Abstract
We tested the effect of chronic leptin treatment on fasting-induced torpor in leptin-deficient A-ZIP/F-1 and ob/ob mice. A-ZIP/F-1 mice have virtually no white adipose tissue and low leptin levels, whereas ob/ob mice have an abundance of fat but no leptin. These two models allowed us to examine the roles of adipose tissue and leptin in the regulation of entry into torpor. Torpor is a short-term hibernation-like state that allows conservation of metabolic fuels. We first characterized the A-ZIP/F-1 animals, which have a 10-fold reduction in total body triglyceride stores. Upon fasting, A-ZIP/F-1 mice develop a lower metabolic rate and decreased plasma glucose, insulin, and triglyceride levels, with no increase in free fatty acids or β-hydroxybutyrate. Unlike control mice, by 24 hr of fasting, they have nearly exhausted their triglycerides and are catabolizing protein. To conserve energy supplies during fasting, A-ZIP/F-1 (but not control) mice entered deep torpor, with a minimum core body temperature of 24°C, 2°C above ambient. In ob/ob mice, fasting-induced torpor was completely reversed by leptin treatment. In contrast, neither leptin nor thyroid hormone prevented torpor in A-ZIP/F-1 mice. These data suggest that there are at least two signals for entry into torpor in mice, a low leptin level and another signal that is independent of leptin and thyroid hormone levels. Studying rodent torpor provides insight into human torpor-like states such as near drowning in cold water and induced hypothermia for surgery.
Keywords: fasting, lipoatrophic diabetes, body temperature, hypothermia, A-ZIP, F-1 mice
Living organisms must cope with food scarcity. The capabilities to store excess fuel and regulate energy expenditure were thus crucial evolutionary adaptations. In higher organisms, white adipocytes store triglycerides that are burned during fasting and starvation (1, 2). These adipocyte triglycerides account for the vast majority of the body’s fuel reserves (3).
Adipose tissues also contribute to energy homeostasis in an endocrine/paracrine manner. Leptin is secreted by adipocytes in direct proportion to body fat mass and regulates energy expenditure, metabolic efficiency, and food intake (4–6). Leptin binds to receptors in the hypothalamus and other sites and conveys the size of adipose tissue lipid stores. Adipocytes also make additional hormones, including tumor necrosis factor α, which affects insulin sensitivity (7, 8). Thus it is clear that adipose tissues contribute to energy metabolism in two ways, as a metabolic regulator and fuel depot.
To analyze the physiologic roles of fat, we generated a transgenic mouse, named A-ZIP/F-1, which has virtually no white adipose tissue and a reduced amount of brown adipose tissue (9). These mice express, selectively in adipose tissue, a dominant negative protein that heterodimerizes with certain basic leucine zipper transcription factors. The A-ZIP/F-1 phenotype strikingly resembles that of humans with severe lipoatrophic diabetes mellitus, a disease characterized by reduced amounts of adipose tissue, insulin resistance, hyperlipidemia, fatty liver, and organomegaly (10, 11). Transgenic mice similar to the A-ZIP/F-1 line, but with less severe phenotypes, resulted from adipose expression of a modified diphtheria toxin (12, 13) or a constitutively active SREBP-1c protein (14).
The ob/ob mouse is another tool for studying the physiology of adipose tissue. These mice have a mutation in the leptin gene, resulting in early-onset obesity and insulin resistance (4, 15). Although leptin has received much attention for its antiobesity properties, leptin may be more important for adaptation to starvation (5, 6, 16). The ob/ob mice provide an important contrast to the A-ZIP/F-1 mice: both have low leptin levels, but the ob/ob mice have massive triglyceride stores, whereas the A-ZIP/F-1 animals have very low energy reserves.
In the present study, we first characterized the metabolic response to fasting in A-ZIP/F-1 mice. These experiments revealed that A-ZIP/F-1 mice use torpor as a major adaptive mechanism during fasting. This discovery led us to examine the role of leptin in regulating the onset of torpor. Using A-ZIP/F-1 and ob/ob mice, we present evidence for at least two signals for entry into torpor. One is a low leptin level, and the other signal is independent of leptin level.
Methods
Mice.
A-ZIP/F-1 mice were hemizygous on the FVB/N background. Male C57BL/6J and C57BL/6J lepob/lepob mice (15) were purchased from The Jackson Laboratory. Wild-type mice were sex-matched littermates of the A-ZIP/F-1 and ob/ob mice. Mice were kept on a 12-hr light-dark cycle with lights on at 6:00 a.m. Rodent laboratory chow (NIH-07; 5% fat by weight) and drinking water were provided ad libitum except during fasting when only drinking water was available.
Biotelemetry.
