Abstract
Strategies to treat cancer have focused primarily on the killing of tumor cells. Here, we describe a differential stress resistance (DSR) method that focuses instead on protecting the organism but not cancer cells against chemotherapy. Short-term starved S. cerevisiae or cells lacking proto-oncogene homologs were up to 1,000 times better protected against oxidative stress or chemotherapy drugs than cells expressing the oncogene homolog Ras2val19. Low-glucose or low-serum media also protected primary glial cells but not six different rat and human glioma and neuroblastoma cancer cell lines against hydrogen peroxide or the chemotherapy drug/pro-oxidant cyclophosphamide. Finally, short-term starvation provided complete protection to mice but not to injected neuroblastoma cells against a high dose of the chemotherapy drug/pro-oxidant etoposide. These studies describe a starvation-based DSR strategy to enhance the efficacy of chemotherapy and suggest that specific agents among those that promote oxidative stress and DNA damage have the potential to maximize the differential toxicity to normal and cancer cells.
Keywords: reactive oxygen species, short-term starvation, maintenance mode
Our studies in S. cerevisiae and those of others in worms, flies, and mice have uncovered a strong association between lifespan extension and resistance to oxidative stress (1–6). This resistance is observed in long-lived yeast cells lacking RAS2 and SCH9, the orthologs of components of the human Ras and Akt/S6K pathways (2, 5, 7), and in long-lived worms and mice with reduced activity of homologs of the IGF1 receptor (IGF1R), implicated in many human cancers (8). Notably, the IGF1R functions upstream of Ras and Akt in mammalian cells (3–6). Stress resistance is also observed in model systems in which calorie intake is reduced by at least 30% (9). This reduced calorie intake, also known as calorie restriction (CR) or dietary restriction (DR), has been studied for many years and is known to extend life span in organisms ranging from yeast to mice (10). CR also protects against spontaneous cancers and against carcinogen-induced cancers (10–12), raising the possibility that CR and reduced IGF1 may increase stress resistance by similar mechanisms.
Our discovery of the role of Ras2 and Sch9 in the negative regulation of antioxidant and other protective systems together with the association between mutations that activate IGF1R, Ras, or Akt and many human cancers prompted our hypothesis that normal but not cancer cells would respond to starvation or down-regulation of Ras/Akt signaling by entering a stress-resistance mode. In fact, one of the major “hallmarks of cancer cells” is the self-sufficiency for growth signals (13). In the majority of cancers, this ability to grow or remain in a growth mode even in the absence of growth factors is provided by the hyperactivation of one or several components of the IGF1R, Ras, Akt, and mTor pathways.
Here, we tested the hypothesis that short-term starvation (STS) or low glucose/low serum can protect mammalian cells, but not or to a lesser extent cancer cells, against high doses of oxidative damage or chemotherapy.
Results
Short-Term Starvation Induces Differential Stress Resistance Against Oxidative Stress in Yeast.
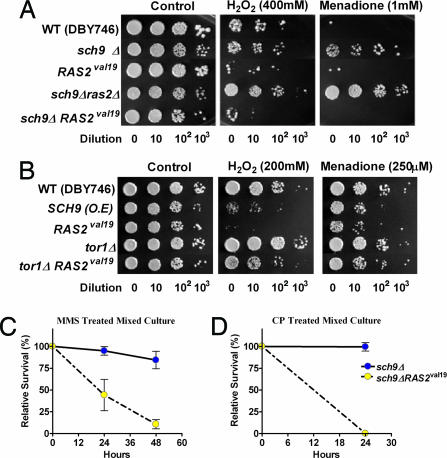
To test the hypothesis that constitutively active oncogenes or oncogene homologs can prevent the switch to a protective maintenance mode in response to starvation, we first determined whether acute starvation would be as effective in increasing oxidative stress resistance as long-term CR has been shown to be (14). We first performed differential stress resistance (DSR) studies in S. cerevisiae. We selected a STS paradigm as well as the deletion of the SCH9 and/or RAS2 genes, each of which mimics in part CR and was shown in our previous studies to cause high resistance to oxidative stress (15–17). Our hypothesis was that the combination of these genetic manipulations with starvation would maximize DSR. Cells were treated with either H2O2 or the superoxide-generating agent menadione. The combination of STS (switch from glucose medium to water at day 1 and incubation in water for 24–48 h) with the deletion of SCH9 or both SCH9 and RAS2 caused resistance to a 30- to 60-min treatment with hydrogen peroxide or menadione that was up to 1,000-fold higher than that of cells expressing the constitutively active oncogene homolog RAS2val19 or cells lacking SCH9 (sch9Δ) but expressing RAS2val19 (sch9ΔRAS2val19) (Fig. 1A). The rationale for this experiment was to model in a simple system the effect of the combination of STS and a genetic approach on the differential protection of normal and cancer cells. The results show that the expression of the oncogene-like RAS2val19 prevents the 1,000-fold protection caused by the combination of STS and inhibition of Sch9 activity. Notably, under these conditions yeast cells are not dividing.
Fig. 1.
