Abstract
Recent experimental evidence suggests that reactive nitrogen oxide species can contribute significantly to postischemic myocardial injury. The aim of the present study was to evaluate the role of two reactive nitrogen oxide species, nitroxyl (NO−) and nitric oxide (NO⋅), in myocardial ischemia and reperfusion injury. Rabbits were subjected to 45 min of regional myocardial ischemia followed by 180 min of reperfusion. Vehicle (0.9% NaCl), 1 μmol/kg S-nitrosoglutathione (GSNO) (an NO⋅ donor), or 3 μmol/kg Angeli’s salt (AS) (a source of NO−) were given i.v. 5 min before reperfusion. Treatment with GSNO markedly attenuated reperfusion injury, as evidenced by improved cardiac function, decreased plasma creatine kinase activity, reduced necrotic size, and decreased myocardial myeloperoxidase activity. In contrast, the administration of AS at a hemodynamically equieffective dose not only failed to attenuate but, rather, aggravated reperfusion injury, indicated by an increased left ventricular end diastolic pressure, myocardial creatine kinase release and necrotic size. Decomposed AS was without effect. Co-administration of AS with ferricyanide, a one-electron oxidant that converts NO− to NO⋅, completely blocked the injurious effects of AS and exerted significant cardioprotective effects similar to those of GSNO. These results demonstrate that, although NO⋅ is protective, NO− increases the tissue damage that occurs during ischemia/reperfusion and suggest that formation of nitroxyl may contribute to postischemic myocardial injury.
Keywords: free radicals, reperfusion, neutrophils
Nitric oxide (NO⋅) is produced from l-arginine by a family of isoenzymes, the so-called nitric oxide synthases (NOS). Whereas the physiological production of NO⋅ from the endothelial isoform (eNOS) plays a critical role in cardiovascular homeostasis, excess production of NO⋅ by the inducible isoform (iNOS) is involved in host defense mechanisms and has been postulated to not only mediate cytotoxicity but also cause tissue injury in a variety of pathological states (1). Several recent studies have demonstrated that NOS inhibition significantly attenuates postischemic myocardial injury (2–4). It has thus been hypothesized that, in addition to reactive oxygen species (ROS), which have been implicated in ischemia/reperfusion injury for a number of years (5), reactive nitrogen oxide species (RNOS) generated from NOS may also contribute to postischemic myocardial injury (6).
Peroxynitrite anion (ONOO−), a RNOS produced from the interaction between NO⋅ and superoxide (O2⨪), has received considerable attention in recent years. A number of in vitro biochemical studies have demonstrated that ONOO− is highly reactive toward a wide variety of compounds and results in an oxidative tissue damage similar to that caused by hydroxyl radicals (⋅OH) (7). Based on these in vitro observations, it has been suggested that ONOO− may play a significant role in tissue damage mediated by RNOS after ischemia and reperfusion (8). However, recent studies from our laboratory as well as those from other investigators have revealed that the effects of ONOO− are critically dependent on the microenvironment in which this oxidant is produced. For example, ONOO− causes aggregation of platelets in buffer solution but inhibits aggregation of platelets in plasma (9). Administration of ONOO− to buffer solution-perfused rat hearts increases reperfusion injury whereas administration of ONOO− to whole blood-perfused hearts significantly diminishes reperfusion injury (10). Moreover, Nossuli et al. (11) have recently reported that administration of a low concentration of ONOO− in vivo attenuates rather than enhances reperfusion injury. These results suggest that the beneficial effects of NOS inhibition on postischemic injury cannot be entirely attributed to a reduced production of NO⋅ and, consequently, ONOO− and imply that other toxic RNOS must be formed from NOS that can contribute to reperfusion injury.
Nitroxyl anion (NO−) is the one electron reduction product of NO⋅. Several in vitro studies have suggested that NO− can be formed by NOS, either directly through the enzymatic activity of NOS or indirectly via metabolism of the decoupled NOS product NG-hydroxyl-l-arginine (HO-Arg) (12–14). Two recent in vitro studies revealed that NO− is considerably more cytotoxic than NO⋅ by causing DNA strand breaks and base oxidation (15, 16). Taken together, these results suggest that NO− may contribute to the cytotoxicity that is associated with an enhanced expression of the l-arginine/NO⋅ pathway under certain pathologic conditions and might also be involved in reperfusion injury.
To date, the detrimental action of NO− has not been evaluated in vivo. The major aim of the present study, therefore, was to investigate the role of NO− in tissue injury under in vivo pathologic conditions. Using a rabbit myocardial ischemia reperfusion model, we directly compared the effects of the NO⋅ donor, S-nitrosoglutathione (GSNO), with that of the NO− donor, Angeli’s salt (AS), on postischemic myocardial injury. We here demonstrate that, although NO⋅ markedly attenuates postischemic myocardial tissue damage, its one-electron reduction product, NO−, exerts completely opposite effects and aggravates myocardial reperfusion injury. The implications of these findings for the pathophysiology of ischemia reperfusion-related tissue injury are discussed.
Materials and Methods
Materials.