Core body temperature (Tb) was continuously monitored in conscious, unrestrained animals by using a VitalView system (Mini-Mitter, Sunriver, OR). Briefly, each animal was anesthetized with halothane and a temperature-sensitive transmitter (PDT-4000) was implanted intra-abdominally. The signal emitted by the transmitter is received and then converted into temperature by the VitalView software. Mice were allowed to recover >7 days after implantation; typically a normal Tb circadian rhythm was re-established 1–2 days after implantation. Tb values were measured each sec and collected as 5-min averages. During temperature measurement mice were individually housed in standard barrier plastic cages at room temperature or at 30 ± 1°C, as indicated.
Leptin Infusion.
For continuous delivery of recombinant mouse leptin (R & D Systems), osmotic mini-pumps (model 2001; Alza) were implanted s.c. These pumps delivered leptin at 30 μg/day for 7 days. Control mice were infused with sterile saline. This dose increased plasma leptin levels by 3–14 ng/ml. In control mice it reduced epididymal fat pad weights from 251 ± 41 mg to 81 ± 49 mg.
Fasting.
Measurement of blood chemistries during the fasting time course was conducted with two groups of mice. Group A was used for measurements in the fed state and at 2 and 6 hr of fasting while group B was used for the 4-, 12-, and 24-hr points. The 2-, 4-, 6-, 12-, and 24-hr fasts started at 11 a.m., 11 a.m., 8 a.m., 8 p.m., and 11 a.m., respectively. Approximately 200 μl of tail venous blood was withdrawn each time, with 5–7 days between bleedings. Body weights were measured before and after each fast. Quantitatively similar results to those reported herein were obtained in an independent replicate experiment.
For biotelemetry experiments, 3-month-old male mice were fasted starting at 10:30 a.m. on day 5 of leptin treatment. Mice were refed on day 6 and sacrificed on day 7 for the collection of retro-orbital sinus blood and organ harvest. There were no deaths during 24-hr fasting in control mice (0/42) and a 15% death rate (6/40) in the A-ZIP/F-1 mice.
Indirect Calorimetry.
Oxygen consumption and carbon dioxide production were measured by using a four-chamber Oxymax system (Columbus Instruments, Columbus, OH), with one mouse per chamber. Transgenic mice were tested simultaneously with controls. Motor activity (total and ambulating) was determined by IR beam interruption (Opto-Varimex mini, Columbus Instruments). Resting oxygen consumption was calculated as the average of the points with less than six ambulating beam breaks per min, omitting the first hour of the experiment. Oxygen consumption data were normalized to (body weight)0.75 (17, 18), although mice of similar weight were used within each experiment. The effect of leptin was measured during day 5 of leptin infusion.
Biochemical Assays.
Glucose was measured by using a Glucometer Elite (Bayer, Elkhart, IN). Insulin (Linco Research Immunoassay, St. Charles, MO, #SRI-13K), testosterone (DPC, Los Angeles, CA), corticosterone (ICN), and T3, T4, and thyroid stimulating hormone (19) were measured by RIA. Triglycerides and glycerol (Sigma, 339–11), free fatty acids (FFA) (Boehringer Mannheim, 1383175), β-hydroxybutyrate (Boehringer Mannheim, 907979), and blood urea nitrogen (Sigma, 640) were assayed by using the indicated kits. Tissue triglyceride content (20) was measured in liver and carcass (minus gastrointestinal tract and liver) after digestion in ethanolic KOH by using a radiometric assay for glycerol (21, 22) with 885 g/mol as the triglyceride molecular weight. No correction was made for glycerol from nontriglyceride sources. Glycogen was measured by using a radiometric assay for glucose after amyloglucosidase digestion, with correction for nonglycogen glucose (21).
Statistical Analysis.
Values are reported as means ± SEM. For blood chemistry data, statistical significance was determined by using t test (paired or unpaired, as appropriate) or ANOVA with Tukey pair-wise tests using sigmastat. Tb, body weight, and food intake were analyzed by t test or ANOVA followed by Scheffe’s pair-wise comparisons to test for statistical differences among groups at individual time points. Two-tailed differences were considered significant at P < 0.05.
Results
Quantitation of the Fuel Deficiency in A-ZIP/F-1 Mice.