DSR against oxidants and genotoxins in yeast. (A and B) Survival of nondividing (day 3) STS-treated yeast cells deficient in Sch9 and/or Ras2 (sch9Δ and sch9Δ ras2Δ), and cells overexpressing Sch9 or expressing constitutively active RAS2 val19 (SCH9, RAS2val19, sch9Δ RAS2val19, and tor1Δ RAS2val19) after treatment with H2O2 (30 min) or menadione (60 min). At day 3, cells were treated with either H2O2 for 30 min or menadione for 60 min. Serial dilution (10-, 102-, and 103-fold dilutions, respectively, in the spots from left to right) of the treated cultures was spotted onto YPD plates and incubated for 2–3 days at 30°C (see detailed methods in SI Materials and Methods). This experiment was repeated at least three times with similar results. A representative experiment is shown. (C and D) Differential stress resistance (DSR) to chronic CP and methylmethane sulfonate (MMS) treatments in mixed yeast cultures: sch9Δ and sch9Δ RAS2val19. To model the mixture of normal and tumor cells in mammalian cancer, sch9Δ and sch9Δ RAS2val19 were mixed in the same flask and incubated for 2 h at 30°C with shaking. The initial sch9Δ:sch9Δ RAS2val19 ratio, measured by growth on selective media, was 25:1. Mixed cultures were then treated with either CP (0.1 M) or MMS (0.01%). Viability was measured after 24–48 h by plating onto appropriate selective media that allows the distinction of the two strains. Data from three independent experiments are shown as means ± SD.
We also tested the effect of increased activity of Sch9 on resistance to oxidants. As with RAS2val19, overexpression of SCH9 sensitized yeast cells to both H2O2 and the superoxide-generating agent menadione (Fig. 1B). Similar to the effect of the deletion of RAS2 and SCH9, the deletion of the homolog of TOR, another gene implicated in oncogenesis, slightly increased the resistance to hydrogen peroxide. Whereas the expression of RAS2val19 completely reversed the protective effect of the deletion of SCH9, it only had a minor effect on the reversal of the protective effect of tor1Δ (Fig. 1B). This is an important difference because it suggests that it may be risky to achieve DSR by inhibiting intracellular targets such as Tor, which may be equally effective in protecting cancer cells.
Short-Term Starvation Induces Differential Stress Resistance in Yeast.
We also tested whether DSR would also be effective against a high concentration of drugs used in chemotherapy. We studied the effect of SCH9 mutations on the toxicity caused by the alkylating agents methylmethane sulfonate (MMS) and cyclophosphamide (CP; widely used in cancer treatment) (19). CP is a prodrug, which must be metabolically activated, mainly in the liver, into its DNA alkylating cytotoxic form. CP treatments have also been shown to increase the generation of reactive oxygen species (ROS) and oxidative DNA damage (8-hydroxyguanosine) in human granulosa cells (20) and to induce oxidative stress and lipid peroxidation as well as GSH reduction (21). As a very simple model system to understand the differential effect of STS on the mixture of normal and cancer cells observed in mammals with metastatic cancer, we mixed in the same flask mutants lacking SCH9 with mutants lacking SCH9 but also expressing RAS2val19 at a 25:1 ratio and exposed them to chronic treatment with CP or MMS. This ratio was selected to be able to start with 10 million RAS2val19-expressing cells while maintaining a relatively high ratio of normal vs. oncogene homolog-expressing cells. The monitoring of the viability of the two mixed populations was possible because each population could be distinguished by the ability to grow on plates containing different selective media. Of the ≈10 million sch9ΔRAS2val19 cells mixed with 250 million sch9Δ cells, <5% of the sch9ΔRAS2val19 cells survived a 48-h treatment with 0.01% MMS (Fig. 1C). By contrast, the great majority of sch9Δ cells survived this treatment (Fig. 1C). Similar results were obtained when mixed cultures of sch9ΔRAS2val19/sch9Δ were treated with CP (Fig. 1D). We also performed an experiment in which each cell type was treated with CP separately and observed a similar DSR between cells expressing RAS2val19 and the cells lacking SCH9 [supporting information (SI) Fig. S1]. Again, in all of the experiments above, the yeast cells are maintained under nondividing conditions, which rules out a role for differential cell division in the difference in stress resistance between the various strains.
Taken together, these results confirm that the overexpression/constitutive activation of oncogene homologs prevents the up to 1,000-fold increase in resistance to oxidative stress or chemotherapy drugs induced by starvation and/or mutations.
Glucose Restriction Protects Primary Glia but Not Cancer Cells Against Oxidative Damage.