S-nitroso-l-glutathione (GSNO) and Angeli’s salt (AS) (sodium trioxodinitrate, Na2N2O3) were synthesized as described earlier (14, 15). All other compounds were purchased from Sigma. GSNO and AS were prepared fresh in saline (0.9% NaCl) immediately before application. For some experiments, AS solution was allowed to decompose for 72 h at room temperature before administration. A total of 76 adult male New Zealand white rabbits (2.8–3.5 kg) were included in this study. The experiments were performed in adherence to National Institutes of Health Guidelines on the Use of Laboratory Animals and were approved by the Thomas Jefferson University Committee on Animal Care.
Experimental Preparation.
Rabbits were anaesthetized with sodium pentobarbital (30 mg/kg, iv.) and were ventilated with a Harvard small animal respirator. A polyethylene catheter was inserted into the right external jugular vein for supplemental pentobarbital injection and for administration of test compounds. The arterial blood pressure was measured via a polyethylene catheter cannulated to the right femoral artery, and the left ventricular pressure was measured via a Millar Mikro-tip catheter transducer, which was inserted into the left ventricular cavity through the left carotid artery.
After midline thoracotomy, a 4-0 silk ligature was placed around the major marginal branch of the left circumflex coronary, 10–12 mm from its origin. After a 20-min period of stabilization, myocardial ischemia (MI) was initiated by complete ligation of the marginal coronary artery. After 45 min of ischemia, the ligature was untied and the ischemic myocardium reperfused (R) for 3 hs. Sham MI/R rabbits were subjected to the same surgical procedures performed on MI/R rabbits, except that the suture was left untied. The rabbits were randomly assigned to one of the following groups: (i) sham MI/R + GSNO or AS (0.1 to 3 μmol/kg, n = 3 for each compound; dose titration group); (ii) MI/R + vehicle (0.9% NaCl, n = 10; control group); (iii) MI/R + GSNO (1.0 μmol/kg, n = 12; NO⋅ treatment group); (iv) MI/R + AS (3 μmol/kg, n = 11; nitroxyl treatment group); (v) MI/R + potassium ferricyanide (PF) (10 μmol/kg, n = 11; oxidant control group); or (vi) MI/R + AS + PF (n = 12; nitroxyl/oxidant combination group). Two additional groups served as further controls for the effect of Angeli’s salt: (vii) MI/R + AS (1 μmol/kg, n = 7; low dose nitroxyl group); and (viii) MI/R + decomposed AS (3 μmol/kg, n = 7; nitrite control group). Each drug or vehicle was given 5 min before reperfusion as i.v. short term infusion over 1 min.
Analysis of Myocardial Injury.
MI/R-induced cardiac contractile dysfunction was continuously monitored during the entire ischemia and reperfusion period. Left ventricular pressure and arterial blood pressure (ABP) were sampled at 250 Hz and were digitally processed via a hemodynamic analyzing system (Digi-Med, Louisville, KY). Mean arterial blood pressure (MABP), left ventricular systolic pressure, left ventricular end diastolic pressure (LVEDP), positive and negative maximal values of the instantaneous first derivative of left ventricular pressure (+dP/dtmax and −dP/dtmax), and heart rate were derived by computer algorithms. The pressure–rate index, calculated as the product of mean arterial blood pressure and heart rate divided by 1,000, was used as an approximation of myocardial oxygen demand.
Arterial blood samples (1 ml) were drawn immediately before ligation (0 min), 45 min after ischemia, and hourly thereafter. Plasma creatine kinase (CK) activity was measured in a blinded manner by using a Sigma kit and was expressed as units per gram of protein.
At the end of the 3.0-h reperfusion period, the ligature around the marginal coronary artery was retied, and 20 ml of 5% Evans blue dye was injected into the left ventricular cavity. The heart was quickly excised, and the atria, right ventricle, and fatty tissues were removed from the heart. The marginal coronary artery was isolated, and a 4- to 5-mm-long segment was removed from below the ligature. Coronary endothelial function was studied as described (17). Endothelial dysfunction was defined as decreased vasorelaxation response to an endothelium-dependent vasodilator, acetylcholine (ACh), but a normal vasorelaxation response to an endothelium-independent vasodilator, acidified sodium nitrite (NaNO2). The left ventricle was then sliced into 2- to 3-mm-thick sections, and the unstained portion of myocardium [i.e., the area at risk (AAR)] was separated from the Evans blue-stained portion of the myocardium (i.e., the area not at risk). The AAR was incubated at 37°C in 0.1% solution of nitro blue tetrazolium in phosphate buffer for 15 min. At the end of this time, unstained necrotic tissue (NEC) was separated from the stained non-necrotic viable tissue in a blinded manner. Samples from all three portions of the left ventricular cardiac tissue (i.e., area not at risk, non-necrotic tissue, and NEC) were weighed. The AAR as a percentage of the total left ventricle mass, the NEC as a percentage of AAR, and the NEC as a percentage of total left ventricle mass were calculated. The three portions of the myocardium were then stored at −70°C for later assay of myeloperoxidase activity (MPO) as described (17). MPO was used as an index of neutrophil accumulation in the myocardial tissue (18).
Measurement of NO⋅ Formation from AS.
