Abstract
Clinical findings suggest that inflammatory disease symptoms are aggravated by ongoing, repeated stress, but not by acute stress. We hypothesized that, compared with single acute stressors, chronic repeated stress may engage different physiological mechanisms that exert qualitatively different effects on the inflammatory response. Because inhibition of plasma extravasation, a critical component of the inflammatory response, has been associated with increased disease severity in experimental arthritis, we tested for a potential repeated stress-induced inhibition of plasma extravasation. Repeated, but not single, exposures to restraint stress produced a profound inhibition of bradykinin-induced synovial plasma extravasation in the rat. Experiments examining the mechanism of inhibition showed that the effect of repeated stress was blocked by adrenalectomy, but not by adrenal medullae denervation, suggesting that the adrenal cortex mediates this effect. Consistent with known effects of stress and with mediation by the adrenal cortex, restraint stress evoked repeated transient elevations of plasma corticosterone levels. This elevated corticosterone was necessary and sufficient to produce inhibition of plasma extravasation because the stress-induced inhibition was blocked by preventing corticosterone synthesis and, conversely, induction of repeated transient elevations in plasma corticosterone levels mimicked the effects of repeated stress. These data suggest that repetition of a mild stressor can induce changes in the physiological state of the animal that enable a previously innocuous stressor to inhibit the inflammatory response. These findings provide a potential explanation for the clinical association between repeated stress and aggravation of inflammatory disease symptoms and provide a model for study of the biological mechanisms underlying the stress-induced aggravation of chronic inflammatory diseases.
There is considerable evidence that stress can exacerbate a number of chronic inflammatory diseases. For example, repeated mild daily stressors have been associated with exacerbation of rheumatoid arthritis (1, 2), ulcerative colitis (3), inflammatory bowel disease (4), and asthma (5). Patients with rheumatoid arthritis (6) cite stress as the most frequent antecedent of symptom flares, and stress management interventions can reduce symptoms (7). Similarly, studies in animal models of arthritis (8, 9) demonstrate that stress can exacerbate disease activity. Very little is known, however, about the biological mechanisms by which stress may exert these effects.
Acute stress (10, 11), as well as acute activation of neuroendocrine circuits known to be activated by stressful stimuli (12, 13), transiently suppresses the inflammatory response. However, exacerbation of inflammatory diseases has been correlated with chronic repeated exposure to stressful stimuli rather than with exposure to a single acute stressor (2–4). We hypothesized that, compared with single acute stressors, chronic repeated stress may engage different physiological mechanisms that exert qualitatively different effects on the inflammatory response. To test this hypothesis, we investigated the effects of chronic repeated stress on the inflammatory response and determined the neuroendocrine mechanism of these effects. Specifically, we used synovial plasma extravasation, induced by the potent inflammatory mediator bradykinin (BK), as a model of the inflammatory response because inhibition of BK-induced synovial plasma extravasation has been associated with increased disease severity in experimental arthritis (14–16).
Materials and Methods
Animals.
The experiments were performed on 300- to 400-g male, Sprague–Dawley rats (Bantin & Kingman, Fremont, CA). Care and handling of animals were in accordance with the American Physiological Society guidelines. The experimental protocols were approved by the University of California San Francisco Committee on Animal Research.
Restraint Stress.
Restraint stress was performed as previously described (17). Rats were placed in acrylic tubes (inner diameter, 5.7 cm; length, 20.3 cm) secured with tape at both ends. Once inside the tubes, rats were placed on a table in a horizontal orientation and remained there for 30 min. The tubes completely restricted the lateral movement of the rats and markedly reduced their front-to-back movement. Restraint stress always took place between 8 a.m. and 11 a.m. We performed these experiments during the trough of the corticosterone circadian rhythm to eliminate any nonspecific effects of circadian rises in corticosterone.
Plasma Extravasation.
As described previously (18), to evaluate BK-induced plasma extravasation, rats were anesthetized with sodium pentobarbital (65 mg/kg, i.p.) and were then given a tail vein injection of Evans blue dye (50 mg/kg in a volume of 2.5 ml/kg), which binds stoichiometrically to serum albumin. Fluid was perfused through the knee joint at a constant rate (250 μl/min), and perfusate samples were collected every 5 min for a 90-min period. After establishing baseline levels of plasma extravasation in the first three samples, the inflammatory mediator BK (150 nM) was added to the perfusing fluid (normal saline) and remained present in the fluid for the duration of the experiment. Using spectrophotometric measurement (absorbance at 620 nm), we then evaluated samples for Evans blue dye concentration, which is linearly related to protein concentration (19).
Adrenalectomy.