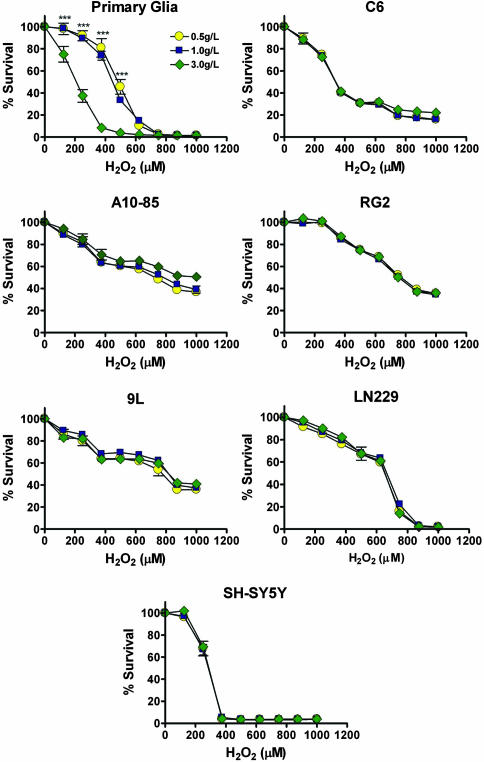
Next, we tested whether STS could also induce DSR against oxidative stress in mammalian cells. We tested primary rat mixed glial cells (astrocytes plus 5–10% microglia), four different rat glioma cell lines (C6, RG2, A10-85, and 9L), one human glioma cell lines (LN229), and one human neuroblastoma cell lines (SH-SY5Y). The concentration of glucose in the media was reduced to mimic STS. The normal physiological blood glucose level for both mice and humans is ≈1.0 g/liter but can reach 0.5 g/liter after starvation. Therefore, we tested the effect of normal glucose (1.0 g/liter), low glucose (0.5 g/liter), and high glucose (3.0 g/liter) on oxidative stress. All cell lines were grown until confluence to minimize proliferation and differences in proliferation between the primary and cancer cells and then switched to medium containing different glucose concentrations with 1% serum. Low serum was used to minimize the addition of serum glucose, which is ≈1.0 g/liter. After a 24-h glucose treatment, cells were challenged with two different oxidants, H2O2 and menadione, for 24 h. In primary glial cells, STS enhanced resistance against H2O2 (0–625 μM), although the effect was more pronounced at 375 μM H2O2 where 80% of the cells pretreated with normal and low glucose were resistant while <10% of cells pretreated with high glucose survived (P < 0.001). However, cytotoxicity of H2O2 toward cancer cells was unaffected by varying glucose concentrations (Fig. 2). Although a reduction in glucose concentration only partially protected primary glial cells treated with menadione, it increased the toxicity of menadione to most cancer cell lines. Thus, STS was still effective in generating DSR to menadione, although the differential resistance was created by a small protection of normal cells but a sensitization of cancer cells (Fig. S2).
Fig. 2.
In vitro DSR to H2O2 treatment. Primary rat glial cells, rat glioma cell lines (C6, A10-85, RG2, and 9L), a human glioma cell line (LN229), and a human neuroblastoma cell line (SH-SY5Y) were tested for glucose restriction-induced DSR. Cells were incubated in low glucose (0.5 g/liter, STS), normal glucose (1.0 g/liter), or high glucose (3.0g/liter), supplemented with 1% serum, for 24 h. Viability (MTT assay) was determined after a 24-h treatment with 0–1,000 μM H2O2. All data are presented as means ± SD. P values were calculated with Student's t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; of 0.5 and 1.0 g/liter vs. 3.0 g/liter glucose).
Glucose Starvation Protects Primary Glia but Not Cancer Cells Against Cyclophosphamide.
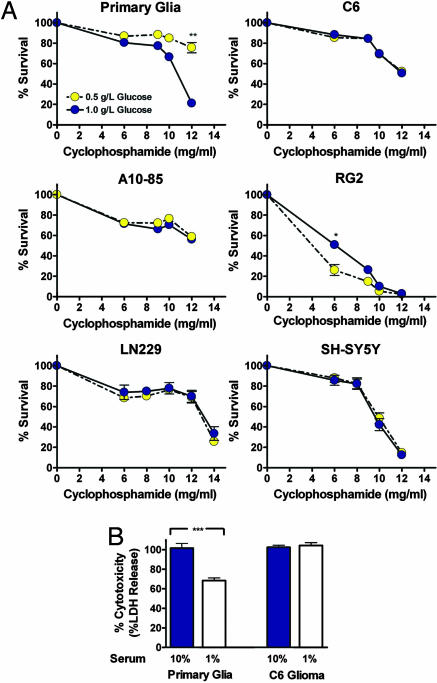
To test the efficacy of the starvation-based DSR method against a chemotherapy drug/pro-oxidant in mammalian cells, we incubated primary rat mixed glial cells (astrocytes plus 5–10% microglia), three different rat glioma cell lines, one human glioma cell line, and one human neuroblastoma cell line in medium containing low serum and either normal (1.0 g/liter) or low (0.5 g/liter) glucose and then treated them with CP for 10 h. All cell lines were grown until confluence to minimize proliferation and differences in proliferation. Although 80% of glial cells were resistant to 12 mg/ml CP in the presence of 0.5 g/liter glucose, only 20% of the cells survived this treatment in 1.0 g/liter glucose (Fig. 3A). The increased stress resistance at the lower concentration of glucose (0.5 g/liter) was observed starting at 6 mg/ml CP but became much more pronounced at 12 mg/ml CP (Fig. 3A). By contrast, the lower glucose concentration did not increase the resistance of cancer cell lines including C6, A10-85, RG2 rat glioma, LN229 human glioma, or human SH-SY5Y neuroblastoma cells to 12–14 mg/ml CP (Fig. 3A). The lower glucose concentration actually decreased the resistance of RG2 glioma cells to CP at 6 and 8 mg/ml doses (Fig. 3A). To determine whether the DSR is affected by the high cell density, we also repeated this experiment with cells that were only 70% confluent, and obtained similar results (Fig. S3).
