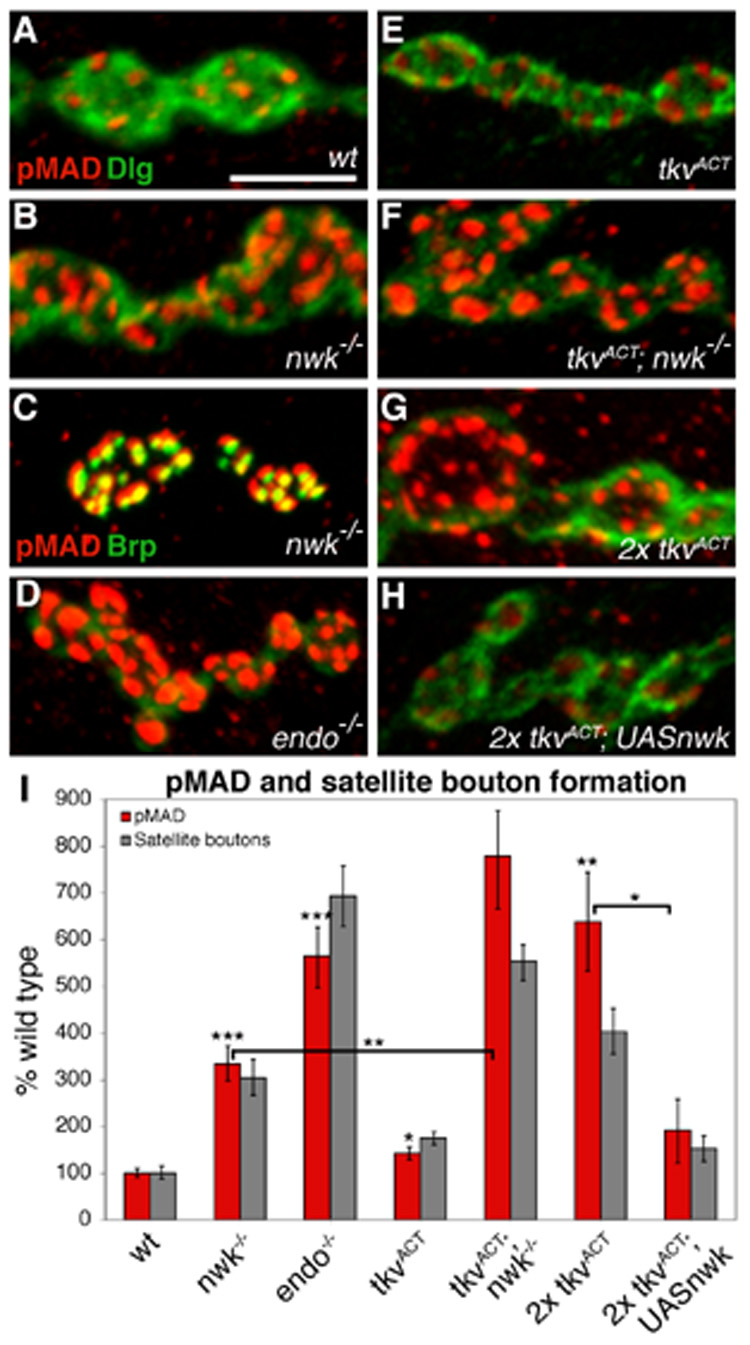
(A–B, D–H) High magnification images of boutons labeled with rabbit anti-pMAD (red) and mouse anti-Dlg (green) in (A) wild type, (B) nwk1/2, (D) endo2730, (E) C155GAL4; UAStkvACT/+, (F) C155GAL4; nwk1/UAStvkACT nwk2, (G) C155GAL4; UAStkvACT/UAStkvACT, and (H) C155GAL4; UAStkvACT UASnwk/UAStkvACT boutons. pMAD levels are increased in endocytic mutants (B,D) and in response to ectopic BMP signaling (E,G). While loss of Nwk enhances (F), overexpression of Nwk suppresses (H) BMP-induced pMAD expression. (C) A high magnification image of nwk boutons labeled with rabbit anti-pMAD (red) and mouse anti-NC82 (green), which labels the active zone protein Bruchpilot (Brp), shows localization of pMAD to presynaptic terminals. Note that in (B), pMAD does not colocalize with the postsynaptic marker Dlg. (I) Quantification of pMAD levels normalized for control (Discs large) intensity (red bars). pMAD levels show a strong correspondence to satellite bouton number (grey bars). * p < 0.05, ** p < 0.01, *** p < 0.001. P-values above individual bars refer to comparisons between wild type and the indicated genotype. Scale bar equals 5 µM.