The generation of NO⋅ was quantified by its gas phase chemiluminescence reaction with ozone. The chemiluminescence detector was connected to the outlet of a water-jacketed, septum-sealed reaction chamber containing deaerated buffer solution (0.1 M phosphate buffer, 37°C, pH 7.4). A stream of either nitrogen gas or dried room air was passed over the surface of the stirred aqueous solution (total sample volume 1.0 ml) at a rate of 100 ml/min. After passage through a cold trap to remove water vapor, the gas stream was introduced directly into the detector and was mixed with ozone, and the resulting chemiluminescence signal was recorded. AS was introduced by injection of an aliquot from a concentrated stock solution (10 mM in 0.1 M NaOH) through the septum of the reaction chamber, followed by addition of either reduced glutathione, PF, or the vehicle (buffer) 2 min thereafter. The detection limit was 2 pmol of NO⋅/ml × min at an integration time of 5 s and a signal to noise ratio of >3:1.
Statistical Analysis.
All values in the text, tables, and figures are presented as means ± SEM of n independent experiments. All data were subjected to ANOVA followed by the Bonferroni correction for post hoc t tests.
Results
Establishment of the Conditions for the Redox Conversion of NO− to NO⋅.
We have shown previously that AS produces NO− but not NO⋅ and that NO− released from AS can be converted to NO⋅ by a one-electron oxidant (15). This offers an elegant way to directly compare the actions of NO− with those of NO⋅ by using the same compound for their generation. However, to what extent the conversion of NO− to NO⋅ may be influenced by the presence of oxygen has not been investigated. Because the generation of NO⋅ from GSNO is known to be markedly enhanced by thiols (19), we additionally investigated the possibility that glutathione, the most abundant thiol in mammalian systems, may catalyze the formation of NO⋅ from AS.
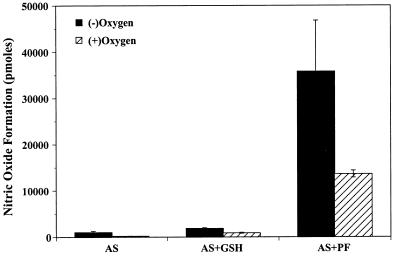
There was little “spontaneous” formation of NO⋅ during AS decomposition in aqueous solution. The total amount of NO⋅ formed during a 30-min incubation at pH 7.4 and 37°C corresponded to <1% and 0.2% of the applied AS in the absence or presence of oxygen, respectively (Fig. 1). Addition of either EDTA or DTPA (100 μM) almost completely abolished the signal, indicating that NO⋅ formation from AS was largely mediated by trace metals present in the buffer (data not shown). Addition of glutathione (0.1–10 μmol) resulted in a small increase in NO⋅ formation (Fig. 1), but only under conditions of an at least 5-fold molar excess of thiol over AS. In contrast, addition of PF (1–100 nmol) led to an immediate and concentration-dependent increase in NO⋅ formation. At equimolar concentrations of PF and AS, NO− was effectively converted to NO⋅ with yields of ≈14 and 35% in the presence and absence of oxygen, respectively (Fig. 1). These results demonstrate that NO⋅ formation from AS only occurs to an appreciable extent in the presence of a one-electron oxidant and suggests that oxygen competes with this oxidant for the reaction with nitroxyl.
Figure 1.
Formation of nitric oxide (NO⋅) from Angeli’s salt (AS) (100 nmol) in the absence and presence of reduced glutathione (1 μmol) and potassium ferricyanide (PF) (100 nmol). Experiments were carried out either in the absence (filled columns) or presence of oxygen (hatched columns). Depicted data are means ± SEM from 3–5 independent experiments.
Determination of the Optimal Doses for GSNO and AS.
Both NO⋅ and NO− are endowed with significant vasorelaxant activity, a property that itself can affect the severity of myocardial reperfusion injury. Therefore, to compare directly the effects of NO⋅ with those of NO− on postischemic myocardial injury, the doses of GSNO and AS that produce comparable hemodynamic effect needed to be determined. In sham MI/R rabbits, administration of GSNO and AS both decreased MABP in a dose-dependent fashion in the range of 0.3–3 μmol/kg, with GSNO being 2- to 3-fold more potent than AS. Comparable hemodynamic effects were obtained with 1 μmol/kg GSNO [maximal MABP decrease −15.2 ± 1.3 mmHg (1 mmHg = 133 Pa); duration of hypotensive response <20 min] and 3 μmol/kg AS (maximal MABP decrease −14.8 ± 1.1 mmHg; duration of response <20 min). These two doses were thus used for a direct comparison between the actions of NO⋅ and NO− in subsequent experiments. The rationale for using a dose of 10 μmol/kg of PF was based on a pilot study that showed that co-administration of PF at either 1 μmol/kg or 3 μmol/kg only insignificantly attenuated the detrimental effects of AS. It was predicted that, during administration of the compound, one- to two-thirds of the oxidant is scavenged by endogenous reductants.
Hemodynamics and Cardiac Function.
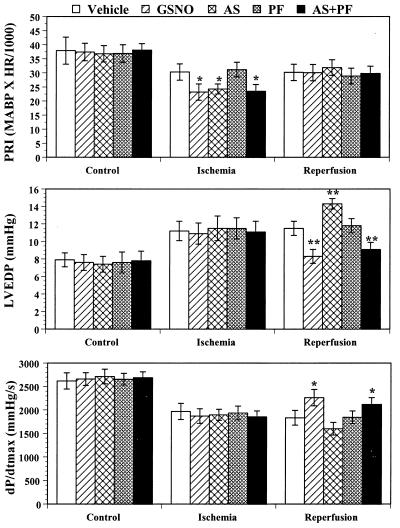
Fig. 2 summarizes the effects of a variety of treatments given 5 min before reperfusion on the hemodynamics at the end of ischemia and reperfusion. In the GSNO-, AS-, and the AS+PF-treated animals, MABP decreased significantly after compound administration. Therefore, at the end of 45 min of ischemia, the pressure–rate index in these three groups was significantly lower than that in the vehicle group. However, there was no significant difference in pressure–rate index either before or after compound administrations among these three groups. Therefore, any difference in the effects of these treatments on postischemic myocardial injury could not be attributed to their different influence on myocardial oxygen demand.