Fig. 3.
In vitro DSR to CP treatments. Primary rat glial cells, rat glioma cell lines (C6, A10-85, and RG2), a human glioma cell line (LN229), and a human neuroblastoma cell line (SH-SY5Y) were tested. (A) Glucose restriction-induced DSR. Cells were incubated in either low glucose (0.5 g/liter, STS) or normal glucose media (1.0 g/liter), supplemented with 1% serum, for 24 h. Cells were then treated with CP (6–12 mg/ml) for 10 h, and viability was determined (MTT assay) (n = 9). (B) Serum restriction-induced DSR. Cells were incubated in medium containing either 1% (STS) or 10% serum for 24 h, followed by a single CP treatment (15 mg/ml) for 10 h. Cytotoxicity was determined by the LDH assay (n = 12). All data are presented as means ± SD. P values were calculated with Student's t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
The experiments above were performed in medium containing 1% serum and different concentrations of glucose. We also tested the effect of only reducing the level of serum from the standard 10% to 1% on the toxicity of high-dose CP. Treatment with 15 mg/ml CP was toxic to primary glial cells in 10% serum, but the switch to 1% serum caused a reduction in toxicity (Fig. 3B). By contrast, the same concentration of CP was as toxic to C6 glioma cells in 10% serum as it was in 1% serum (Fig. 3B).
These results strongly suggest that STS achieved by lowering the concentration of glucose or other nutrients/factors contained in serum can be very effective in protecting normal but not cancer cells against chemotherapy. In some cases, low glucose/serum even increased toxicity to cancer cells.
Short-Term Starvation Induces Differential Stress Resistance Against Oxidative Stress/Chemotherapy in Mice.
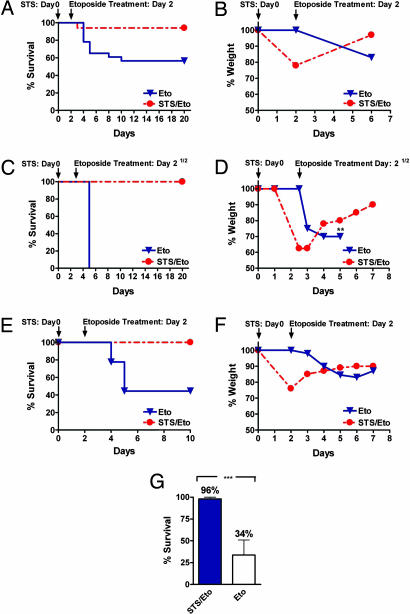
We examined whether STS could also enhance resistance of mice against etoposide, a widely used chemotherapy drug that damages DNA by multiple mechanisms and displays a generalized toxicity profile ranging from myelosuppression to liver and neurologic damage (22–24). Furthermore, etoposide has been reported to increase the production of ROS in human glioblastoma cells, leading to cellular apoptosis possibly mediated by p53 (25), and to increase the production of ROS and MnSOD expression in myeloid leukemia cells (26). We administered an unusually high dose of etoposide (80 mg/kg) to A/J mice that had been starved for 48 h. In humans, one-third of this concentration of etoposide (30–45 mg/kg) is considered to be a high dose and therefore in the maximum allowable range (27). Whereas 80 mg/kg etoposide killed 43% of control mice by day 10 (Eto, n = 23, two experiments), only one of the mice that were prestarved (STS/Eto, n = 17) died after etoposide treatment (Fig. 4A; P < 0.05). A/J mice were considered to be survivors if they were alive at day 20. Remarkably, STS-pretreated mice, which lost 20% of their weight during the 48 h of starvation, regained most of the weight in the 4 days after chemotherapy (Fig. 4B), whereas in the same period, the control mice lost ≈20% of their weight (Fig. 4B). Control mice treated with etoposide showed signs of toxicity including reduced mobility, ruffled hair, and hunched back posture (Fig. S4B), whereas STS-pretreated mice showed no visible signs of stress or pain after etoposide treatment (Fig. S4A).
Fig. 4.
Short-term starvation protects against high-dose chemotherapy in vivo. (A) A/J mice were treated (i.v.) with 80 mg/kg etoposide with (STS/Eto, n = 17) or without (Eto, n = 23) a prior 48-h starvation (STS). (B) Percent weight loss (a measure of toxicity) after etoposide treatment in STS-treated (n = 17) or untreated (n = 23) A/J mice. (C) CD-1 mice were treated (i.v.) with 110 mg/kg etoposide with (STS/Eto, n = 5) or without (Eto, n = 5) a 60-h prior starvation. (D) Percent weight loss after etoposide treatment in STS-treated (n = 5) or untreated (n = 5) CD-1 mice. Asterisks indicate the day at which all mice died of toxicity. (E) Athymic (Nude-nu) mice were treated (i.v.) with 100 mg/kg etoposide with (STS/Eto, n = 6) or without (Eto, n = 9) a 48-h prior starvation. (F) Percent weight loss after etoposide treatment in the treated (STS/Eto, n = 6) or untreated (Eto, n = 9) athymic (Nude-nu) mice. (G) Comparison of survival of all of the mice that were either prestarved (STS/Eto) or not (Eto) before etoposide injection. The survival of all STS-treated (n = 28) and untreated (n = 37) mice from all genetic backgrounds above (A/J, CD1, and Nude-nu) has been averaged (***, P < 0.05).