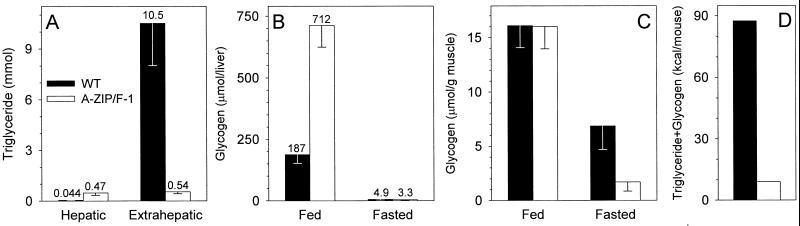
To understand energy homeostasis in A-ZIP/F-1 mice, we quantitated their energy reserves. In 28-week-old males, total body triglycerides were reduced ≈10-fold (Fig. 1A). Extrahepatic triglycerides were reduced 20-fold, whereas hepatic triglycerides increased 11-fold relative to wild-type levels. Normally, triglyceride is the predominant form of stored energy, but some also is stored as glycogen. We measured glycogen levels to determine whether they were increased. Total liver glycogen was 3.8-fold elevated in the A-ZIP/F-1 mice whereas muscle glycogen was not changed (Fig. 1 B and C). Glycogen accounts for 6.8% of the estimated triglyceride + glycogen stores in fed A-ZIP/F-1 mice. This increase is 26-fold over the 0.28% in the wild-type mice, but does not adequately compensate for the triglyceride energy deficit of the A-ZIP/F-1 mice. Because the resting metabolic rate of wild-type FVB/N mice is ≈12 kcal/day (O.G., unpublished work), the magnitude of the triglyceride + glycogen fuel reduction in the A-ZIP/F-1 mice is staggering (87.5 vs. 8.9 kcal/mouse, Fig. 1D).
Figure 1.
Reduced energy stores in A-ZIP/F-1 mice. (A) Triglyceride content in 28-week-old male mice (n = 4 per group). (B and C) Glycogen content in liver and muscle of 30- to 33-week-old male mice either fed or after a 24-hr fast (n = 5–6 per group). (D) Energy stored in triglyceride + glycogen was estimated by using data from A–C, assuming that muscle is 40% of body weight, glycogen is 4.18 kcal/g, and fat is 9.35 kcal/g (42). Wild-type (WT) data are depicted with filled bars and A-ZIP/F-1 with open bars.
A-ZIP/F-1 Mice Adapt to Fasting at an Accelerated Rate.
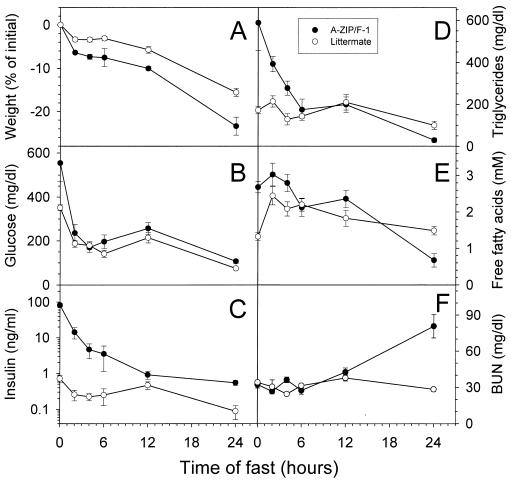
The fuel deficit of the A-ZIP/F-1 mice should affect their metabolic response to fasting, and this was demonstrated in preliminary experiments (9). We examined adaptation to fasting in more detail. Weight loss was more rapid in the A-ZIP/F-1 mice compared with littermate controls (Fig. 2A). Initially the plasma glucose was markedly elevated in A-ZIP/F-1 mice, but by 2 hr after food removal it was 67% decreased and comparable to (or just slightly higher than) control mice (Fig. 2B). Baseline insulin and triglyceride levels were 114-fold and 3.4-fold higher in the A-ZIP/F-1 mice and fell more rapidly than in the controls (Fig. 2 C and D). FFA concentrations initially were elevated, but did not rise further in A-ZIP/F-1 mice, unlike the controls (Fig. 2E).
Figure 2.
Time course of fasting in A-ZIP/F-1 mice. Female A-ZIP/F-1 and littermate controls (wild type; 4–6 weeks old, n = 5 per group) were fasted for 0, 2, 4, 6, 12, and 24 hr (see Methods). Body weight (A) and plasma glucose (B), insulin (C), triglyceride (D), FFA (E), and blood urea nitrogen (BUN) (F) concentrations were measured. At 24 hr of fasting, the A-ZIP/F-1 mice were different from the wild-type mice (P < 0.01) for all parameters shown.
By 24 hr of fasting, the A-ZIP/F-1 mice had lost more weight and had lower triglyceride and FFA levels than control mice. Glucose levels were comparable, whereas insulin levels dropped more in the A-ZIP/F-1 mice (150-fold vs. 8-fold), but were still higher than the controls, suggesting that the mice are still somewhat insulin resistant. The level of plasma β-hydroxybutyrate increased 21-fold with a 24-hr fast in the wild-type mice but changed <2-fold in the A-ZIP/F-1 animals (not shown). Urea is a waste product of protein catabolism. By 24 hr of fasting, the A-ZIP/F-1 mice had a 2.5-fold elevated blood urea, compared with no change in the controls (Fig. 2F).