Animals were anesthetized with sodium pentobarbital (65 mg/kg). An incision was then made in the dorsal abdominal wall, and the adrenal glands were exposed and removed. Following surgery and throughout the recovery and experimental periods, rats received drinking water (ad libitum) containing 0.5% saline and 25 μg/ml corticosterone (20). This dose of exogenous corticosterone produces low (i.e., 8 μg/dl peak circadian value) but still phasic plasma levels of plasma corticosterone and inhibits both the enhanced corticotropin (ACTH) secretion and decreased body-weight gain observed in adrenalectomized rats without corticosterone replacement (20). Corticosterone drinking water was replaced with normal tap water 2 hr prior to the beginning of knee-joint perfusion to avoid any acute increases in corticosterone during the experiment. Adrenalectomy was performed 1 wk prior to restraint stress experiments. Successful surgery was confirmed by measuring corticosterone levels in blood samples collected from anesthetized animals prior to sacrifice. Plasma corticosterone levels were below 1 μg/dl in all adrenalectomized animals (data not shown). In intact, anesthetized animals, plasma corticosterone ranged from 12 to 16 μg/dl (Fig. 1A, Control).
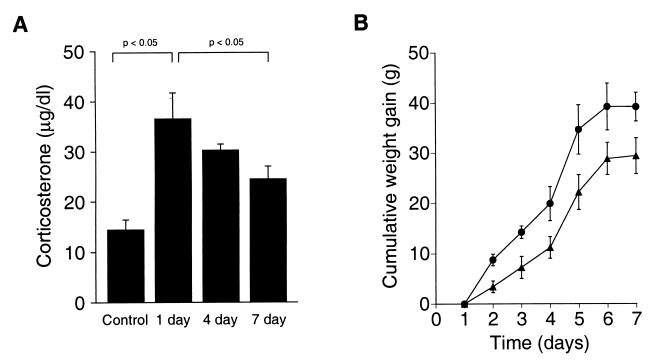
Figure 1.
Daily restraint is stressful to rats. (A) Plasma corticosterone was measured in anesthetized rats 1 hr after exposure to restraint stress. One (1 day, n = 8), four (4 day, n = 5), or seven daily (7 day, n = 7) exposures to restraint stress induced a significant increase in plasma corticosterone compared with home-cage control rats (Control, n = 10). After seven daily exposures to restraint stress, the adrenal gland habituated to the stressful stimulus. (B) Rats exposed daily to restraint stress (▴) gained significantly less body weight over time compared with home-cage controls (●), providing an independent verification that daily restraint is perceived by the rats as stressful.
Adrenal Medullae Denervation.
Innervation of the adrenal medullae was ablated as previously described (21, 22). Animals were anesthetized with sodium pentobarbital (65 mg/kg), and an incision was made in the lateral abdominal wall. The greater splanchnic nerve innervating each adrenal gland was exposed, and the adrenal innervation region was isolated close to the adrenal gland and cut. Adrenal denervation was performed 1 wk prior to restraint stress experiments. Because it is conceivable that the adrenal cortex could be injured during this surgical procedure, adrenal cortical function was assessed by measuring plasma corticosterone levels in stressed animals. In all cases, except one, plasma corticosterone levels were similar in stressed intact and stressed adrenal medullae-denervated rats (data not shown). All data from the one exception were excluded from analysis.
Analysis of Plasma Corticosterone.
Blood (≈100 μl) was collected for corticosterone measurement from the tail vein of rats with an i.v. infusion set ≈10 min after administration of anesthesia. Blood was collected 1 hr after the onset of restraint stress or drug (metyrapone, ACTHar, or saline) treatment. Blood samples were immediately spun in a tabletop centrifuge, and plasma was collected. Samples were stored at −20°C until assayed (≤3 mo). Corticosterone was measured with a radioimmunoassay kit (ICN), as previously described (17).
Metyrapone Administration.
Metyrapone (100 mg/kg, dissolved in DMSO), a corticosterone synthesis inhibitor, was administered i.p. 5 min prior to restraint stress, daily for 6 days. On the seventh day, no drug was administered prior to exposure to stress. Control rats received six daily metyrapone injections, but were not otherwise subjected to stress and remained in their home cages at all times, except to receive their injections.
ACTHar Administration.
A single injection of ACTHar (4 units/kg dissolved in normal saline), a clinically approved, slow-release formulation of ACTH, was administered s.c. daily for 7 days between 8 a.m. and 11 a.m. Control rats received s.c. injections of saline on the same schedule as ACTHar injections.
Statistics.
Plasma extravasation data were analyzed with repeated measures ANOVA. Plasma corticosterone data were also analyzed with ANOVA. Games–Howell tests were used for all post hoc comparisons.
Materials.
Evans blue dye, BK triacetate, corticosterone, and DMSO were obtained from Sigma. Metyrapone was obtained from Aldrich, and ACTHar was obtained from Rhône-Poulenc Rorer (Collegeville, PA).