We also tested the effect of STS on the protection of mice of another genetic background (CD-1). To determine whether an extended STS strategy can be effective against a higher dose of etoposide, we administered 110 mg/kg etoposide and also increased the starvation period to 60 h. Based on our previous experiments with resistance to oxidative stress, we determined that this period is the maximum STS that provides protection to mice. Longer starvation periods can weaken the animals and have the opposite effect (data not shown). This concentration of etoposide killed all of the control mice (Eto) by day 5 but none of the STS-pretreated mice (STS/Eto, n = 5) (Fig. 4C; P < 0.01). CD-1 mice were considered to be survivors if they were alive at day 20. As with the A/J mice, prestarved CD-1 mice lost 40% of the weight during the 60 h of starvation but regained nearly all of the weight in the week after the etoposide treatment and showed no visible signs of toxicity (Fig. 4D).
The effect of our STS-based method was similar in athymic (Nude-nu) mice, which are widely used in cancer research because they allow the study of human tumors in the mouse model. Whereas 100 mg/kg etoposide killed 56% of the nude mice by day 5 (n = 9), none of the STS/Eto-treated mice (48-h starvation) died (n = 6) (Fig. 4E; P < 0.05). Nude mice were considered to be survivors if they were alive at day 10. As observed with the other two genetic backgrounds, the prestarved mice gained weight during the period in which the Eto-treated mice lost weight (Fig. 4F).
In summary, of 28 mice from three genetic backgrounds that were starved for 48–60 h before etoposide treatment, only one mouse died (Fig. 4G). By contrast, of the 37 mice treated with etoposide alone, 20 died of toxicity (Fig. 4G). These results are consistent with our yeast and glia/glioma data showing increased resistance to oxidative damage and chemotherapy toxicity in response to starvation.
Short-Term Starvation Prevents the Death of Mice but Not of Injected Cancer Cells Treated with High-Dose Etoposide.
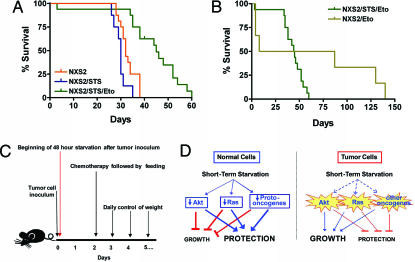
To determine whether the DSR observed in yeast and mammalian cells would also occur in vivo, we followed the survival of mice injected with cancer cells and treated with etoposide (Fig. 5C). We selected a particularly aggressive tumor line (NXS2) that models neuroblastoma (NB), the most common extracranial solid tumor and the first cause of lethality in preschool-age children. Advanced NB patients, who represent ≈50% of the cases, show metastatic dissemination at diagnosis and have a long-term survival rate of only 20% despite aggressive chemotherapy with autologous hematopoietic stem cell support (28, 29).
Fig. 5.
DSR in mice. (A and B) Survival of neuroblastoma (NXS2)-bearing mice. All mice were inoculated (i.v.) with 200,000 NXS2 cells per mouse. The different groups were treated as follows: NXS2 (control group, 16 mice), i.v. inoculation with NSX2 tumor cells on time 0; NXS2/STS (STS, 8 mice), i.v. inoculation with NSX2 tumor cells at time 0 followed by a 48-h starvation; NXS2/STS/Eto (STS/Eto, 16 mice), i.v. inoculation with NSX2 tumor cells at time 0, followed by a 48-h starvation, followed by an i.v. injection with 80 mg/kg etoposide and feeding at 48 h; NXS2/Eto (Eto, 6 mice, two deaths caused by the injection procedure), i.v. inoculation with NSX2 tumor cells at time 0, followed by an i.v. injection of 80 mg/kg etoposide at 48 h. The survival period of the NXS2 (control) and NXS2/STS/Eto groups was significantly different (P < 0.001), whereas that of the NXS2 (control) and Eto groups was not (P = 0.20). In addition, the survival periods of the NXS2/STS/Eto and NXS2/Eto groups were not significantly different (P = 0.12). (C) Procedure for the in vivo experiment. (D) Model for DSR in response to STS. In normal cells, downstream elements of the IGF1 and other growth factor pathways, including the Akt, Ras, and other proto-oncogenes, are down-regulated in response to the reduction in growth factors caused by starvation. This down-regulation blocks/reduces growth and promotes protection to chemotherapy. By contrast, oncogenic mutations render tumor cells less responsive to STS because of their independence from growth signals. Therefore, cancer cells fail to or only partially respond to starvation conditions and continue to promote growth instead of protection against oxidative stress and high-dose chemotherapy.