Figure 2.
Pressure–rate index (PRI), left ventricular end diastolic pressure (LVEDP), and maximal first derivative of left ventricular pressure (dP/dtmax) in rabbits under control conditions and after animals have been subjected to MI/R treated with vehicle (saline), S-nitrosoglutathione (GSNO) (1 μmol/kg), Angeli’s salt (AS) (3 μmol/kg), potassium ferricyanide (PF) (10 μmol/kg), or a combination of AS and PF (3 and 10 μmol/kg, respectively). *, P < 0.05, vs. the vehicle-treated rabbits.
There was no significant difference in basal LVEDP among groups. After occlusion of the coronary artery, LVEDP increased significantly in all five MI/R groups. Upon reperfusion, LVEDP decreased slightly in the first 30 min of reperfusion and increased again thereafter in vehicle, AS, and PF groups. Administration of GSNO prevented this secondary increase in LVEDP, which occurred during the late phase reperfusion. Thus, LVEDP was significantly lower in the GSNO-treated group than in the vehicle-treated group at the end of the experimental period (Fig. 2). In contrast, in the AS-treated group, LVEDP not only did not decrease after reperfusion, it increased progressively, reaching a significantly higher level at 180 min of reperfusion at the 3 μmol/kg dose level and was marginally higher than control at 1 μmol/kg AS. Although administration of PF alone had little effect on LVEDP, co-administration of PF with AS completely reversed the effect of AS on this parameter. At the end of reperfusion, LVEDP in the combination group reached a level that was significantly lower than that of the vehicle-treated group, resembling that seen after GSNO application.
All treatment groups showed comparable initial values for dP/dtmax. Upon coronary occlusion, dP/dtmax decreased dramatically over the first 20 min and recovered slowly thereafter. There was no significant difference among groups during the entire ischemia period and during the first 120 min of reperfusion. However, in GSNO-treated rabbits, myocardial contractile function recovered significantly after reperfusion. Hence, dP/dtmax was significantly higher compared with the vehicle-treated group. In contrast, dP/dtmax continuously decreased after 60 min of reperfusion in the AS-treated group. Administration of PF alone did not change dP/dtmax; however, co-administration of PF with AS improved the recovery of contractile function (Fig. 2).
Myocardial Cellular Injury, Neutrophil Accumulation, and Endothelial Dysfunction.
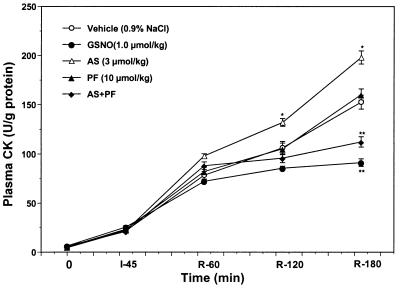
As illustrated in Fig. 3, plasma CK activity only slightly increased at the end of the 45-min ischemic period, and there was no significant difference among groups. However, CK activity increased markedly during reperfusion. MI/R rabbits treated with GSNO developed significantly lower plasma CK activities compared with that of MI/R rabbits treated with vehicle at 120 and 180 min after reperfusion. In contrast, the administration of AS at a dose (3 μmol/kg) that induced changes in blood pressure comparable to those observed with GSNO not only did not attenuate myocardial CK release, but markedly enhanced plasma enzyme activity after reperfusion (P < 0.05 vs. vehicle). This effect was not apparent at a threefold lower dose of AS (see Table 1). Moreover, in MI/R animals that received a solution of decomposed AS (3 μmol/kg), plasma CK activity was comparable to that of vehicle-treated controls (Table 1), indicating that the decomposition products of AS were not responsible for the deleterious effect of AS. Administration of PF alone had no effect on CK release after ischemia and reperfusion. However, co-administration of PF with AS totally reversed the effect of AS on myocardial CK release (Fig. 3).
Figure 3.
Plasma creatine kinase (CK) activity expressed as units per gram of protein measured before ischemia (0), at the end of ischemia (I-45), and hourly thereafter for the main five treatment groups. *, P <0.05; **, P < 0.01 vs. the vehicle-treated group.
Table 1.
Effects of AS, decomposed AS, and GSNO in rabbits subjected to 45 min of myocardial ischemia and 180 min of reperfusion
| Vehicle | GSNO (1 μmol/kg) | AS (1 μmol/kg) | AS (3 μmol/kg) | Decomposed AS (3 μmol/kg) | |
|---|---|---|---|---|---|
| CK at R180 | 153 ± 7.1 | 91 ± 3.9* | 161 ± 8.1 | 198 ± 6.7* | 159 ± 7.3 |
| NEC/AAR, % | 53.4 ± 2.9 | 23.2 ± 1.2* | 57.9 ± 2.3 | 72.4 ± 1.7* | 56.8 ± 3.6 |
| MPO, units/100 g | 0.18 ± 0.03 | 0.04 ± 0.01* | 0.17 ± 0.03 | 0.22 ± 0.03 | 0.19 ± 0.02 |
CK at R180, plasma creatine kinase activity 180 min after the onset of reperfusion; NEC, necrotic tissue; AAR, area at risk; MPO, myeloperoxidase activity in myocardial tissue from the area at risk.