The model that Nwk is functioning with the endocytic machinery to regulate BMP signaling, suggests that mutations in other endocytic proteins will also result in increased pMAD levels. In fact, a recent study of the Drosophila spastic paraplegias protein Spichthyin (Spict), an early endosome-associated protein that negatively regulates synaptic growth, reported a more than 3-fold increase in presynaptic pMAD levels at spict NMJs (Wang et al., 2007). We investigated pMAD levels in dap160, shi, and endo mutants and observed increases in all three backgrounds, with the most significant increase (5.7-fold) found in endo mutants (Figures 8D and I). Our model further predicts a correlation between BMP-induced synaptic growth and pMAD levels and the modulation of these levels by Nwk. A previous study found an approximately 50% increase in nuclear pMAD levels in larvae expressing TkvACT (Collins et al., 2006). We observe a similar TkvACT-induced increase both in motor neuron nuclei and at the NMJ, where pMAD levels are 42% higher than wild type (Figures 8E and I). Consistent with our model, we observe a much larger (6.4-fold) increase in presynaptic pMAD when two copies of TkvACT are expressed (Figures 8G and I). Significantly, we also found that these levels are regulated by Nwk. Removing Nwk from larvae expressing one copy of TkvACT, which we have found causes a further increase in synaptic growth, results in a 7.8-fold increase in pMAD levels over wild type and a 2.3-fold increase over nwk levels (Figures 8F and I). Conversely, overexpression of Nwk in larvae expressing two copies of TkvACT reduces pMAD levels 3.3-fold, which is consistent with Nwk’s ability to suppress BMP-induced synaptic overgrowth (Figures 8H and I). Finally, we observe a correspondence between pMAD levels and satellite bouton formation (Figure 8I). These data demonstrate a clear relationship between synapse morphology and BMP signaling levels, and confirm that Nwk regulates these levels.
Discussion
This work presents a model for the endocytic regulation of retrograde BMP signaling at synapses, integrating the roles of known positive and negative regulators of synaptic growth into a common molecular pathway. Specifically, our findings indicate that Nwk negatively regulates Tkv trafficking through an endocytic mechanism to limit retrograde BMP signaling, thereby constraining synaptic growth.
A role for Nwk in receptor endocytosis
Nwk interacts functionally and physically with a number of known endocytic proteins, notably dynamin and Dap160. FM1-43 uptake experiments showed that Nwk does not function in an internalization step of synaptic vesicle endocytosis (Coyle et al., 2004), suggesting Nwk affects a later step in endocytic trafficking. In agreement, we observe colocalization between Nwk and the recycling endosome-associated Rab GTPase Rab11, but not between Nwk and Rab proteins associated with either early or late endosomes (unpublished results). Consistent with this observation, Khodosh et al. (2006) found that hypomorphic mutations in Drosophila rab11 result in a synaptic overgrowth phenotype that very closely resembles nwk.
Importantly, Nwk, Dap160 and dynamin are all linked to regulation of actin assembly (Coyle et al., 2004; Engqvist-Goldstein and Drubin, 2003; Hussain et al., 2001). Recent experiments demonstrate that dynamin–mediated vesicle fission requires actin polymerization. For example, Itoh et al. (2005) found that inhibiting actin polymerization blocks fission. Nwk likely facilitates a critical interaction between the endocytic machinery and actin polymerization at NMJs since it directly binds both dynamin and Wasp. Nwk also contains an F-BAR domain, which promotes membrane invagination (Itoh et al., 2005). These domains are found almost exclusively in adaptor proteins that associate both with actin regulators and the endocytic machinery, highlighting the important links between these cellular processes.