The NXS2 neuroblastoma line in mice induces consistent and reproducible metastases in a pattern that resembles the clinical scenario observed in neuroblastoma patients at advanced stages of the disease (31). Experimental metastases in the liver, kidneys, adrenal gland, and ovaries were observed after 25–30 days of the inoculation with 200,000 NXS2 cells (Table S1) as described in ref. 31. The tumor development and survival of the NXS2/STS/Eto group was significantly different from that of the NXS2 group (P < 0.001; Fig. 5A and Table S1), indicating that STS was highly effective in protecting the mice but only provided partial protection to cancer cells against etoposide. In fact, at least 50% of the NXS2/STS/ETO mice lived 10–20 days longer compared with the NXS2 mice (P < 0.05; Fig. 5A). Considering that it takes the cells <30 days to go from the injected 200,000 to the metastasis that kill the mouse, this 10- to 20-day-longer survival indicates that many and possibly the majority of the cancer cells have died. As also shown in Fig. 4, ≈50% of the mice treated with etoposide in the absence of STS died of chemotherapy toxicity, but the few mice that survived died of cancer between day 80 and day 140, confirming that STS also partially protects cancer cells (Fig. 5B). Naturally, the high initial toxicity in the etoposide-alone group would prevent the use of high-dose etoposide in the absence of STS.
In summary, these results suggest that STS greatly improves early survival by ameliorating chemotherapy toxicity but reduces the effect of a highly toxic dose of etoposide on metastases and cancer-dependent death by partially protecting NXS2 cells. However, the improved survival of the NXS2/STS/ETO compared with the NXS2 group suggests that STS allows the etoposide to kill a major portion of the cancer cells or slows their growth and ability to form lethal metastases. Notably, the increased survival observed in the NXS2/STS/ETO group is unlikely to be due to slower cancer growth because STS is only performed for the initial 48 h, whereas it takes 35–60 days for metastasis to cause mortality.
Because a significant survival extension was obtained with a single treatment with high-dose etoposide after STS and considering that the STS-pretreated mice did not show signs of toxicity during the initial chemotherapy treatment, these results suggest that multiple treatments with high-dose chemotherapy in combination with STS have the potential to kill most or all cancer cells without causing significant toxicity to the host. Our attempts to perform weekly injections of etoposide in combination with STS were discontinued because of tail damages caused by the multiple i.v. injections. Thus, future experiments will be necessary to develop a paradigm that allows the testing of the effect of multiple STS/Eto cycles on metastatic cancer.
Discussion
The data above indicate that STS protects normal cells and mice but not a variety of cancer cells treated with ROS or certain chemotherapy drugs that are also implicated in the generation of ROS. In yeast, worms, and mice, starvation or the genetic manipulation of starvation response pathways causes a major increase in life span and protection against multiple stresses including heat shock and oxidative damage. In mammals, starvation causes a reduction in IGF1 signaling, which is associated with increased stress resistance (5). For example, CR protects mice against liver cell death caused by acetaminophen (9) and against carcinogen-induced cancer (11). Furthermore, CR protects against the development of spontaneous tumors in mice (12, 31).
Here, we show that yeast Ras and Sch9, orthologs of components of two of the major oncogenic pathways activated by IGF1, regulate starvation-dependent resistance to oxidants or alkylating agents. As anticipated based on the constitutive activation of pathways that included homologs of yeast Ras and Sch9 in cancer cells, starvation (STS) was highly effective in protecting mammalian cells and mice but not cancer cells against the toxicity of chemotherapy drugs including oxidants and alkylating agents. Although we have not investigated the role of IGF1 in the mediation of DSR in mammalian cells and mice, others have shown a 40% decrease in IGF1 in CD-1 mice that were starved for 36 h (32), raising the possibility that decreasing IGF1 signaling may mediate in part the protective effect of starvation. One of the most surprising findings of this study is the ability of mice of three different genetic backgrounds that have been starved for 48–60 h to show no visible signs of toxicity in response to doses of chemotherapy highly toxic to control animals and gain back the 20–40% of weight that was lost during starvation even in the presence of doses of etoposide that caused a 20–30% weight loss and killed >40% of the control mice. This high resistance to a drug that damages the DNA of dividing cells, particularly blood cells, would be consistent with the entry of most or all of the normally dividing cells into a high-protection/cell-cycle-arrested mode in response to the 48- to 60-h starvation (Fig. 5D). Because etoposide is rapidly excreted (up to 90% within 48 h in humans), such a “protective mode” may only need to last for a few days. Our recent results in S. cerevisiae indicate that the lack of SCH9, and to a lesser extent starvation, protected against DNA damage in cells lacking the RecQ helicase SGS1, which forms a DNA repair complex with topoisomerase III, by reducing errors during DNA repair (18). It will be important to establish whether STS or reduction of IGF1/Akt/S6K signaling can protect mammalian cells against the topoisomerase II inhibitor etoposide by similar mechanisms.
Chemotherapy treatment often relies on the combination of several DNA-damaging agents such as etoposide, CP, and doxorubicin. Although these agents are supposedly much more toxic to cancer cells than to normal cells, our in vitro studies show that CP, for example, can be as or more toxic to primary glial cells than it is to glioma cancer cells. This implies that the combination of multiple chemotherapy drugs causes massive damage not only to blood cells but also other tissues, especially at high doses. Notably, the DSR of mammalian cells to the alkylating agent CP by our starvation-response methods was <10-fold, whereas starved yeast lacking SCH9 reached a 1,000-fold higher resistance to menadione and hydrogen peroxide compared with RAS2val19-expressing yeast cells (Fig. 1). Furthermore, the 1,000-fold differential toxicity in yeast was obtained after only 30 min with hydrogen peroxide compared with the several days required for the differential toxicity of MMS or CP. Although toxic molecules such as hydrogen peroxide are not suitable for human cancer treatments, these results suggest that the identification of novel chemotherapy drugs and possibly agents that generate a high level of ROS in combination with DSR has the potential to result in an even more rapid and effective toxicity to cancer cells.