We were interested in whether or not the stored fuels in A-ZIP/F-1 mice could be mobilized. Livers of A-ZIP/F-1 mice lost more weight with fasting than did controls (controls went from 1.09 ± 0.07 to 0.81 ± 0.05 g, whereas A-ZIP/F-1 went from 2.16 ± 0.26 to 0.95 ± 0.18 g), suggesting that hepatic lipid and glycogen were indeed catabolized. Next, fuel stores were measured directly. After a 24-hr fast, hepatic triglyceride dropped by 95% in A-ZIP/F-1 mice (from 149 ± 11 to 7.8 ± 1.6 μmol/liver). Liver glycogen was used to the point of exhaustion (down 99.5%), whereas muscle glycogen was depleted in A-ZIP/F-1 mice to a greater extent than in controls (depleted 89% vs. 57%; Fig. 1 B and C). Thus the triglyceride and glycogen in A-ZIP/F-1 mice are available and are used during fasting.
The above results support the following scenario. Because of the lack of white adipose tissue, the A-ZIP/F-1 mice exhaust their triglyceride stores during a 24-hr fast. The FFA levels fall because they cannot be replenished and ketone body levels do not rise, possibly because of the low FFA. Notably, the A-ZIP/F-1 glucose levels are preserved and remain comparable to the controls for the duration of the fast, demonstrating that these mice maintain their blood glucose concentration for at least 24 hr. To do this, the mice catabolize protein, releasing amino acids that are used for gluconeogenesis.
Metabolic Rate in Fasting A-ZIP/F-1 Mice.
Metabolic rate decreases with fasting (23). We used indirect calorimetry to determine how rapidly this occurs in the A-ZIP/F-1 mice. The fed resting oxygen consumption was slightly lower in A-ZIP/F-1 mice than in controls (Fig. 3). A short (5 hr) fast had no effect in control mice, but caused a 30% reduction in oxygen consumption in the A-ZIP/F-1 mice (Fig. 3). Because A-ZIP/F-1 mice have a low serum leptin level (9), we infused leptin to see whether it prevented this reduction. Leptin had no effect on metabolic rate in the A-ZIP/F-1 mice, either in the fed or fasted state (not shown). In summary, fasting in A-ZIP/F-1 mice causes a greater decrease in metabolic rate.
Figure 3.
Decreased resting metabolic rate in fasting A-ZIP/F-1 mice. Female mice (23–27 weeks old, n = 5) were fasted at 22°C. Oxygen consumption was measured from 3 to 5 hr after the start of the fast (10 a.m.). Fed data were obtained by using the same animals at the same time of the day a week later. The difference between wild type (WT) and A-ZIP/F-1 was significant in the fed (P = 0.045) and fasted (P < 0.001) states; the drop with fasting was significant for the A-ZIP/F-1 (P = 0.001) but not control mice. Weights of the A-ZIP/F-1 and control mice were not different.
Endocrine Status of Fed and Fasting A-ZIP/F-1 Mice.
Endocrine adaptations to fasting include down-regulation of reproductive and thyroid function and increased glucocorticoid production (16). Testosterone levels and testis weight were used as acute and chronic measures of gonadal axis status. Both were reduced in A-ZIP/F-1 mice as compared with wild-type mice, and 24-hr fasting lowered testosterone in wild-type mice to the level of the A-ZIP/F-1 animals (Table 1). Corticosterone was elevated in A-ZIP/F-1 mice and did not increase further with fasting, whereas fasting did cause an increase in wild-type animals (Table 1). These data are consistent with the A-ZIP/F-1 mice being chronically ill.
Table 1.
Endocrine parameters in 7-week male A-ZIP/F-1 and control mice
| FVB/N, fed | A-ZIP/F-1, fed | FVB/N, fasted | A-ZIP/F-1, fasted | |
|---|---|---|---|---|
| T4 (μg/dl) | 3.6 ± 0.1 | 3.4 ± 0.3 | 2.6 ± 0.2* | 3.5 ± 0.4 |
| T3 (ng/dl) | 119 ± 5 | 116 ± 8 | 87 ± 9* | 88 ± 15 |
| TSH (ng/ml) | 0.049 ± 0.008 | 0.044 ± 0.011 | 0.031 ± 0.007 | 0.032 ± 0.006 |
| Corticosterone (ng/ml) | 51 ± 12 | 377 ± 133* | 212 ± 35* | 305 ± 43 |
| Testosterone (ng/ml) | 1.3 ± 0.7 | 0.5 ± 0.3 | 0.4 ± 0.1* | 0.5 ± 0.3 |
| Testes weight (mg) | 177 ± 5 | 139 ± 5* | 171 ± 5 | 134 ± 7 |
Data are mean ± SEM, n = 5–8 per group; * indicates different from fed FVB/N, P < 0.05. Mice were fasted for 24 hr or fed, as indicated. Corticosterone and testosterone samples were obtained at 9–11 a.m.; T4, T3, and thyroid stimulating hormone (TSH) were obtained at 2–7 p.m. Testes weight is the sum of both glands.