The critical role of endocytic accessory proteins in linking the core endocytic machinery to cell signaling molecules is becoming increasingly clear (Conner and Schmid, 2003; Di Fiore and De Camilli, 2001). In addition to attenuating signaling by targeting receptors for degradation, endocytic adaptor proteins play key roles in spatial and temporal regulation of signal transduction from ligand-activated receptors. Recent work in other systems also suggests a critical role for endocytic trafficking during TGF-β/BMP signal transduction. For example, Smad phosphorylation and nuclear translocation in vertebrates depend on localization of the endosomal protein, Smad anchor for receptor activation (SARA), and activated type I and II receptors to EEA1-positive endosomal compartments (Hayes et al., 2002). Similarly, in the Drosophila wing, targeting of Sara, Tkv and the ligand Decapentaplegic (Dpp) to early endosomes is required for productive signaling (Bokel et al., 2006). Nwk is ideally situated to bridge endocytosis and growth signal regulation at presynaptic terminals because of its links to actin assembly and endocytosis as well as its capacity for binding a number of proteins – including Tkv – through its multiple protein-protein interaction domains.
Endocytosis, growth signaling and satellite bouton formation
Many cellular processes have been implicated in negative regulation of synaptic growth, including cell adhesion, ubiquitin-dependent protein degradation, actin dynamics and endocytosis, while studies in Drosophila and vertebrates demonstrate a direct correlation between morphological growth and physiological plasticity (Collins and DiAntonio, 2007; Dillon and Goda, 2005). Loss of endocytic proteins results in the specific phenotype of excessive satellite bouton formation (Dickman et al., 2006), as do mutations in actin-associated proteins, including Nwk, Wasp and components of the Scar complex (Coyle et al., 2004; Koh et al., 2004; Marie et al., 2004; Schenck et al., 2004). These observations suggest that satellite bouton formation is a specific result of impairment of an actin-dependent step in endocytosis. Dickman et al. (2006) suggested the possible misregulation of a signaling pathway responsible for bouton growth and morphology in endocytic mutants and, because satellite bouton formation had not been linked with any known pathway, postulated existence of either an unidentified positive growth signal downregulated by endocytosis or an endocytosis-dependent negative growth signal. Here we show that BMP signaling regulates satellite bouton formation. Increasing levels of BMP signaling, either by expressing UAStkvACT or by reducing endogenous negative regulation of the pathway, generates a significant increase in satellite bouton formation. Further, overexpression of Nwk or Dap160 suppresses BMP-induced overgrowth, including satellite bouton formation. Together, these results indicate that impaired endocytic regulation of retrograde BMP signaling results in generation of satellite boutons at NMJs. In the case of endocytic and Dad mutants, downregulation of endogenous BMP signaling is impaired, while in TkvACT-expressing larvae, ectopic BMP signaling apparently overwhelms the usual mechanisms of negative regulation.
A model of Nwk-dependent endocytic regulation of BMP signaling
Although BMP signaling is required for NMJ growth, it has remained unclear whether the signal acts merely as a switch to initiate or permit growth or instead plays a more instructive role in regulating and coordinating synaptic growth (Aberle et al., 2002; Marques et al., 2002; McCabe et al., 2004; McCabe et al., 2003; Rawson et al., 2003; Sweeney and Davis, 2002). Here, we demonstrate a direct relationship between levels of BMP signaling and extent of synaptic growth. Neuronal expression of a single copy of TkvACT results in modest increase in pMAD and no significant increase in synaptic growth, whereas expression of two copies induces a dramatic increase in pMAD levels and extensive synaptic overgrowth – both of which are suppressed by overexpression of Nwk. Further, in agreement with Sweeney and Davis (2002), we find that mutations in the endogenous negative regulator Dad also cause increased synaptic growth (Sweeney and Davis, 2002). These data indicate that the level of BMP signaling has an instructive role in governing synaptic size and complexity, and reveal the importance of interactions between positive and negative regulators that modulate the growth signal in response to internal and external cues.
Our results demonstrate that endocytosis is an important regulatory mechanism for attenuating BMP signaling at synapses. Previous work suggested that Hiw, an E3 ubiquitin ligase and negative regulator of synaptic growth, also acted to limit BMP signaling (McCabe et al., 2004). However, subsequent work demonstrated that pMAD levels are not increased in hiw and no effects of Hiw overexpression on BMP signaling have been described (Collins et al., 2006). In addition, hiw synapses are extremely expansive, elaborately branched, and contain numerous small boutons, but no satellite boutons (Wan et al., 2000). This phenotype is distinct from that associated with TkvACT overexpression or other genotypes believed to elevate BMP signaling, including Dad, nwk, and known endocytic genes – all of which exhibit satellite boutons. Together with the recent finding that Hiw regulates MAPKKK-dependent Fos activity, these observations suggest Hiw and BMP signaling regulate different aspects of synaptic growth.