Results
Repeated but Not Single Exposures to Restraint Stress Inhibit BK-Induced Plasma Extravasation.
Restraint induced a large increase in plasma corticosterone, indicating that restraint was stressful [Fig. 1A; F (3, 26) = 8.4, P < 0.001; post hoc 1-day restraint vs. home-cage, anesthetized control, P < 0.05)]. Similar to the findings of others (23, 24), the adrenal showed habituation of this response because, after seven daily exposures to restraint, the evoked plasma corticosterone response to restraint was significantly less than the response to one exposure [Fig. 1A; F (3, 26) = 8.4, P < 0.001; post hoc 7-day restraint vs. 1-day restraint, P < 0.05]. As an independent confirmation that restraint was perceived as stressful throughout the 7-day exposure period, we measured rat body weights. Rats exposed to daily restraint gained significantly less body weight than control rats exposed to similar housing conditions [Fig. 1B; F (1, 12) = 7.8, P < 0.05].
To determine the effect of stress on synovial plasma extravasation induced by the inflammatory mediator BK, we exposed rats to 30 min of restraint stress daily for 1, 4, or 7 days. We then measured BK-induced plasma extravasation 1 hr after the last stress exposure. BK induced a rapid and sustained increase from baseline plasma extravasation in home-cage control rats (Fig. 2A). BK-induced plasma extravasation in rats exposed to one session of restraint stress was not significantly different from BK-induced plasma extravasation in unstressed, home-cage control rats [Fig. 2A; F (3, 48) = 15.9, P < 0.001; post hoc 1-day restraint vs. control, not significant (NS)]. However, when rats were exposed to the same stressor daily for 4 or 7 days, BK-induced plasma extravasation was markedly inhibited [Fig. 2A; F (3, 48) = 15.9, P < 0.001; post hoc 4-day or 7-day restraint vs. control, P < 0.01]. These data suggest that repeated exposure to stress alters the physiology of the animal in such a way that a stimulus (restraint stress) that previously had no effect now inhibits the inflammatory response.
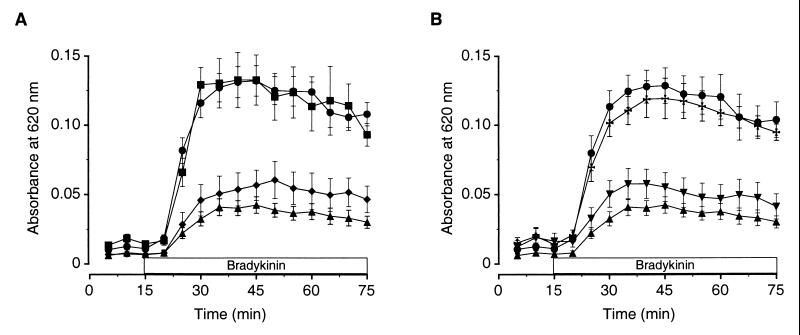
Figure 2.
Repeated but not a single exposure to restraint stress inhibits BK-induced plasma extravasation. (A) BK induces sustained plasma extravasation (●, n = 14). One exposure to restraint stress does not affect BK-induced plasma extravasation (■, n = 13), while four (♦, n = 12) or seven (▴, n = 14) daily exposures to restraint stress markedly inhibit it. (B) BK-induced plasma extravasation is markedly inhibited 1 (▴, n = 14) and 5 hr (▾, n = 5) after the seventh daily exposure to restraint stress and returns to control levels (●, n = 14) 24 hr after the seventh daily exposure to restraint stress (✠, n = 5).
To determine the duration of the effects of repeated stress, we exposed rats to restraint stress daily for 7 days and measured BK-induced plasma extravasation 1, 5, or 24 hr after cessation of the last restraint-stress session. Experiments investigating the 5-hr post stress time point took place, as did all other experiments, in the trough of the corticosterone circadian rhythm to eliminate any nonspecific effects of circadian rises in corticosterone. BK-induced plasma extravasation was significantly inhibited 1 and 5 hr after cessation of the seventh daily exposure to restraint stress and returned to baseline by 24 hr after that exposure [Fig. 2B; F (3, 34) = 18.2, P < 0.001; post hoc 1 hr or 5 hr post restraint vs. control or 24 hr post restraint, P < 0.01; post hoc 24 hr post restraint vs. home-cage control, NS]. These data suggest that frequent exposure to the stressor is required to maintain the animal in the altered physiological state in which its response to inflammatory challenge is inhibited.
The Hypothalamic–Pituitary–Adrenal (HPA) Axis Mediates Repeated Stress-Induced Inhibition of Plasma Extravasation.