The ability to reach a 1,000-fold or much more modest differential toxicity between cancer cells and normal human cells would lead to improved therapies for many cancers. Naturally, we do not know whether such an elevated DSR can be achieved in cancer patients, but considering the results obtained with a single treatment with etoposide in mice bearing metastasis of the aggressive NXS2 neuroblastoma line that we injected, we are optimistic about the potential efficacy of multiple cycles of STS/etoposide treatment against different types of cancers.
Materials and Methods
Yeast Growth Conditions and Oxidative Stress Assays.
See methods in SI Materials and Methods and Table S2.
Cell Cultures.
See methods in SI Materials and Methods.
In Vitro Drug Treatments.
See detailed methods in SI Materials and Methods. Briefly, primary glia, glioma, or neuroblastoma cells were seeded into 96-well microtiter plates at 20,000–30,000 cells per well and incubated for 2 days. Glucose restriction was done by incubating cells in glucose-free DMEM (Invitrogen) supplemented with either low glucose (0.5 g/liter) or normal glucose (1.0 g/liter) for 24 h in 1% serum. Serum restriction was done by incubating cells in DMEM/F12 with either 10% or 1% FBS for 24 h. After STS treatments, cells were treated with H2O2 or menadione for 24 h. CP (Sigma) was used for in vitro chemotherapy studies. After STS treatments, cells were incubated with varying concentrations of CP (6–15 mg/ml) for 10 h in DMEM/F12 with 1% FBS. Glial cells have been reported to express cytochrome P450 and thus are capable of metabolizing the prodrug CP (33, 34). Survival was determined by the MTT/LDH assay (see SI Materials and Methods) and presented as percent ratio of treated to control.
In Vivo Studies in Mice.
See detailed methods in SI Materials and Methods. Briefly, to evaluate resistance to high-dose etoposide, three different genetic backgrounds—i.e., A/J, CD-1, and Nude/nude mice—were used. Six-week-old female A/J mice (Harlan) weighing 15–18 g and 4-week-old female athymic (Nude-nu) mice (Harlan) weighing 20–22 g were starved for 48 h and then i.v. injected with 80 and 100 mg/kg etoposide (Teva Pharma), respectively. Four-week-old female CD-1 mice weighing 18–20 g were starved for 60 h and then i.v. injected with 110 mg/kg etoposide. In all experiments the mice were offered food after chemotherapy and were monitored daily for weight loss and general behavior. Survival time was used as the main criterion for determining DSR.
For in vivo cancer studies, 6- to 7-week-old female A/J mice weighing 15–18 g were injected i.v. with murine neuroblastoma NXS2 cell line (200,000 per mouse), as described in ref. 30. After tumor-cell injection, some groups of animals were starved for 48 h and then i.v. treated with etoposide, administered as a single dose. Control groups (NXS2 group) of mice without diet starvation were also investigated. Treatment schedule: time 0, 200,000 NXS2 per mouse; time 0–48 h, STS; 48 h, etoposide (80 mg/kg), followed by feeding. To determine toxicity and efficacy, mice were monitored routinely for weight loss and general behavior.
Statistical Analyses.
The significance of the differences between groups in mouse experiments was determined by using Kaplan–Meier curves and Peto's log-rank test in StatDirect (CamCode). The differences were considered significant if the P value was <0.05.
Comparisons between groups in the in vitro mammalian DSR experiments were done with Student's t test using GraphPad Prism v.4.00. Comparisons were between different glucose treatment groups for a specific drug concentration. All statistical analyses were two-sided and P values <0.05 were considered significant.
Supplementary Material
Acknowledgments.
We thank Vito Pistoia (Laboratory of Oncology, Gaslini Institute) for reading the manuscript and for helpful comments, and T. Chen (University of Southern California) for providing glioma cell lines. This work was supported in part by National Institutes of Health/National Institute on Aging Grants AG20642 and AG025135 and a University of Southern California Norris Cancer Center pilot grant (to V.D.L.). L.R. is the recipient of a Fondazione Italiana per la Lotta al Neuroblastoma fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708100105/DCSupplemental.