In their recent study of Spict, Wang et al. (2007) demonstrated the partial colocalization of Spict with early endosomes along with data suggesting that Spict plays a role in BMP receptor trafficking (Wang et al., 2007). For example, Spict overexpression in S2 cells caused Wit to relocalize to early endosomes, suggesting negative regulation of BMP signaling by sequestering Wit receptor and/or Wit-Gbb signaling complexes. However, studies of Wit trafficking at spict NMJs, which are limited by the small size of boutons and the lack of reliable antibodies, did not uncover this trafficking defect but instead revealed increased Wit levels consistent with a role for Spict in the degradation of Wit. Nonetheless, the results from the analysis of Spict support with the idea that endocytic regulation of BMP signaling is required for proper synaptic growth. It will be interesting to determine whether Spict interacts with Nwk or plays a distinct role in the regulation of BMP signaling at synapses.
Overall, our findings support a model in which Nwk constrains synaptic growth by regulating endocytic trafficking of Tkv to attenuate positive retrograde growth signaling. While the simplest model is that Nwk targets Tkv for degradation, we do not observe gross differences in levels of ectopically expressed Tkv-GFP in otherwise wild type, nwk and C155GAL4; UAS-nwk larvae (unpublished results). A caveat is that without the necessary antibody reagents we are unable to look at endogenous Tkv. Thus, these observations might not accurately reflect normal receptor trafficking but rather the fact that high levels of Tkv can override endogenous regulation. Nonetheless, together with our observation that Nwk colocalizes with Rab11, this finding is consistent with a role for Nwk in BMP receptor recycling. For example, Nwk might attenuate BMP signaling levels by regulating the rate at which vacant Tkv receptors are recycled back to the plasma membrane following activation and internalization. Nwk might also regulate the trafficking of unbound receptors from the plasma membrane. Previous work in vertebrates demonstrates that TGF-β receptors are recycled through a Rab11-dependent mechanism independent of ligand binding, possibly as a means of rapidly and dynamically regulating surface receptor number and, thus, sensitivity to TGF-β (Mitchell et al., 2004). Interestingly, we observe the re-localization of Nwk from a more uniform to a more punctate expression pattern upon overexpression of Tkv (See Figure 7A), consistent with recruitment of Nwk to regulate trafficking of ectopic Tkv. Nwk might also have localized effects within boutons, for example, by restricting sites of BMP signaling through spatial regulation of receptor recycling. It is intriguing to speculate that disruption of spatial constraints on BMP signaling results in ectopic bouton division and, thus, satellite bouton formation. Such a mechanism could provide a critical means for effecting localized changes to existing synapses that underlie neural plasticity. A conceptually similar Rab11-dependent process for the asymmetric activation of Notch signaling in the developing nervous system has recently been described (Emery et al., 2005; Jafar-Nejad et al., 2005). A future challenge will be to further dissect the regulatory mechanisms that control the levels, timing and localization of BMP signaling at synapses. These studies will advance our understanding of the dynamic regulation of synaptic growth and plasticity and likely provide additional general insights into the intricate role of endocytosis in signal transduction.
Experimental Procedures
Fly Stocks
The following fly stocks were used: nwk1, nwk2 and UASnwk33D (Coyle et al., 2004); dap160EMS, dap160Δ,1 and UASdap160 (Koh et al., 2004); mad12 (Sekelsky et al., 1995); gbbD20 (Chen et al., 1998); elavGAL4 (A. DiAntonio); UAStkvAB3 (Hoodless et al., 1996); Dad271-68 (Tsuneizumi et al., 1997); UASrab5-GFP and UASrab7-GFP (M. González-Gaitán); and UAStkv-GFP (Hsiung et al., 2005). All other stocks were obtained from Bloomington Stock Center.