We used surgical interventions to determine whether stress-activated neural endocrine circuits (i.e., HPA axis, sympathoadrenal axis) mediate the repeated stress-induced inhibition of plasma extravasation observed in these animals. To determine the contribution of each stress axis, we lesioned both the HPA and sympathoadrenal axes, by removing the adrenal glands, before exposing rats to 7 days of repeated restraint stress. BK-induced plasma extravasation was not significantly different in adrenalectomized rats compared with adrenalectomized rats that had been exposed to repeated restraint stress. Stressed, intact rats showed significantly inhibited plasma extravasation compared with these two groups [Fig. 3A; F (2, 35) = 7.2, P < 0.001; post hoc adrenalectomized vs. adrenalectomized plus restraint stress, NS; post hoc adrenalectomized or adrenalectomized plus restraint stress vs. intact plus restraint stress, P < 0.01]. These data show that repeated stress-induced inhibition of plasma extravasation is blocked in adrenalectomized rats and indicate that the HPA and/or sympathoadrenal axes are involved in mediating the inhibition.
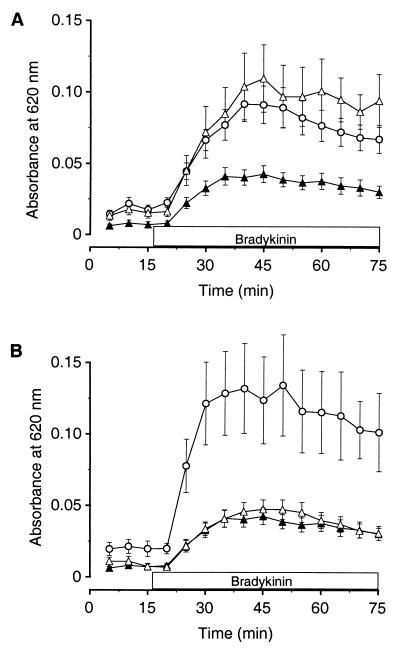
Figure 3.
Stress-induced inhibition of BK-induced knee joint plasma extravasation is mediated by the HPA axis. (A) Seven-day restraint stress-induced inhibition of plasma extravasation (▴, n = 14) was completely blocked in animals that had been adrenalectomized 1 wk prior to receiving restraint stress (▵, n = 11). BK-induced plasma extravasation was not significantly different in these animals compared with control animals that had been adrenalectomized but not subjected to restraint stress (○, n = 15). (B) Seven-day restraint stress-induced inhibition of plasma extravasation (▴, n = 14) was not affected in animals that had undergone adrenal medullae denervation 1 wk prior to receiving restraint stress (▵, n = 10). A curve depicting BK-induced plasma extravasation in control rats that had undergone adrenal denervation but had not received restraint stress (○, n = 6) is included for comparison.
To distinguish between the contribution of the HPA axis and the sympathoadrenal axis, we surgically denervated the adrenal medullae, leaving the adrenal cortexes intact, and then exposed rats to 7 days of repeated restraint stress. Repeated stress induced a significant inhibition of plasma extravasation in the adrenal medullae-denervated rats that was nearly identical to the stress-induced inhibition observed in intact rats [Fig. 3B; F (2, 25) = 13.7, P < 0.001; post hoc adrenal denervated plus restraint stress vs. adrenal denervated, P < 0.01; post hoc adrenal denervated plus restraint stress vs. intact plus restraint stress, NS]. Because repeated stress-induced inhibition of plasma extravasation was blocked in adrenalectomized rats but not in adrenal medullae-denervated rats, these data suggest that the HPA axis and specifically the adrenal cortex mediates the repeated stress-induced physiological change in the animals that results in inhibition of plasma extravasation (Fig. 3B). Because stress induces rapid synthesis and release of corticosterone from the adrenal cortex, these data implicate corticosterone as a potential mediator of these effects.
Repeated Daily Pulses of Corticosterone Reproduce Repeated Stress-Induced Suppression of Plasma Extravasation, and Inhibiting Corticosterone Synthesis Blocks This Suppression.
Next, we tested the hypothesis that repeated elevations of plasma corticosterone evoked by daily restraint stress mediate repeated stress-induced inhibition of plasma extravasation. To test this hypothesis, we blocked the ability of the stressor to induce production of corticosterone on the first six days of daily restraint stress by administering the corticosterone synthesis inhibitor metyrapone prior to the daily stress session. Metyrapone was not administered prior to the seventh daily restraint session so that, in terms of corticosterone secretion, these animals would respond similarly to acutely stressed animals. In terms of HPA axis functions not affected by corticosterone, they would be expected to respond similarly to chronically stressed animals. In this way, we determined the effect of repeated corticosterone pulses on plasma extravasation. As shown in Fig. 4A Inset, metyrapone treatment affected corticosterone production as expected because chronically stressed animals that had been treated with metyrapone for 6 days exhibited levels of corticosterone on day 7 that were not significantly different from those observed in acutely stressed animals [F (4, 33) = 6.4, P < 0.001; post hoc, NS]. These levels were significantly enhanced compared with animals subjected to repeated stress without metyrapone treatment [F (4, 33) = 6.4, P < 0.001; post hoc, P < 0.05]. Animals exposed to six daily metyrapone injections without restraint stress showed corticosterone levels on day 7 that were not significantly different from levels measured in home-cage control animals [F (4, 33) = 6.4, P < 0.001; post hoc, NS].