*P < 0.01 vs. vehicle.
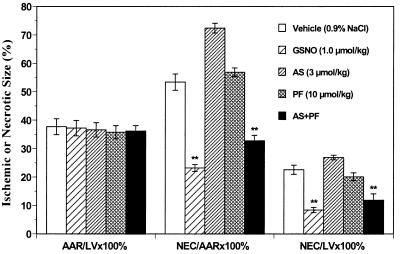
To ascertain the effects of GSNO and AS on the degree of myocardial salvage of ischemic/reperfused tissue, we measured the amount of necrotic cardiac tissue and expressed it as a percentage of either the area at risk or of the total left ventricular mass (Fig. 4). There was no significant difference among groups in the area at risk expressed as a percentage of the total left ventricle, indicating that a comparable degree of ischemic jeopardy existed in all MI/R groups. After 45 min of coronary occlusion and 180 min of reperfusion, in the vehicle group, ≈53% of the ischemic-reperfused myocardial tissue evolved to necrosis. Administration of GSNO before reperfusion significantly decreased the size of necrotic tissue when compared with the vehicle group. In contrast, the administration of a hemodynamically equieffective dose of AS (3 μmol/kg) enlarged the necrotic area. However, treatment of rabbits with 1 μmol/kg AS, i.e., a dose equimolar to that of GSNO, or with decomposed AS (3 μmol/kg) did not show any significant effect on the size of the necrotic area (Table 1). Consistent with the CK data, administration of PF alone had no effect on necrotic size whereas the co-administration of PF with AS not only completely prevented the detrimental effect of AS but allowed AS to now exert significant protective effects against reperfusion injury (Fig. 4).
Figure 4.
Tissue wet weight of the area at risk (AAR) as a percentage of the total left ventricular (LV) wet weight and of necrotic tissue (NEC) as a percentage of area at risk and of the total left ventricle for the main five treatment groups. **, P < 0.01, vs. the vehicle-treated rabbits.
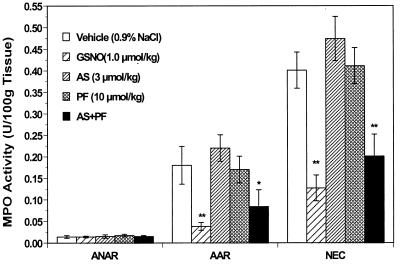
In the non-ischemic myocardium (i.e., the area not at risk), MPO activity was very low in all groups, and there was no significant difference among them. However, MPO activity in the ischemic region was found to be markedly enhanced in vehicle-treated animals. Treatment with GSNO markedly decreased MPO activity both in ischemic non-necrotic myocardial tissue and in necrotic tissue whereas an equimolar dose of AS did not exert any protective effects (Fig. 5; Table 1). Treatment with either AS or PF alone had no or only marginal effects on MPO activity in any region. There was a slight increase in neutrophil-associated enzyme activity with AS, but this did not reach statistical significance (Table 1). In contrast, the combination of AS and PF offered a protective effect that was similar to that of GSNO and led to a significant reduction of MPO activity (Fig. 5).
Figure 5.
Tissue myeloperoxidase (MPO) activity in the area not at risk (ANAR), area at risk (AAR), and necrotic area (NEC) expressed as units/100 mg tissue wet weight in hearts from the main five treatment groups. *, P < 0.05; **, P < 0.01 vs. the vehicle-treated rabbits.
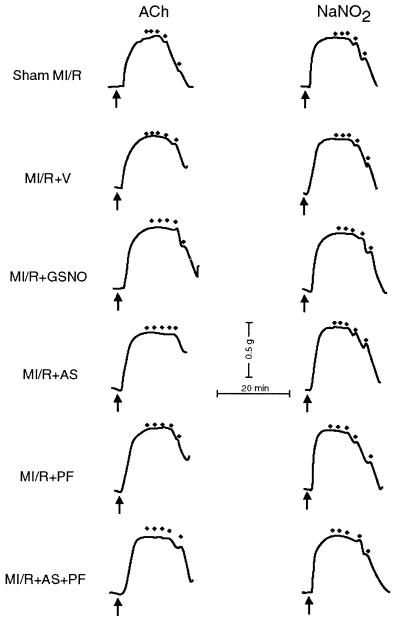
Fig. 6 illustrates the vasorelaxant responses of isolated coronary artery rings to ACh and NaNO2. In the coronary artery rings isolated from sham MI/R rabbits, ACh induced a concentration-dependent vascular relaxation with 93.2 ± 4.2% (n = 15 rings from three rabbits) relaxation occurring at a concentration of 10 μM. In contrast, maximal vasorelaxation to ACh was significantly decreased in coronary artery rings from vehicle-treated MI rabbits (62.6 ± 2.6%, n = 17 rings from four rabbits, P < 0.01 vs. sham). Treatment with GSNO markedly improved ex vivo vasorelaxation responses of coronary artery rings to ACh (84.5 ± 3.9%, n = 16 rings from four rabbits, P < 0.01 vs. vehicle). However, treatment with 3 μmol/kg AS (but not 1 μmol/kg AS or 3 μmol/kg decomposed AS) further blunted the vasorelaxation response to ACh (41.6 ± 4.1%, n = 16 rings from four rabbits, P < 0.01 vs. vehicle), an effect that was completely prevented by co-administration of PF (77.2 ± 3.5%, n = 15 rings from three rabbits, P < 0.05 vs. vehicle and P < 0.005 vs. AS).