Immunofluorescence studies
All larvae were obtained from crosses of 10 females and 10 males, to control for crowding, conducted at 25°C. shits1 crosses were kept at room temperature and progeny shifted to 25°C during embryogenesis. Wandering third instar larvae were dissected in ice-cold Ca2+-free saline and fixed for 15 minutes in 4% formaldehyde in PBS. Larvae were incubated in primary antibodies overnight at 4°C or for 4 hours at room temperature and secondary antibodies for 1–2 hours at room temperature then mounted in Vectashield (Vector Laboratories) for microscopic analysis. For comparison of pMAD levels, larvae of all genotypes analyzed were stained in the same dish. wit null mutants were analyzed as a control for potential cross-reactivity with Mad. We used the following antibodies: FITC-conjugated anti-HRP at 1:100 (Jackson ImmunoResearch); mouse anti-NC82 (Bruchpilot) at 1:100 (E. Buchner), rat anti-Nwk at 1:1000 (Coyle et al., 2004); rabbit anti-Dap160 and anti-dynamin at 1:100 (Roos and Kelly, 1998); rabbit anti-GFP at 1:500 (Molecular Probes); rabbit anti-PS1 at 1:500 (Persson et al., 1998); rabbit anti-pMAD at 1:100 (Cell Signaling) and mouse anti-Dlg at 1:500 (Developmental Hybridoma Studies Bank). Species-specific Alexa-488 and Alexa-568 (Molecular Probes) were used at 1:200.
Imaging and quantification
Quantification of bouton number was performed at NMJ 4 in segments A2-A4 as described (Coyle et al., 2004). The muscle size of all larvae analyzed was similar. Satellite boutons were defined as extensions of 5 or fewer boutons emanating from the main branch of the nerve terminal. The same control data sets were used for multiple comparisons. We performed the statistical analysis by ANOVA and conducted post hoc tests with the appropriate Bonferroni adjustment for multiple comparisons to ensure an experiment-wise simultaneous significance level of 0.05. While we conducted each comparison at the reduced critical p-value, we report the significance levels at the corresponding experiment-wise levels of less than 0.05, less than 0.01, or less than 0.001, by 1, 2, and 3 stars respectively. Error bars denote SEM. Confocal images were obtained on a Bio-Rad Radiance 2100 MP Rainbow mounted on a Nikon Eclipse TE2000 and processed in NIH ImageJ. Brightness and contrast were adjusted using Adobe Photoshop. Quantification of pMAD intensity was performed on confocal images captured with identical settings using NIH ImageJ software.
Yeast two-hybrid analysis
Yeast two-hybrid experiments were performed using the Clontech Matchmaker system according to their protocols. Drop tests were performed by plating 5µl of serial dilutions of mid-log-phase liquid cultures on appropriate selection media.
GST pulldowns
Nwk, dynamin, Dap160 and Tkv constructs were cloned into the pGEX-6P-3 vector using standard molecular techniques and expressed in bacteria. Fusion proteins were immobilized on glutathione-sepharose beads and incubated with cell extracts prepared from C155GAL4; UASnwk-RFP or C155GAL4; UAStkv-GFP adult flies. Beads were washed three times in lysis buffer. Bound proteins were eluted and analyzed by western analysis using rabbit anti-dsRed (Clontech) at 1:750 or purified rabbit anti-GFP (Abcam) at 1:1000.