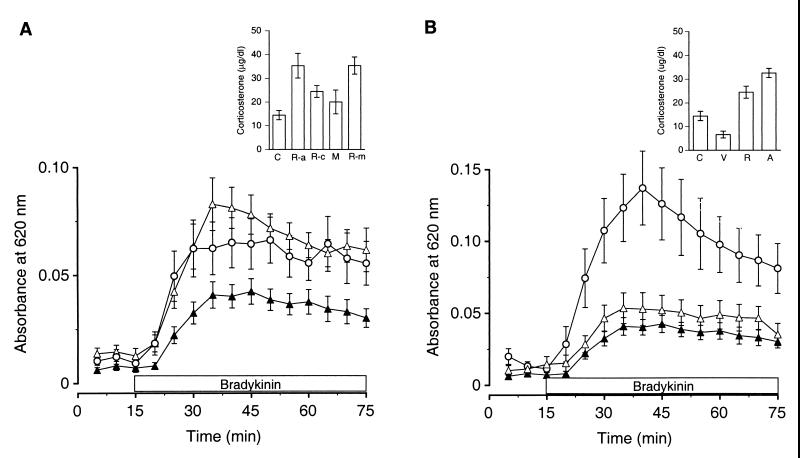
Figure 4.
Repeated daily pulses of corticosterone reproduce 7-day restraint stress-induced suppression of plasma extravasation, and inhibiting corticosterone synthesis blocks this suppression. (A) Seven-day restraint stress-induced inhibition of plasma extravasation (▴, n = 14) was completely blocked in animals that had been treated with metyrapone, on the first 6 days of the seven daily exposures to restraint stress (▵, n = 11). BK-induced plasma extravasation was not significantly different in these animals compared with control animals treated daily for 6 days with metyrapone but not exposed to restraint stress (○, n = 13). (Inset) Animals treated with the corticosterone synthesis inhibitor metyrapone prior to restraint stress on the first 6 days of the seven daily exposures (R-m, n = 6) showed plasma corticosterone levels that were not significantly different from those of untreated animals exposed to only one restraint session (R-a, n = 8). These levels were significantly elevated compared with those of animals exposed to 7-day restraint without metyrapone treatment (R-c, n = 7). Metyrapone treatment alone (M, n = 7) evoked plasma corticosterone levels that were not significantly different than those measured in home-cage control animals (C, n = 10). (B) Seven-day restraint stress-induced inhibition of plasma extravasation (▴, n = 14) was mimicked by daily injections of ACTHar (▵, n = 8). A curve depicting BK-induced plasma extravasation in control animals injected daily with saline vehicle (○, n = 5) is included for comparison. (Inset) Animals injected with ACTHar daily for 7 days (A, n = 6) showed plasma corticosterone levels that were not significantly different from those exhibited by animals exposed to seven daily sessions of restraint stress (R, n = 7). Plasma corticosterone was significantly elevated in these groups compared with control animals injected daily with saline vehicle (V, n = 3) or to home-cage control animals (C, n = 10).
Animals treated with metyrapone prior to their daily exposure to stress did not show repeated stress-induced inhibition of BK-induced plasma extravasation. Instead, they exhibited levels of BK-induced plasma extravasation similar to animals treated with metyrapone and not subjected to restraint stress [Fig. 4A; F (4, 61) = 10.7, P < 0.001; post hoc, NS]. Animals exposed to repeated restraint stress without metyrapone pretreatment showed inhibition of BK-induced plasma extravasation when compared with either animals treated with metyrapone alone or with metyrapone plus repeated restraint stress [Fig. 4A; F (4, 61) = 10.7, P < 0.001; post hoc, P < 0.01]. These data suggest that repeated daily pulses of corticosterone are necessary to produce inhibition of plasma extravasation.