The thyroid axis is the major regulator of basal metabolic rate. Thyroid function tests were the same in fed A-ZIP/F-1 mice as in controls (Table 1). With fasting, T3 decreased equally in A-ZIP/F-1 and littermate mice. T4 did not decrease in fasted A-ZIP/F-1 mice. No significant changes in thyroid stimulating hormone were detected. Thus, augmented down-regulation of the thyroid axis does not explain either the reduced resting metabolism or the rapid metabolic adaptation to fasting in the A-ZIP/F-1 mice.
A-ZIP/F-1 Mice Enter Torpor When Fasted.
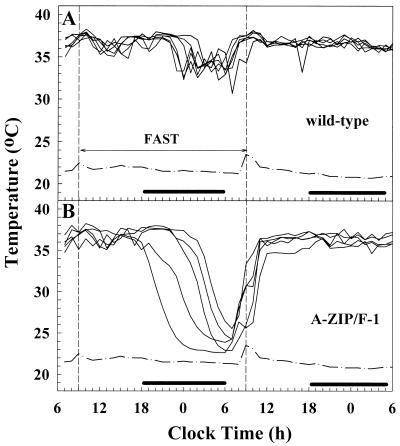
In small mammals, a large percentage of the metabolic rate goes toward maintaining Tb (24, 25). Thus, A-ZIP/F-1 mice could conserve fuel by lowering their Tb. We used telemetry to measure Tb while avoiding the stress of handling. At room temperature, the A-ZIP/F-1 mice had the same Tb and circadian rhythm as control mice. Fasting wild-type mice lowered their Tb by 4°C, which rapidly normalized with refeeding (Fig. 4). Fasted A-ZIP/F-1 mice showed a more rapid and greater drop, falling 13°C and reaching 2°C above the ambient temperature (Fig. 4). Similar results were observed with mice of both sexes ranging in age from 2 to 7 months. The A-ZIP/F-1 mice enter deep torpor, which is a hibernation-like state characterized by inactivity, a lowered Tb, and a low metabolic rate. Three conditions are thought to be needed for torpor: a quiet environment, reduced food availability, and a low ambient temperature (26). Given the severe fast, room temperature is sufficiently cool for A-ZIP/F-1 mice to enter torpor; however, mice housed at 30°C did not go into torpor (not shown). A quiet environment also is needed. For example, in the experiment shown in Fig. 4, the A-ZIP/F-1 mice began coming out of torpor because of activity in the room 2 hr before they were refed.
Figure 4.
A-ZIP/F-1 mice enter torpor upon fasting. Tb was measured in 19- to 20-week-old male wild-type (A, n = 6) and A-ZIP/F-1 (B, n = 5) mice by telemetry. Mice were fasted for the indicated 24 hr starting at 9:30 a.m. During fasting, Tb fell to 33.88 ± 0.44°C in wild-type mice and 24.12 ± 0.69°C in A-ZIP/F-1 mice. The dash-dot line indicates ambient temperature and the black bar indicates lights out.
Recovery from torpor is a coordinated multitissue process in which brown adipose tissue (BAT) is thought to have a major role. Because A-ZIP/F-1 mice have diminished amounts of BAT, appearing inactive histologically, we examined the rate of rewarming. The maximum rate of increase in Tb of the A-ZIP/F-1 mice was 0.57°C/min, which compares with ≈0.4°C/min reported for C57BL/6 mice (27). Thus it appears that rewarming from the torpid state is not impaired in the A-ZIP/F-1 mice and that tissues besides BAT contribute to rewarming.
Leptin Does Not Prevent Fasted A-ZIP/F-1 Mice From Entering Torpor.
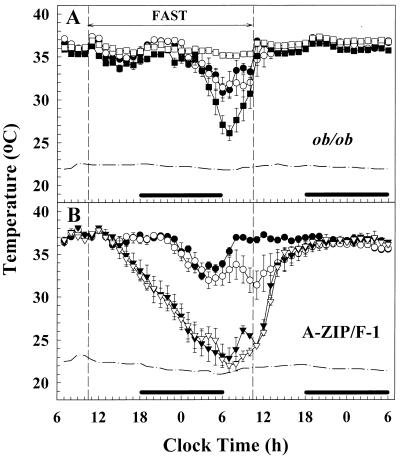
The molecular signals that instruct an animal to enter torpor are not known. Treatment of A-ZIP/F-1 mice with T3 (2 μg/g i.p. daily for 4 days, last dose at food removal) or CL316243 (28), a β3-adrenergic agonist (1 μg/g i.p. once at food removal) did not prevent fasting-induced torpor (not shown). Another possible signal is a low circulating leptin level. In support of this hypothesis, ob/ob mice enter torpor during fasting (27, 29) (Fig. 5). Conversely, high leptin levels might be predicted to prevent torpor. Indeed, upon leptin replacement, ob/ob mice did not go into torpor (Fig. 5). Thus, a low leptin level is one signal for entering torpor.
Figure 5.