Figure 6.
Representative recordings of the relaxation elicited by the endothelium-dependent vasodilator, acetylcholine (ACh), and the endothelium-independent vasodilator, acidified nitrite (NaNO2), in U-46619 precontracted coronary artery rings isolated from sham MI rabbit (control) or from MI/R rabbits that had received different treatments. The arrows indicate addition of U-46619 (50 nM); dots on top indicate addition of ACh (0.001–10 μM) or NaNO2 (0.1–100 μM).
To determine whether MI/R may have altered the responsiveness of the vascular smooth muscle to exogenous NO⋅, we additionally investigated the vasorelaxant effect to acidified NaNO2, an exogenous NO⋅ donor, in coronary artery rings isolated from all eight groups. As illustrated in Fig. 6, 100 μM of acidified NaNO2 induced a full relaxation in vascular rings isolated from all eight groups (P > 0.05 among the groups). These findings indicate that MI/R resulted in a selective vascular endothelial dysfunction with no alteration in the sensitivity or effectiveness of NO⋅ on the smooth muscle, and that this endothelial dysfunction was significantly attenuated by NO⋅, but aggravated by NO−.
Discussion
The redox products of NO⋅ and their chemical reactions with biological molecules have been studied extensively in the last few years. Interestingly, as the chemistry of NO⋅ reveals its richness, the pharmacological and pathophysiological role of NO⋅ becomes more diverse as well as confusing. Numerous studies have demonstrated that administration of NO⋅ donors significantly attenuates myocardial reperfusion injury (20–23). In contrast, many other studies have demonstrated that administration of NOS inhibitors to reduce endogenous NO⋅ production also significantly attenuates postischemic myocardial injury (2–4). To date, there is no satisfactory explanation as to why supplementation of exogenous NO⋅ as well as inhibition of endogenous NO⋅ both attenuate postischemic myocardial injury. The favored explanation for the cardioprotective effect of NOS inhibition is that NO⋅ reacts with O2⨪ to generate ONOO−. Because ONOO− is a highly reactive molecule that can cause significant tissue injury, NOS blockade is expected to lead to a decrease in ONOO− production, thus reducing its deleterious effects (8). Although biochemical experimental results obtained in vitro suggest this explanation has merits, several studies have revealed that in vivo effects of ONOO− on postischemic myocardial injury are much more complicated. Our recent studies in rabbit MI/R have demonstrated that the formation of ONOO− can be a “double-edged sword:” on the one hand, ONOO− can directly produce cell injury; on the other hand, it can indirectly prevent cell injury by inhibiting neutrophil accumulation in postischemic myocardial tissue and thus reduce neutrophil-induced myocardial and endothelial injury (24). These results suggest that, besides ONOO−, there must exist some other toxic RNOS that may mediate postischemic myocardial injury.
Our present experimental results provide key evidence for a deleterious role of NO− and a protective role of NO⋅ in postischemic myocardial injury. Using Angeli’s salt (AS) as a source for NO−, we here demonstrate that nitroxyl is a highly toxic molecule that significantly aggravates postischemic myocardial and endothelial injury when administered in vivo. The observed effects were dose-dependent, unrelated to the hemodynamic action of AS and not caused by its decomposition products, nitrite and nitrous oxide. Although we cannot exclude the possibility that some NO− generated from AS might have reacted with oxygen present in the tissue to form ONOO− (NO− + O2 → ONOO−), the observed detrimental action of AS is likely to have been caused by NO− directly. Unlike ONOO−, which possesses significant antineutrophil activity and thus attenuates neutrophil-induced myocardial injury, NO− exerted no beneficial effect on neutrophil accumulation in the ischemic-reperfused myocardial tissue. Treatment with AS actually tended to further enhance MPO activity in the ischemic tissue, which may be explained by a nitroxyl-mediated stimulation of neutrophil migration (25). NO− is also a powerful thiol oxidant and by this mechanism may further enhance the ROS-mediated increase in neutrophil adhesion. Therefore, in contrast to the “double-edged sword” role of ONOO− in reperfusion injury, NO− is a “one-edged sword” that only causes direct myocardial damage.
These results let NO− appear a more likely candidate responsible for RNOS-mediated tissue injury under myocardial ischemia and reperfusion. Moreover, our present experimental results clearly demonstrate that the effects on postischemic myocardial injury exerted by NO⋅ are completely opposite to those exerted by its one electron reduction product, NO−. Most interestingly, we have shown that co-administration of AS with PF, a one-electron oxidant that effectively converts NO− to NO⋅ in vitro, fully reversed the deleterious effects of NO−, turning them into myocardial and endothelial protective effect similar to those observed upon administration of NO⋅. These results suggest that it is the redox product of NO⋅, nitroxyl, and not NO⋅ itself, that is cytotoxic and responsible for the RNOS-induced tissue injury in myocardial ischemia and reperfusion.