Acknowledgements
We thank Hugo Bellen, Erich Buchner, Aaron DiAntonio, Marcos González-Gaitán, Regis Kelly, Thomas Kornberg, Sean Sweeney, Peter ten Dijke, Kristi Wharton, the Bloomington Stock Center and the Developmental Studies Hybridoma Bank for generously providing fly stocks and antibodies. We are grateful to members of the Ganetzky lab and Avital Rodal for helpful discussions and to Heather Broihier, Tim Fergestad and Daniel Miller for critical comments on the manuscript. We also thank Katherine Hannon and Veena Rao for the many dissections they performed during the course of this work and Noah Rozich for assistance with biochemical experiments. This research was supported by an NIH grant (NS-15390) to B.G. and a fellowship from The Jane Coffin Childs Memorial Fund for Medical Research to K.M.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. doi: 10.1016/s0896-6273(02)00589-5. [DOI] [PubMed] [Google Scholar]
- Allan DW, St Pierre SE, Miguel-Aliaga I, Thor S. Specification of neuropeptide cell identity by the integration of retrograde BMP signaling and a combinatorial transcription factor code. Cell. 2003;113:73–86. doi: 10.1016/s0092-8674(03)00204-6. [DOI] [PubMed] [Google Scholar]
- Bokel C, Schwabedissen A, Entchev E, Renaud O, Gonzalez-Gaitan M. Sara endosomes and the maintenance of Dpp signaling levels across mitosis. Science (New York, N.Y. 2006;314:1135–1139. doi: 10.1126/science.1132524. [DOI] [PubMed] [Google Scholar]
- Budnik V, Zhong Y, Wu CF. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci. 1990;10:3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Riese MJ, Killinger MA, Hoffmann FM. A genetic screen for modifiers of Drosophila decapentaplegic signaling identifies mutations in punt, Mothers against dpp and the BMP-7 homologue, 60A. Development (Cambridge, England) 1998;125:1759–1768. doi: 10.1242/dev.125.9.1759. [DOI] [PubMed] [Google Scholar]
- Collins CA, DiAntonio A. Synaptic development: insights from Drosophila. Current opinion in neurobiology. 2007;17:35–42. doi: 10.1016/j.conb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Collins CA, Wairkar YP, Johnson SL, DiAntonio A. Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron. 2006;51:57–69. doi: 10.1016/j.neuron.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Coyle IP, Koh YH, Lee WC, Slind J, Fergestad T, Littleton JT, Ganetzky B. Nervous wreck, an SH3 adaptor protein that interacts with Wsp, regulates synaptic growth in Drosophila. Neuron. 2004;41:521–534. doi: 10.1016/s0896-6273(04)00016-9. [DOI] [PubMed] [Google Scholar]
- Di Fiore PP, De Camilli P. Endocytosis and signaling. an inseparable partnership. Cell. 2001;106:1–4. doi: 10.1016/s0092-8674(01)00428-7. [DOI] [PubMed] [Google Scholar]
- Dickman DK, Lu Z, Meinertzhagen IA, Schwarz TL. Altered synaptic development and active zone spacing in endocytosis mutants. Curr Biol. 2006;16:591–598. doi: 10.1016/j.cub.2006.02.058. [DOI] [PubMed] [Google Scholar]
- Dillon C, Goda Y. The actin cytoskeleton: integrating form and function at the synapse. Annual review of neuroscience. 2005;28:25–55. doi: 10.1146/annurev.neuro.28.061604.135757. [DOI] [PubMed] [Google Scholar]
- Dudu V, Bittig T, Entchev E, Kicheva A, Julicher F, Gonzalez-Gaitan M. Postsynaptic mad signaling at the Drosophila neuromuscular junction. Curr Biol. 2006;16:625–635. doi: 10.1016/j.cub.2006.02.061. [DOI] [PubMed] [Google Scholar]
- Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, Gaitan MG, Knoblich JA. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122:763–773. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein AE, Drubin DG. Actin assembly and endocytosis: from yeast to mammals. Annual review of cell and developmental biology. 2003;19:287–332. doi: 10.1146/annurev.cellbio.19.111401.093127. [DOI] [PubMed] [Google Scholar]
- Estes PS, Roos J, van der Bliek A, Kelly RB, Krishnan KS, Ramaswami M. Traffic of dynamin within individual Drosophila synaptic boutons relative to compartment-specific markers. J Neurosci. 1996;16:5443–5456. doi: 10.1523/JNEUROSCI.16-17-05443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi AP, McCabe BD, Fetter RD, Palmer JE, Hom S, Goodman CS. Retrograde control of synaptic transmission by postsynaptic CaMKII at the Drosophila neuromuscular junction. Neuron. 2003;39:255–267. doi: 10.1016/s0896-6273(03)00427-6. [DOI] [PubMed] [Google Scholar]