Leptin prevents fasting-induced torpor in ob/ob but not A-ZIP/F-1 mice. Tb was measured in 12- to 13-week-old male ob/ob and C57BL/6J control (A) and A-ZIP/F-1 and littermate FVB/N control (B) mice. Leptin pumps were inserted (day 0) and mice regained normal circadian Tb rhythms by day 3. The 24-hr fast was begun on day 5. Data are hourly averages ± SEM (■, ob/ob + saline; □, ob/ob + leptin; ●, wild-type + saline; ○, wild-type + leptin; ▾, A-ZIP/F-1 + saline; ▿, A-ZIP/F-1 + leptin). The dash-dot line indicates ambient temperature and the black bar indicates lights out. One A-ZIP/F-1 + saline mouse died (at 12:30 p.m.), and three A-ZIP/F-1 + leptin mice died (at 9:30 a.m., 11:30 a.m., and 11:30 a.m.).
In comparison to the ob/ob mice with their low leptin but ample energy stores, the A-ZIP/F-1 mice have both low leptin levels and low energy stores. Upon fasting, leptin-treated A-ZIP/F-1 mice entered torpor indistinguishably from saline-treated A-ZIP/F-1 mice (Tb dropped 14.3°C in saline-treated and 14.1°C in leptin-treated). Thus, although a low leptin level typically will occur under conditions conducive to torpor, this experiment demonstrates that it is not the sole signal for entry into torpor.
Discussion
A-ZIP/F-1 Mice Respond Rapidly to Fasting.
The concept that fat is the major fuel source during fasting is old and established, yet has been difficult to study directly. Metabolic adaptations to fasting include changes in fuel usage, increased metabolic efficiency, and minimization of energy expenditure. The A-ZIP/F-1 mice, having a 10-fold reduction in triglyceride content, are a useful model system for studying the contributions of white adipose tissue to these adaptations.
In A-ZIP/F-1 mice, plasma glucose drops within 2 hr of fasting from the diabetic to the normal range. The plasma FFA concentration initially fails to rise and then falls, which we attribute to the lack of normal triglyceride stores. The accumulated hepatic lipid was mobilizable and was exhausted by 24 hr of fasting. Liver glycogen is metabolized before muscle glycogen, but in fasted A-ZIP/F-1 mice both sources are depleted. By 24 hr of fasting, the A-ZIP/F-1 mice are catabolizing protein to provide energy, which can no longer be supplied from triglyceride or glycogen stores. A-ZIP/F-1 mice down-regulate their metabolic rate early in fasting and then go into deep torpor to conserve energy. Taken together, these adaptations illustrate the severity of the energy deficit and the intactness of regulatory loops in the A-ZIP/F-1 mice, resulting in an accelerated metabolic response to fasting.
Leptin and Torpor.
Torpor is a state of physical inactivity, reduced Tb, and reduced metabolic expenditure. Some birds and small mammals routinely use daily torpor to conserve energy, although the drop in Tb may be only a few degrees. Seasonal hibernation is a state of deep torpor lasting days to even months in which the Tb falls severely (e.g., to ≈1°C over ambient, when ambient is in the 5–15°C range) and metabolic rate drops by 90%. Mice and other very small mammals do not use hibernation, possibly because they cannot store enough fuel for an extended hibernation (30).
Although not widely appreciated, it has been documented that mice can enter torpor when there is a quiet environment, reduced food availability, and a low ambient temperature (reviewed in ref. 26). During deep torpor, mice will maintain their Tb at 1–2°C above ambient, down to a minimum Tb of 16–19°C (31). Because of plentiful food and adequate room temperatures, deep torpor infrequently is seen in laboratory mice and recognized even more rarely.
The ob/ob mice are an exception, entering deep torpor upon fasting and occasionally entering torpor even when well-fed and housed at room temperature (27, 29). We found that leptin treatment had no effect on the 4°C drop that occurs with fasting in wild-type mice. In contrast, leptin replacement in ob/ob mice not only prevented the deep torpor, but also the modest 4°C fall. These data demonstrate that leptin plus large energy stores (i.e., leptin-treated ob/ob mice) prevents both deep torpor and the modest hypothermic state.
Low leptin levels signal low energy stores (6, 16) and the role of leptin in regulating daily torpor has been examined in a few cases (32). The marsupial Sminthopsis macroura undergoes significant daily torpor, with an ≈80% reduction in metabolic rate and a 15°C fall in Tb. Leptin treatment decreased the duration and lessened the drop in Tb (33). Leptin treatment of rat pups increased Tb during their shallow daily torpor by ≈1°C (34). In mice, leptin increased the Tb of ob/ob mice housed at room temperature (35). Here we show that leptin also prevents torpor in ob/ob mice. These results demonstrate that leptin has a role in regulating Tb and torpor in a variety of model systems.