To test the hypothesis that daily pulses of corticosterone, similar to those evoked by restraint stress, are sufficient to produce inhibition of BK-induced plasma extravasation, we injected ACTHar (4 units/kg) daily for 7 days. ACTHar, a slow-release form of ACTH, induces a sustained rise in plasma corticosterone in the rat that is not significantly different in level or in time course to that induced by restraint stress (25). Consistent with previous work (25), in anesthetized animals, ACTHar (4 units/kg) produced similar levels of corticosterone as 7-day restraint stress, and both of these groups showed significantly higher corticosterone levels than vehicle-injected or home-cage control animals [Fig. 4B Inset; F (3, 22) = 19.3, P < 0.001; post hoc ACTHar vs. 7-day restraint, NS; post hoc ACTHar or 7-day restraint vs. vehicle or home-cage control, P < 0.01]. As shown in Fig. 4B, ACTHar injections (4 units/kg) were sufficient to produce inhibition of BK-induced plasma extravasation similar to that produced by 7-day restraint stress. BK-induced plasma extravasation was inhibited in these two groups compared with that in control animals injected daily with saline vehicle. [F (3, 37) = 12.3, P < 0.001; post hoc ACTHar vs. 7-day restraint, NS; post hoc ACTHar or 7-day restraint vs. vehicle or home-cage control, P < 0.01]. These data indicate that daily pulses of corticosterone evoked by stressful stimulation are sufficient to mediate inhibition of synovial plasma extravasation.
Discussion
In this study, we demonstrate that repeated but not single exposures to restraint stress induce a marked inhibition of plasma extravasation, a fundamental component of the inflammatory response. Repeated exposure to stress appears to alter the physiological state of the animal in such a way that exposure to a stressful stimulus that previously did not affect the animal’s inflammatory response now induces a marked inhibition of it. The effects of repeated stress are sustained, persisting for at least 5 hr after the last exposure to the stressor and, upon reexposure to the stressful stimulus, can be reevoked for at least 3 days.
Inhibition of rat knee joint plasma extravasation is associated with worsening of joint injury in experimental arthritis (14–16). Plasma extravasation may exert a protective effect by increasing extravasation of plasma proteinase inhibitors (e.g., α1-proteinase inhibitor, α1-anti-chymotrypsin, and α2-macroglobulin) that control excessive proteolytic activity and thereby protect against connective tissue damage (26), or by increasing the rate of removal of tissue-injurious products of the inflammatory response. Therefore, our observation of stress-induced inhibition of plasma extravasation provides a mechanism for the clinical findings that repeated stress can aggravate inflammatory disease symptoms (2–4) and suggests that this phenomenon may be due, at least in part, to direct effects on the inflammatory response.
While we observed no effect on plasma extravasation 1 hr after acute stress, we did not test for an effect of acute stress on plasma extravasation during the stressful stimulus. Earlier reports have indicated that acute stress can inhibit plasma extravasation if the inflammatory challenge is presented during the stressful stimulus (10, 11). However, the inhibition of plasma extravasation was transient and recovered soon after removal of the stressor. Therefore, it seems unlikely that these transient actions would have noticeable effects on full-blown inflammatory disease, such as the aggravation of symptoms reported clinically (2–4). Transient inhibition of plasma extravasation during an acute stressor is mediated by the stress-activated sympathoadrenal axis (11) and may involve physiological changes (i.e., catechol-mediated increases in heart rate and blood pressure) that subside quickly after the stressor is removed.
Our data support the hypothesis that acute and repeated stress engage fundamentally different mechanisms. These data clearly indicate that the sympathoadrenal axis does not play a role in the mechanism of repeated stress effects. Instead, the physiological change induced by repeated stress is mediated by the HPA axis and specifically by corticosterone because it is blocked by inhibition of corticosterone synthesis and because repeated exposure to mild stress levels of corticosterone are sufficient to produce it.
Our data demonstrate that repeated elevations in plasma corticosterone are necessary and sufficient to alter the physiological state of the animal in such a way that a stimulus that previously had no effect on its inflammatory response now profoundly suppresses it. However, the mechanism by which corticosterone induces this altered state is, as yet, unknown. Repeated elevations in plasma corticosterone may sensitize or up-regulate expression of glucocorticoid receptors. Alternatively, other physiological changes that could potentially mediate inhibition of plasma extravasation have been observed in chronically stressed animals. For example, up-regulation of β-adrenergic receptors (27) and decreases in plasma testosterone (28) have been both observed in chronically stressed animals and implicated in regulation of plasma extravasation (15, 29). Further investigation is required to elucidate this underlying mechanism.