NO− is the one-electron reduction product of NO⋅. However, in vivo, direct bioreduction of NO⋅ generated from a biological source is an unlikely source of NO− because the reduction potential of NO⋅ is higher than that of oxygen, and oxygen is generally at least 100× in excess over NO⋅. Therefore, oxygen will be preferentially reduced over NO⋅ under in vivo conditions. Moreover, even if reduction of NO⋅ did occur, the product would be distinct from that generated from AS and other sources of NO− (i.e., triplet instead of singlet). The most likely biological sources of NO− are from NOS itself, the oxidation of HO-Arg, and the decomposition of S-nitrosothiols with proximal reduced thiols. Xia and Zweier have recently reported that the production of l-citrulline by NOS is always higher than that of NO⋅, suggesting that NOS may also generate other reactive nitrogen species (26). Two recent studies have reported that the primary product of NOS is NO−, which can be converted to NO⋅ by superoxide dismutase and other suitable electron acceptors (12, 14). It has also been reported that the NOS intermediate, HO-Arg, can be oxidized by catalase and hydrogen peroxide or cytochrome P450 enzymes to produce NO− (13). These results suggest that NO− may be produced either directly from NOS or from precursors such as HO-Arg. Consistent with this idea, ischemia followed by reperfusion has been shown to be associated with high oxidative stress, significant decreases in superoxide dismutase activity, and an increased iNOS expression in myocardial tissue (27, 28). It is possible that, in the pathologic setting of ischemia and reperfusion, the generation of NO− by NOS is increased whereas its conversion to NO⋅ by endogenous oxidants or copper-containing enzymes such as superoxide dismutase is decreased. According to this scenario, the administration of NOS inhibitors may thus prevent the (direct or indirect) formation of NO−, and attenuate NO−-induced myocardial injury. Conceivably, NO⋅ donors may exert their protective action via two chemical mechanisms: the inactivation of ROS such as ⋅OH and O2⨪ (29), and RNOS such as ONOO− (30), as well as through the scavenging of NO− [NO⋅ + NO− → (NO)2−] (31). Thus, NO⋅ may act mainly as a scavenger of NO− rather than O2⨪ or ONOO−, as hitherto assumed. Electron acceptors such as superoxide dismutase may thus be protective not only by preventing ROS-mediated damage but also by converting the potentially deleterious species NO− into a protective entity: i.e., NO⋅. This conversion may occur on a continuous and quantitative basis unless the capacity of the system exceeds a critical limit. This may be reached with the onset of a massive up-regulation of ROS production during the first minutes of reperfusion (32). Our finding that ferricyanide alone was not protective may be explained by the possibility that either the applied dose was too low or that, as a charged inorganic complex, the oxidant did not gain access to the intracellular compartment, where endogenous NO− is expected to be produced. The above assumptions are difficult to proof or disproof conclusively on the basis of pharmacological experiments only and underscore the necessity for specific and sensitive analytical methods for the detection of nitroxyl in biological systems, which are not available to date.
Throughout the last couple of years, the literature on NO⋅ and ischemia/reperfusion has focused almost entirely on peroxynitrite. The results of the present investigation offer an alternative explanation for some apparently contradictory results on the role of NO⋅ in reperfusion injury (i.e., the fact that NOS inhibitors and NO⋅ donors both exert significant protection). Inhibition of NOS can decrease enzymatic NO− generation and, by this mechanism, inhibit NO−-induced myocardial injury. On the other hand, because direct reduction of NO⋅ to NO− cannot occur in vivo, the administration of NO⋅ donors will exert significant beneficial effects against postischemic myocardial injury via their vasodilatory, antineutrophil, and antiplatelet actions, and possibly also via direct trapping of endogenously generated NO−. Moreover, we have demonstrated that co-administration of a one-electron oxidant, PF, with a nitroxyl generating compound, AS, not only completely prevented the detrimental effects of NO−, but afforded significant cardiac and endothelial protective effects similar to those exerted by GSNO, an NO⋅ donor. This suggests that antioxidants and redox switches that increase the flux of NO⋅ by converting NO− to NO⋅ may be beneficial in ischemia/reperfusion and abate tissue damage.