Both A-ZIP/F-1 and ob/ob mice have low leptin levels, but in A-ZIP/F-1 mice, unlike ob/ob animals, leptin treatment did not prevent torpor. This finding suggests that there are at least two signals for entry into torpor, one of which is a low leptin level. There are a number of possible mechanisms for a second signal. Adipose tissue could supply a signal(s), besides leptin, that prevents torpor. Another possibility is that a nonadipose hormonal regulator of metabolism, responding to the fasting state, induces torpor. This role has been ruled out for thyroid hormone, the most significant regulator of basal metabolic rate. Similarly, treatment with a β3 agonist did not prevent torpor. Finally, direct sensing of dwindling energy reserves, for example in the hypothalamus, could be the leptin-independent signal. In support of this hypothesis, 2-deoxyglucose, an inhibitor of glucose utilization, induces torpor, particularly when given intracerebrally (36). Both A-ZIP/F-1 and control mice entered torpor after systemic injection of 2-deoxyglucose (unpublished observations). We favor the idea that depletion of central energy stores is the second signal, but further experiments are needed to confirm this hypothesis.
Torpor in Humans.
Maintaining Tb is easier for humans than for mice. However, there are at least three situations in which humans enter a torpor-like state. After the 1985 Mexico City earthquake, six surviving newborns were rescued after being trapped for 6–8 days (see ref. 37). Contemporary speculation was that these survivors had entered a “hibernation-like” state. Another example is the survival of children submerged in cold water. In an extreme case, a child who was immersed for 66 min recovered with mild neurologic deficit (38). Survival in this situation requires two conditions, the diving response and core hypothermia from surface cooling (39).
A third torpor-like condition in humans is the hypothermic and hypometabolic state induced clinically for some types of surgery (40). Understanding of torpor in mice could apply to improving the establishment and management of surgical hypothermia. For example, blocking leptin action preoperatively might facilitate both cooling (by reducing the body’s ability and/or drive to fight hypothermia) and tissue preservation (by adapting tissues to a lower metabolic rate). Another possibility is selective β3-adrenergic agonist treatment (41) to aid recovery from hypothermia.
In summary, our observation that fat-deficient mice enter deep torpor during fasting has allowed us to dissect the mechanisms that regulate torpor in mice and to determine that a low leptin is not the sole signal to enter torpor. These investigations provide insight into the mechanisms underlying torpor-like states in rodents and humans.
Acknowledgments
We thank Drs. D. Accili, E. Arioglu, A. Ginsberg, J. Moitra, S. Taylor, and L. Weinstein for comments on the manuscript and Wyeth-Ayerst for the CL316243. This work was supported in part by National Institutes of Health Grant DK 15070 to S.R.
Abbreviations
- Tb
core body temperature
- FFA
free fatty acid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Keys A, Brozek J, Henschel A, Mickelsen O, Taylor H L. The Biology of Human Starvation. Vol. 1. Minneapolis: University of Minnesota Press; 1950. pp. 161–183. [Google Scholar]
- 2.Cahill G F., Jr N Engl J Med. 1970;282:668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- 3.Arner P, Eckel R H. In: Handbook of Obesity. Bray G A, Bouchard C, James W P T, editors. New York: Dekker; 1998. pp. 379–395. [Google Scholar]
- 4.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 5.Friedman J M, Halaas J L. Nature (London) 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 6.Flier J S. J Clin Endocrinol Metab. 1998;83:1407–1413. doi: 10.1210/jcem.83.5.4779. [DOI] [PubMed] [Google Scholar]
- 7.Uysal K T, Wiesbrock S M, Marino M W, Hotamisligil G S. Nature (London) 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 8.Schreyer S A, Chua S C, Jr, LeBoeuf R C. J Clin Invest. 1998;102:402–411. doi: 10.1172/JCI2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moitra J, Mason M M, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, et al. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster D W. In: Harrison’s Principles of Internal Medicine. Isselbacher K J, Braunwald E, Wilson J D, Martin J B, Fauci A S, Kasper D L, editors. New York: McGraw–Hill; 1994. pp. 2131–2136. [Google Scholar]