We have hypothesized that stress-induced release of glucocorticoids could aggravate tissue injury in inflammatory disease. However, glucocorticoids are administered therapeutically to lessen inflammatory disease activity. Additionally, Fischer rats, which have hyperfunctioning HPA axes, are resistant to development of streptococcal cell wall (SCW) fragment-induced arthritis. These rats exhibit very high plasma corticosterone levels (i.e., 2-fold higher than restraint stress levels in our study) in response to SCW injection (30). This apparent contradiction may result from dose-dependent effects of glucocorticoids such that high doses of glucocorticoids, similar to doses used therapeutically, lessen disease activity, while lower doses exacerbate it. This hypothesis is supported by the observation that mild, repeated stress (lower glucocorticoid levels) is associated with aggravation of arthritis symptoms, while intense stress (higher glucocorticoid levels) is associated with lessening of disease activity in both humans and animals (1, 9). The restraint stress employed in the present study is classified as a mild stress because it induces significantly lower plasma corticosterone levels than severe stressors (data not shown). A biphasic dose-dependence in the effect of glucocorticoids on inflammatory disease activity could be the result of their dose-dependent induction of the cytokine macrophage migration inhibitory factor (MIF). MIF production is induced by low, but not high, doses of glucocorticoids, as well as by mild stress (31). MIF overrides glucocorticoid-induced suppression of gene expression of proinflammatory cytokines, including tumor necrosis factor-α, IL-1β, IL-6, and IL-8 (31, 32), all of which have been associated with increases in inflammatory disease activity (33–37).
The parallel between our findings and clinical observations suggests that determining the mechanism by which repeated stress suppresses plasma extravasation is likely to be important for further understanding of the relationship between stress and inflammatory disease. Furthermore, in view of the current trend toward the use of lower therapeutic doses of glucocorticoids, understanding dose-dependent effects of glucocorticoids on the inflammatory response could have a significant impact on treatment strategies for chronic inflammatory disease.
Acknowledgments
We thank Dr. Fred Miao for performing adrenal denervation surgeries and Drs. David Reichling and Paul Green for helpful discussions on the manuscript. This work was funded by National Institutes of Health Grant AM32634.
Abbreviations
- BK
bradykinin
- ACTH
corticotropin
- NS
not significant
- HPA
hypothalamic–pituitary–adrenal
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Potter P T, Zautra A J. J Consult Clin Psychol. 1997;65:319–323. doi: 10.1037//0022-006x.65.2.319. [DOI] [PubMed] [Google Scholar]
- 2.Zautra A J, Burleson M H, Matt K S, Roth S, Burrows L. Health Psychol. 1994;13:139–148. doi: 10.1037//0278-6133.13.2.139. [DOI] [PubMed] [Google Scholar]
- 3.Levenstein S, Prantera C, Varvo V, Scribano M L, Berto E, Andreoli A, Luzi C. Am J Gastroenterol. 1994;89:1219–1225. [PubMed] [Google Scholar]
- 4.Dancey C P, Taghavi M, Fox R J. J Psychosom Res. 1998;44:537–545. doi: 10.1016/s0022-3999(97)00255-9. [DOI] [PubMed] [Google Scholar]
- 5.Sekas G, Wile M Z. J Med Educ. 1980;55:440–446. doi: 10.1097/00001888-198005000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Affleck G, Pfeiffer C, Tennen H, Fifield J. Arthritis Rheum. 1987;30:927–931. doi: 10.1002/art.1780300813. [DOI] [PubMed] [Google Scholar]
- 7.Parker J C, Smarr K L, Buckelew S P, Stucky-Ropp R C, Hewett J E, Johnson J C, Wright G E, Irvin W S, Walker S E. Arthritis Rheum. 1995;38:1807–1818. doi: 10.1002/art.1780381214. [DOI] [PubMed] [Google Scholar]
- 8.Amkraut A A, Solomon G F, Kraemer H C. Psychosom Med. 1971;33:203–214. doi: 10.1097/00006842-197105000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Rogers M P, Trentham D E, Dynesius-Trentham R, Daffner K, Reich P. J Rheumatol. 1983;10:651–654. [PubMed] [Google Scholar]
- 10.Harmsen A G, Turney T H. Inflammation. 1985;9:9–20. doi: 10.1007/BF00915407. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharya S K, Das N, Sarkar M K. Res Exp Med (Berlin) 1987;187:303–313. doi: 10.1007/BF01852056. [DOI] [PubMed] [Google Scholar]