Abbreviations
- AAR
area at risk
- ACh
acetylcholine
- AS
Angeli’s salt
- CK
creatine kinase
- GSNO
S-nitrosoglutathione
- LVEDP
left ventricular end diastolic pressure
- MABP
mean arterial blood pressure
- MI/R
myocardial ischemia/reperfusion
- MPO
myeloperoxidase
- NEC
necrotic tissue
- NOS
nitric oxide synthase
- PF
potassium ferricyanide
- RNOS
reactive nitrogen oxide species
- ROS
reactive oxygen species
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Moncada S, Higgs E A. FASEB J. 1995;9:1319–1330. [PubMed] [Google Scholar]
- 2.Schulz R, Wambolt R. Cardiovasc Res. 1995;30:432–439. [PubMed] [Google Scholar]
- 3.Wang P H, Zweier J L. J Biol Chem. 1996;271:29223–29230. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- 4.Woolfson R G, Patel V C, Neild G H, Yellon D M. Circulation. 1995;91:1545–1551. doi: 10.1161/01.cir.91.5.1545. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari R. Am J Cardiol. 1995;76:17B–24B. [PubMed] [Google Scholar]
- 6.Darley-Usmar V, Halliwell B. Pharm Res. 1996;13:649–662. doi: 10.1023/a:1016079012214. [DOI] [PubMed] [Google Scholar]
- 7.Beckman J S, Koppenol W H. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 8.Szabo C. Shock. 1996;6:79–88. doi: 10.1097/00024382-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Moro M A, Darley-Usmar V M, Goodwin D A, Read N G, Zamora-Pino R, Feelisch M, Radomski M W, Moncada S. Proc Natl Acad Sci USA. 1994;91:6702–6706. doi: 10.1073/pnas.91.14.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez B L, Christopher T A, Ma X L. Endothelium. 1995;3:s76–s76. [Google Scholar]
- 11.Nossuli T O, Hayward R, Scalia R, Lefer A M. Circulation. 1997;96:2317–2324. doi: 10.1161/01.cir.96.7.2317. [DOI] [PubMed] [Google Scholar]
- 12.Hobbs A J, Fukuto J M, Ignarro L J. Proc Natl Acad Sci USA. 1994;91:10992–10996. doi: 10.1073/pnas.91.23.10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pufahl R A, Wishnok J S, Marletta M A. Biochemistry. 1995;34:1930–1941. doi: 10.1021/bi00006a014. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt H H H W, Hofmann H, Schindler U, Shutenko Z S, Cunningham D D, Feelisch M. Proc Natl Acad Sci USA. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wink D A, Feelisch M, Fukuto J, Chistodoulou D, Jourd’heuil D, Grisham M B, Vodovotz Y, Cook J A, Krishna M, DeGraff W G, et al. Arch Biochem Biophys. 1998;351:66–74. doi: 10.1006/abbi.1997.0565. [DOI] [PubMed] [Google Scholar]
- 16.Ohshima H, Gilibert I, Bianchini F. Free Radical Biol Med. 1999;26:1305–1313. doi: 10.1016/s0891-5849(98)00327-x. [DOI] [PubMed] [Google Scholar]
- 17.Liu G L, Christopher T A, Lopez B L, Gao F, Guo Y P, Gao E, Knuettel A, Feelisch M, Ma X L. J Pharmacol Exp Ther. 1998;287:527–537. [PubMed] [Google Scholar]
- 18.Mullane K M, Kraemer R, Smith B. J Pharmacol Methods. 1985;14:157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- 19.Jourd’heuil D, Laroux F S, Miles A M, Wink D A, Grisham M B. Arch Biochem Biophys. 1999;361:323–330. doi: 10.1006/abbi.1998.1010. [DOI] [PubMed] [Google Scholar]
- 20.Lefer A M. Ann Thorac Surg. 1995;60:847–851. doi: 10.1016/0003-4975(95)00423-I. [DOI] [PubMed] [Google Scholar]
- 21.Siegfried M R, Erhardt J, Rider T, Ma X L, Lefer A M. J Pharmacol Exp Ther. 1992;260:668–675. [PubMed] [Google Scholar]
- 22.Vinten-Johansen J, Sato H, Zhao Z Q. Int J Cardiol. 1995;50:273–281. doi: 10.1016/0167-5273(95)02388-d. [DOI] [PubMed] [Google Scholar]
- 23.Jones S P, Girod W G, Palazzo A J, Granger D N, Grisham M B, Jourd’heuil D, Huang P L, Lefer D J. Am J Physiol. 1999;276:H1567–H1573. doi: 10.1152/ajpheart.1999.276.5.H1567. [DOI] [PubMed] [Google Scholar]
- 24.Ma, X. L., Gao, F., Lopez, B. L., Christopher, T. A. & Vinten-Johansen, J. (2000) J. Pharmacol. Exp. Ther., in press. [PubMed]
- 25.VanUffelen B E, Van Der Z J, De Koster B M, VanSteveninck J, Elferink J G. Biochem J. 1998;330:719–722. doi: 10.1042/bj3300719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia Y, Zweier J L. Proc Natl Acad Sci USA. 1997;94:12705–12710. doi: 10.1073/pnas.94.23.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dudek R R, Wildhirt S, Conforto A, Pinto V, Suzuki H, Winder S, Bing R J. Biochem Biophys Res Commun. 1994;205:1671–1680. doi: 10.1006/bbrc.1994.2860. [DOI] [PubMed] [Google Scholar]
- 28.Bing R J, Suzuki H. Mol Cell Biochem. 1996;161:303–306. doi: 10.1007/BF00240063. [DOI] [PubMed] [Google Scholar]
- 29.Wink D A, Hanbauer I, Laval F, Cook J A, Krishna M C, Mitchell J B. Ann NY Acad Sci. 1994;738:265–278. doi: 10.1111/j.1749-6632.1994.tb21812.x. [DOI] [PubMed] [Google Scholar]
- 30.Yasmin W, Strynadka K D, Schulz R. Cardiovasc Res. 1997;33:422–432. doi: 10.1016/s0008-6363(96)00254-4. [DOI] [PubMed] [Google Scholar]
- 31.Seddon W A, Fletcher J W, Sopchyshyn F C. Can J Chem. 1973;51:1123–1130. [Google Scholar]
- 32.Zweier J L, Flaherty J T, Weisfeldt M L. Proc Natl Acad Sci USA. 1987;84:1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]