- Hayes S, Chawla A, Corvera S. TGF beta receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. The Journal of cell biology. 2002;158:1239–1249. doi: 10.1083/jcb.200204088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang B, Chiba A. Single-cell analysis of Drosophila larval neuromuscular synapses. Developmental biology. 2001;229:55–70. doi: 10.1006/dbio.2000.9983. [DOI] [PubMed] [Google Scholar]
- Hoodless PA, Haerry T, Abdollah S, Stapleton M, O'Connor MB, Attisano L, Wrana JL. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell. 1996;85:489–500. doi: 10.1016/s0092-8674(00)81250-7. [DOI] [PubMed] [Google Scholar]
- Hsiung F, Ramirez-Weber FA, Iwaki DD, Kornberg TB. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature. 2005;437:560–563. doi: 10.1038/nature03951. [DOI] [PubMed] [Google Scholar]
- Hussain NK, Jenna S, Glogauer M, Quinn CC, Wasiak S, Guipponi M, Antonarakis SE, Kay BK, Stossel TP, Lamarche-Vane N, McPherson PS. Endocytic protein intersectin-l regulates actin assembly via Cdc42 and N-WASP. Nature cell biology. 2001;3:927–932. doi: 10.1038/ncb1001-927. [DOI] [PubMed] [Google Scholar]
- Itoh T, De Camilli P. BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochimica et biophysica acta. 2006;1761:897–912. doi: 10.1016/j.bbalip.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Itoh T, Erdmann KS, Roux A, Habermann B, Werner H, De Camilli P. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Developmental cell. 2005;9:791–804. doi: 10.1016/j.devcel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Jafar-Nejad H, Andrews HK, Acar M, Bayat V, Wirtz-Peitz F, Mehta SQ, Knoblich JA, Bellen HJ. Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Developmental cell. 2005;9:351–363. doi: 10.1016/j.devcel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Keshishian H, Kim YS. Orchestrating development and function: retrograde BMP signaling in the Drosophila nervous system. Trends in neurosciences. 2004;27:143–147. doi: 10.1016/j.tins.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Khodosh R, Augsburger A, Schwarz TL, Garrity PA. Bchs, a BEACH domain protein, antagonizes Rab11 in synapse morphogenesis and other developmental events. Development (Cambridge, England) 2006;133:4655–4665. doi: 10.1242/dev.02650. [DOI] [PubMed] [Google Scholar]
- Kim Y, Chang S. Ever-expanding network of dynamin-interacting proteins. Molecular neurobiology. 2006;34:129–136. doi: 10.1385/MN:34:2:129. [DOI] [PubMed] [Google Scholar]
- Koh TW, Verstreken P, Bellen HJ. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron. 2004;43:193–205. doi: 10.1016/j.neuron.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Marie B, Sweeney ST, Poskanzer KE, Roos J, Kelly RB, Davis GW. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron. 2004;43:207–219. doi: 10.1016/j.neuron.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Marques G, Bao H, Haerry TE, Shimell MJ, Duchek P, Zhang B, O'Connor MB. The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron. 2002;33:529–543. doi: 10.1016/s0896-6273(02)00595-0. [DOI] [PubMed] [Google Scholar]
- Marrus SB, DiAntonio A. Preferential localization of glutamate receptors opposite sites of high presynaptic release. Curr Biol. 2004;14:924–931. doi: 10.1016/j.cub.2004.05.047. [DOI] [PubMed] [Google Scholar]
- McCabe BD, Hom S, Aberle H, Fetter RD, Marques G, Haerry TE, Wan H, O'Connor MB, Goodman CS, Haghighi AP. Highwire regulates presynaptic BMP signaling essential for synaptic growth. Neuron. 2004;41:891–905. doi: 10.1016/s0896-6273(04)00073-x. [DOI] [PubMed] [Google Scholar]
- McCabe BD, Marques G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, Goodman CS, O'Connor MB. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39:241–254. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- Mitchell H, Choudhury A, Pagano RE, Leof EB. Ligand-dependent and -independent transforming growth factor-beta receptor recycling regulated by clathrin-mediated endocytosis and Rab11. Molecular biology of the cell. 2004;15:4166–4178. doi: 10.1091/mbc.E04-03-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. Journal of cellular physiology. 2001;187:265–276. doi: 10.1002/jcp.1080. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Kazama H, Nose A, Morimoto-Tanifuji T. Activity-dependent regulation of synaptic size in Drosophila neuromuscular junctions. Journal of neurobiology. 2006;66:929–939. doi: 10.1002/neu.20292. [DOI] [PubMed] [Google Scholar]
- Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson U, Izumi H, Souchelnytskyi S, Itoh S, Grimsby S, Engstrom U, Heldin CH, Funa K, ten Dijke P. The L45 loop in type I receptors for TGF-beta family members is a critical determinant in specifying Smad isoform activation. FEBS letters. 1998;434:83–87. doi: 10.1016/s0014-5793(98)00954-5. [DOI] [PubMed] [Google Scholar]
- Pielage J, Fetter RD, Davis GW. A postsynaptic spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. The Journal of cell biology. 2006;175:491–503. doi: 10.1083/jcb.200607036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson JM, Lee M, Kennedy EL, Selleck SB. Drosophila neuromuscular synapse assembly and function require the TGF-beta type I receptor saxophone and the transcription factor Mad. Journal of neurobiology. 2003;55:134–150. doi: 10.1002/neu.10189. [DOI] [PubMed] [Google Scholar]
- Roos J, Kelly RB. Dap160, a neural-specific Eps15 homology and multiple SH3 domain-containing protein that interacts with Drosophila dynamin. The Journal of biological chemistry. 1998;273:19108–19119. doi: 10.1074/jbc.273.30.19108. [DOI] [PubMed] [Google Scholar]
- Roos J, Kelly RB. The endocytic machinery in nerve terminals surrounds sites of exocytosis. Curr Biol. 1999;9:1411–1414. doi: 10.1016/s0960-9822(00)80087-1. [DOI] [PubMed] [Google Scholar]
- Saitoe M, Schwarz TL, Umbach JA, Gundersen CB, Kidokoro Y. Absence of junctional glutamate receptor clusters in Drosophila mutants lacking spontaneous transmitter release. Science (New York, N.Y. 2001;293:514–517. doi: 10.1126/science.1061270. [DOI] [PubMed] [Google Scholar]
- Schenck A, Qurashi A, Carrera P, Bardoni B, Diebold C, Schejter E, Mandel JL, Giangrande A. WAVE/SCAR, a multifunctional complex coordinating different aspects of neuronal connectivity. Developmental biology. 2004;274:260–270. doi: 10.1016/j.ydbio.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Sekelsky JJ, Newfeld SJ, Raftery LA, Chartoff EH, Gelbart WM. Genetic characterization and cloning of mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster. Genetics. 1995;139:1347–1358. doi: 10.1093/genetics/139.3.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist SJ, Reiff DF, Thiel PR, Steinert JR, Schuster CM. Experience-dependent strengthening of Drosophila neuromuscular junctions. J Neurosci. 2003;23:6546–6556. doi: 10.1523/JNEUROSCI.23-16-06546.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist SJ, Thiel PR, Reiff DF, Schuster CM. The postsynaptic glutamate receptor subunit DGluR-IIA mediates long-term plasticity in Drosophila. J Neurosci. 2002;22:7362–7372. doi: 10.1523/JNEUROSCI.22-17-07362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe E, Ayscough KR. Actin regulation in endocytosis. Journal of cell science. 2006;119:4589–4598. doi: 10.1242/jcs.03247. [DOI] [PubMed] [Google Scholar]
- Sweeney ST, Davis GW. Unrestricted synaptic growth in spinster-a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron. 2002;36:403–416. doi: 10.1016/s0896-6273(02)01014-0. [DOI] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nature reviews. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- Tsuneizumi K, Nakayama T, Kamoshida Y, Kornberg TB, Christian JL, Tabata T. Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature. 1997;389:627–631. doi: 10.1038/39362. [DOI] [PubMed] [Google Scholar]
- Wan HI, DiAntonio A, Fetter RD, Bergstrom K, Strauss R, Goodman CS. Highwire regulates synaptic growth in Drosophila. Neuron. 2000;26:313–329. doi: 10.1016/s0896-6273(00)81166-6. [DOI] [PubMed] [Google Scholar]
- Wang X, Shaw WR, Tsang HT, Reid E, O'Kane CJ. Drosophila spichthyin inhibits BMP signaling and regulates synaptic growth and axonal microtubules. Nature neuroscience. 2007;10:177–185. doi: 10.1038/nn1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Kelly RB. Intersectin 1L guanine nucleotide exchange activity is regulated by adjacent src homology 3 domains that are also involved in endocytosis. Molecular biology of the cell. 2003;14:1624–1637. doi: 10.1091/mbc.E02-08-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]