- 12.Green P G, Miao F J-P, Jänig W, Levine J D. J Neurosci. 1995;15:4678–4686. doi: 10.1523/JNEUROSCI.15-06-04678.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miao, F. J.-P. & Levine, J. D. (1999) J. Pharmacol. Exp. Ther., in press. [PubMed]
- 14.Green P G, Basbaum A I, Helms C, Levine J D. Proc Natl Acad Sci USA. 1991;88:4162–4165. doi: 10.1073/pnas.88.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coderre T, Chan A, Helms C, Basbaum A, Levine J. Neuroscience. 1991;40:185–189. doi: 10.1016/0306-4522(91)90184-p. [DOI] [PubMed] [Google Scholar]
- 16.Miao F J, Helms C, Benowitz N L, Basbaum A I, Heller P H, Levine J D. Neuroscience. 1992;51:649–655. doi: 10.1016/0306-4522(92)90304-k. [DOI] [PubMed] [Google Scholar]
- 17.Akana S F, Dallman M F, Bradbury M J, Scribner K A, Strack A M, Walker C D. Endocrinology. 1992;131:57–68. doi: 10.1210/endo.131.1.1319329. [DOI] [PubMed] [Google Scholar]
- 18.Coderre T J, Basbaum A I, Levine J D. J Neurophysiol. 1989;62:48–58. doi: 10.1152/jn.1989.62.1.48. [DOI] [PubMed] [Google Scholar]
- 19.Carr J, Wilhelm D L. Austr J Exp Biol Med. 1964;42:511–522. doi: 10.1038/icb.1964.48. [DOI] [PubMed] [Google Scholar]
- 20.Akana S F, Cascio C S, Shinsako J, Dallman M F. Am J Physiol. 1985;249:R527–R532. doi: 10.1152/ajpregu.1985.249.5.R527. [DOI] [PubMed] [Google Scholar]
- 21.Celler B G, Schramm L P. Am J Physiol. 1981;241:R55–R61. doi: 10.1152/ajpregu.1981.241.1.R55. [DOI] [PubMed] [Google Scholar]
- 22.Miao F J, Dallman M F, Benowitz N L, Basbaum A I, Levine J D. J Pharmacol Exp Ther. 1993;264:839–844. [PubMed] [Google Scholar]
- 23.De Boer S F, Koopmans S J, Slangen J L, Van der Gugten J. Physiol Behav. 1990;47:1117–1124. doi: 10.1016/0031-9384(90)90361-7. [DOI] [PubMed] [Google Scholar]
- 24.Dhabhar F S, McEwen B S, Spencer R L. Neuroendocrinology. 1997;65:360–368. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- 25.Dallman M F, Jones M T. Endocrinology. 1973;92:1367–1375. doi: 10.1210/endo-92-5-1367. [DOI] [PubMed] [Google Scholar]
- 26.Kozik A, Moore R B, Potempa J, Imamura T, Rapala-Kozik M, Travis J. J Biol Chem. 1998;273:33224–33229. doi: 10.1074/jbc.273.50.33224. [DOI] [PubMed] [Google Scholar]
- 27.Mak J C, Nishikawa M, Shirasaki H, Miyayasu K, Barnes P J. J Clin Invest. 1995;96:99–106. doi: 10.1172/JCI118084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batuman O A, Sajewski D, Ottenweller J E, Pitman D L, Natelson B H. Brain Behav Immun. 1990;4:105–117. doi: 10.1016/0889-1591(90)90013-g. [DOI] [PubMed] [Google Scholar]
- 29.Green P G, Dahlqvist S R, Isenberg W M, Strausbaugh H J, Miao F J, Levine J D. J Neurosci. 1999;19:4082–4089. doi: 10.1523/JNEUROSCI.19-10-04082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sternberg E, Hill J, Chrousos G, Kamilaris T, Listwak S, Gold P, Wilder R. Proc Natl Acad Sci USA. 1989;86:2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calandra T, Bernhagen J, Metz C N, Spiegel L A, Bacher M, Donnelly T, Cerami A, Bucala R. Nature (London) 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 32.Calandra T, Bucala R. Crit Rev Immunol. 1997;17:77–88. doi: 10.1615/critrevimmunol.v17.i1.30. [DOI] [PubMed] [Google Scholar]
- 33.Elliott M J, Maini R N, Feldmann M, Long-Fox A, Charles P, Bijl H, Woody J N. Lancet. 1994;344:1125–1127. doi: 10.1016/s0140-6736(94)90632-7. [DOI] [PubMed] [Google Scholar]
- 34.Elliott M J, Maini R N, Feldmann M, Long-Fox A, Charles P, Katsikis P, Brennan F M, Walker J, Bijl H, Ghrayeb J, et al. Arthritis Rheum. 1993;36:1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- 35.van den Berg W B, Joosten L A, Helsen M, van de Loo F A. Clin Exp Immunol. 1994;95:237–243. doi: 10.1111/j.1365-2249.1994.tb06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuno H, Sawai T, Nezuka T, Uzuki M, Tsuji H, Nishimoto N, Yoshizaki K. Arthritis Rheum. 1998;41:2014–2021. doi: 10.1002/1529-0131(199811)41:11<2014::AID-ART17>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura A, Akahoshi T, Takahashi M, Takagishi K, Itoman M, Kondo H, Takahashi Y, Yokoi K, Mukaida N, Matsushima K. J Leukocyte Biol. 1997;62:444–449. doi: 10.1002/jlb.62.4.444. [DOI] [PubMed] [Google Scholar]