References
- 1.Longo VD, Gralla EB, Valentine JS. J Biol Chem. 1996;271:12275–12280. doi: 10.1074/jbc.271.21.12275. [DOI] [PubMed] [Google Scholar]
- 2.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 3.Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, Le Bouc Y. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 4.Lithgow GJ, White TM, Hinerfeld DA, Johnson TE. J Gerontol. 1994;49:B270–B276. doi: 10.1093/geronj/49.6.b270. [DOI] [PubMed] [Google Scholar]
- 5.Longo VD, Finch CE. Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- 6.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 7.Fabrizio P, Liou LL, Moy VN, Diaspro A, Valentine JS, Gralla EB, Longo VD. Genetics. 2003;163:35–46. doi: 10.1093/genetics/163.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollak MN, Schernhammer ES, Hankinson SE. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 9.Harper JM, Salmon AB, Chang Y, Bonkowski M, Bartke A, Miller RA. Mech Ageing Dev. 2006;127:687–694. doi: 10.1016/j.mad.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weindruch R, Walford R. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Thomas; 1988. [Google Scholar]
- 11.Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, Barrett JC. Cancer Res. 1997;57:4667–4672. [PubMed] [Google Scholar]
- 12.Kritchevsky D. J Nutr. 2003;133(Suppl 1):3827S–3829S. doi: 10.1093/jn/133.11.3827S. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 14.Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- 15.Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, McGrew K, Longo VD. Cell. 2005;123:655–667. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 16.Kaeberlein M, Powers RW, III, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 17.Lin S-J, Defossez P-A, Guarente L. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 18.Madia F, Gattazzo C, Wei M, Fabrizio P, Burhans WC, Weinberger M, Galbani A, Smith JR, Nguyen C, Huey S, et al. J Cell Biol. 2008;180:67–81. doi: 10.1083/jcb.200707154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poole CJ, Earl HM, Hiller L, Dunn JA, Bathers S, Grieve RJ, Spooner DA, Agrawal RK, Fernando IN, Brunt AM, et al. N Engl J Med. 2006;355:1851–1862. doi: 10.1056/NEJMoa052084. [DOI] [PubMed] [Google Scholar]
- 20.Tsai-Turton M, Luong BT, Tan Y, Luderer U. Toxicol Sci. 2007;98:216–230. doi: 10.1093/toxsci/kfm087. [DOI] [PubMed] [Google Scholar]
- 21.Manda K, Bhatia AL. Cell Biol Toxicol. 2003;19:367–372. doi: 10.1023/b:cbto.0000013342.17370.16. [DOI] [PubMed] [Google Scholar]
- 22.Grunberg SM. Drugs. 1999;58(Suppl 3):11–15. doi: 10.2165/00003495-199958003-00002. [DOI] [PubMed] [Google Scholar]
- 23.Mistry AR, Felix CA, Whitmarsh RJ, Mason A, Reiter A, Cassinat B, Parry A, Walz C, Wiemels JL, Segal MR, et al. N Engl J Med. 2005;352:1529–1538. doi: 10.1056/NEJMoa042715. [DOI] [PubMed] [Google Scholar]
- 24.Vinolas N, Graus F, Mellado B, Caralt L, Estapé J. J Neurooncol. 1997;35:145–148. doi: 10.1023/a:1005835430489. [DOI] [PubMed] [Google Scholar]
- 25.Sawada M, Nakashima S, Kiyono T, Nakagawa M, Yamada J, Yamakawa H, Banno Y, Shinoda J, Nishimura Y, Nozawa Y, Sakai N. Oncogene. 2001;20:1368–1378. doi: 10.1038/sj.onc.1204207. [DOI] [PubMed] [Google Scholar]
- 26.Mantymaa P, Siitonen T, Guttorm T, Säily M, Kinnula V, Savolainen ER, Koistinen P. Br J Haematol. 2000;108:574–581. doi: 10.1046/j.1365-2141.2000.01852.x. [DOI] [PubMed] [Google Scholar]
- 27.Kroger N, Hoffknecht M, Hänel M, Krüger W, Zeller W, Stockschläder M, de Wit M, Weh HJ, Kabisch H, Erttmann R, Zander AR. Bone Marrow Transplant. 1998;21:1171–1175. doi: 10.1038/sj.bmt.1701245. [DOI] [PubMed] [Google Scholar]
- 28.De Bernardi B, Nicolas B, Boni L, Indolfi P, Carli M, Cordero Di Montezemolo L, Donfrancesco A, Pession A, Provenzi M, et al. J Clin Oncol. 2003;21:1592–1601. doi: 10.1200/JCO.2003.05.191. [DOI] [PubMed] [Google Scholar]
- 29.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, et al. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 30.Lode HN, Xiang R, Varki NM, Dolman CS, Gillies SD, Reisfeld RA. J Natl Cancer Inst. 1997;89:1586–1594. doi: 10.1093/jnci/89.21.1586. [DOI] [PubMed] [Google Scholar]
- 31.Dirx MJ, Zeegers MP, Dagnelie PC, van den Bogaard T, van den Brandt PA. Int J Cancer. 2003;106:766–770. doi: 10.1002/ijc.11277. [DOI] [PubMed] [Google Scholar]
- 32.O'Sullivan U, Gluckman PD, Breier BH, Woodall S, Siddiqui RA, McCutcheon SN. Endocrinology. 1989;125:2793–2794. doi: 10.1210/endo-125-5-2793. [DOI] [PubMed] [Google Scholar]
- 33.Geng J, Strobel HW. Brain Res. 1998;784:276–283. doi: 10.1016/s0006-8993(97)01346-2. [DOI] [PubMed] [Google Scholar]
- 34.Kempermann G, Knoth R, Gebicke-Haerter PJ, Stolz BJ, Volk B. J Neurosci Res. 1994;39:576–588. doi: 10.1002/jnr.490390509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.