- 11.Seip M, Trygstad O. Acta Paediatr Suppl. 1996;413:2–28. doi: 10.1111/j.1651-2227.1996.tb14262.x. [DOI] [PubMed] [Google Scholar]
- 12.Ross S R, Graves R A, Spiegelman B M. Genes Dev. 1993;7:1318–1324. doi: 10.1101/gad.7.7b.1318. [DOI] [PubMed] [Google Scholar]
- 13.Burant C F, Sreenan S, Hirano K, Tai T A, Lohmiller J, Lukens J, Davidson N O, Ross S, Graves R A. J Clin Invest. 1997;100:2900–2908. doi: 10.1172/JCI119839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimomura I, Hammer R E, Richardson J A, Ikemoto S, Bashmakov Y, Goldstein J L, Brown M S. Genes Dev. 1998;12:3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman D L. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 16.Ahima R S, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier J S. Nature (London) 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 17.Kleiber M. The Fire of Life: An Introduction to Animal Energetics. New York: Wiley; 1961. [Google Scholar]
- 18.West G B, Brown J H, Enquist B J. Science. 1999;284:1677–1679. doi: 10.1126/science.284.5420.1677. [DOI] [PubMed] [Google Scholar]
- 19.Pohlenz, J., Maqueem, A., Cua, K., Weiss, R. E., Van Sande, J. & Refetoff, S. (1999) Thyroid, in press. [DOI] [PubMed]
- 20.Salmon D M, Flatt J P. Int J Obes. 1985;9:443–449. [PubMed] [Google Scholar]
- 21.Bradley D C, Kaslow H R. Anal Biochem. 1989;180:11–16. doi: 10.1016/0003-2697(89)90081-x. [DOI] [PubMed] [Google Scholar]
- 22.Brasaemle, D. L., Levin, D. M., Adler-Wailes, D. C. & Londos, C. (1999) Biochim. Biophys. Acta, in press. [DOI] [PubMed]
- 23.Keys A, Brozek J, Henschel A, Mickelsen O, Taylor H L. The Biology of Human Starvation. Vol. 1. Minneapolis: University of Minnesota Press; 1950. pp. 303–339. [Google Scholar]
- 24.Herrington L P. Am J Physiol. 1940;129:123–139. [Google Scholar]
- 25.Pennycuik P R. Aust J Exp Biol Med Sci. 1967;45:331–346. doi: 10.1038/icb.1967.33. [DOI] [PubMed] [Google Scholar]
- 26.Jakobson M E. Symp Zool Soc London. 1981;47:301–335. [Google Scholar]
- 27.Webb G P, Jagot S A, Jakobson M E. Comp Biochem Physiol A. 1982;72:211–219. doi: 10.1016/0300-9629(82)90035-4. [DOI] [PubMed] [Google Scholar]
- 28.Bloom J D, Dutia M D, Johnson B D, Wissner A, Burns M G, Largis E E, Dolan J A, Claus T H. J Med Chem. 1992;35:3081–3084. doi: 10.1021/jm00094a025. [DOI] [PubMed] [Google Scholar]
- 29.Himms-Hagen J. Am J Physiol. 1985;248:E531–E539. doi: 10.1152/ajpendo.1985.248.5.E531. [DOI] [PubMed] [Google Scholar]
- 30.Eckert R. In: Animal Physiology: Mechanisms and Adaptations. Eckert R, Randall D, Augustine G, editors. New York: Freeman; 1988. pp. 555–605. [Google Scholar]
- 31.Hudson J W, Scott I M. Physiol Zool. 1979;52:205–218. [Google Scholar]
- 32.Doring H, Schwarzer K, Nuesslein-Hildesheim B, Schmidt I. Int J Obes Relat Metab Disord. 1998;22:83–88. doi: 10.1038/sj.ijo.0800547. [DOI] [PubMed] [Google Scholar]
- 33.Geiser F, Kortner G, Schmidt I. Am J Physiol. 1998;275:R1627–R1632. doi: 10.1152/ajpregu.1998.275.5.R1627. [DOI] [PubMed] [Google Scholar]
- 34.Stehling O, Doring H, Ertl J, Preibisch G, Schmidt I. Am J Physiol. 1996;271:R1770–R1774. doi: 10.1152/ajpregu.1996.271.6.R1770. [DOI] [PubMed] [Google Scholar]
- 35.Pelleymounter M A, Cullen M J, Baker M B, Hecht R, Winters D, Boone T, Collins F. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 36.Freinkel N, Metzger B E, Harris E, Robinson S, Mager M. N Engl J Med. 1972;287:841–845. doi: 10.1056/NEJM197210262871702. [DOI] [PubMed] [Google Scholar]
- 37.Lopez M I, Leon N A. Hillside J Clin Psychiatry. 1989;11:147–168. [PubMed] [Google Scholar]
- 38.Bolte R G, Black P G, Bowers R S, Thorne J K, Corneli H M. J Am Med Assoc. 1988;260:377–379. [PubMed] [Google Scholar]
- 39.Gooden B A. Med J Aust. 1992;157:629–632. doi: 10.5694/j.1326-5377.1992.tb137408.x. [DOI] [PubMed] [Google Scholar]
- 40.Taylor C A. In: Thermoregulation: Pathology, Pharmacology, and Therapy. Schonbaum E, Lomax P, editors. New York: Pergamon; 1991. pp. 363–396. [Google Scholar]
- 41.Lowell B B, Flier J S. Annu Rev Med. 1997;48:307–316. doi: 10.1146/annurev.med.48.1.307. [DOI] [PubMed] [Google Scholar]
- 42.McLean J A, Tobin G. Animal and Human Calorimetry. Cambridge: Cambridge Univ. Press; 1987. [Google Scholar]