Abstract
The mammalian target of rapamycin (mTOR) assembles a signaling network essential for the regulation of cell growth, which has emerged as a major target of anticancer therapies. The tuberous sclerosis complex 1 and 2 (TSC1/2) proteins and their target, the small GTPase Rheb, constitute a key regulatory pathway upstream of mTOR. Phospholipase D (PLD) and its product phosphatidic acid are also upstream regulators of the mitogenic mTOR signaling. However, how the TSC/Rheb and PLD pathways interact or integrate in the rapamycin-sensitive signaling network has not been examined before. Here, we find that PLD1, but not PLD2, is required for Rheb activation of the mTOR pathway, as demonstrated by the effects of RNAi. The overexpression of Rheb activates PLD1 in cells in the absence of mitogenic stimulation, and the knockdown of Rheb impairs serum stimulation of PLD activation. Furthermore, the overexpression of TSC2 suppresses PLD1 activation, whereas the knockdown or deletion of TSC2 leads to elevated basal activity of PLD. Consistent with a TSC-Rheb-PLD signaling cascade, AMPK and PI3K, both established regulators of TSC2, appear to lie upstream of PLD as revealed by the effects of pharmacological inhibitors, and serum activation of PLD is also dependent on amino acid sufficiency. Finally, Rheb binds and activates PLD1 in vitro in a GTP-dependent manner, strongly suggesting that PLD1 is a bona fide effector for Rheb. Hence, our findings reveal an unexpected interaction between two cascades in the mTOR signaling pathways and open up additional possibilities for targeting this important growth-regulating network for the development of anticancer drugs.
Keywords: rapamycin, tuberous sclerosis complex, phosphatidic acid
Mammalian target of rapamycin (mTOR) is a Ser/Thr kinase serving a pivotal role in the regulation of cell growth and proliferation by mediating nutrient availability and mitogenic signals (1–3). The best characterized downstream effectors of the rapamycin-sensitive mTOR signaling complex (mTORC1) are the ribosomal S6 kinase 1 (S6K1) and the eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), both of which are critically involved in the regulation of protein synthesis in response to mitogenic stimuli, nutrient sufficiency, and cellular energy levels (1–3). The lipid second messenger phosphatidic acid (PA) has been found to mediate mitogenic activation of mTOR signaling (4, 5). As one of the enzymes responsible for the production of cellular PA, phospholipase D (PLD) is an upstream regulator in the mTOR pathway in both mitogenesis (6, 7) and the mechanical stimulation of skeletal muscle growth (8).
PLD generates PA and choline by hydrolyzing phosphatidylcholine (PC) in response to a variety of stimuli (9, 10). Two major isoforms of PC-specific PLD have been identified in mammals, namely PLD1 and PLD2. PLD1 can be directly activated by three families of proteins, the conventional protein kinase C (cPKC), Rho GTPases (RhoA, Cdc42, and Rac1), and ARF GTPases (9, 10), whereas PLD2 exhibits a high basal activity. Previously, we have shown that PLD1 is required for mTOR signaling and cell size regulation and that Cdc42 activates S6K1 by regulating PLD1 (6).
The tumor suppressors tuberous sclerosis complex 1 and 2 (TSC1/2) are negative regulators of mTOR signaling in the control of cell size and cell cycle progression (11, 12). The GTPase-activating protein (GAP) activity of TSC2 targets the small G protein Rheb (Ras homologue enriched in brain), which activates mTOR signaling and regulates cell size (11, 12). The TSC/Rheb pathway appears to be a signal integrator at which multiple inputs including cellular energy levels, growth factors, and hypoxia converge (3). Despite the initial speculation of the involvement of nutrient signals, TSC1/2 was not found to be absolutely essential in mediating amino acid sufficiency signals (13–15). The mTOR-associating protein Raptor may mediate nutrient-sensing signals (16), and hVps34, a class III PI3K, has also emerged as a component of the amino acid-regulated mTOR pathway parallel to TSC/Rheb (13, 17). The current model of Rheb activation of mTOR involves direct interaction between these two proteins independent of Rheb GTP loading and activation of mTOR kinase by Rheb in a GTP-dependent manner (14, 18). In this study, we have identified another mechanism of Rheb action in the amino acid-sensing mTOR signaling pathway. We find that PLD1 is required for Rheb activation of mTORC1 and that the TSC/Rheb pathway and amino acid sufficiency are upstream regulators of PLD in cells. Furthermore, we present evidence that Rheb binds and activates PLD1 in vitro in a GTP-dependent manner. Our observations strongly suggest that PLD1 is a bona fide effector for Rheb in the amino acid-sensing mTOR signaling pathway.
Results
PLD1 Is Required for Rheb Activation of mTOR Signaling.
To examine the relationship between TSC/Rheb and PLD pathways, which are both upstream regulators of mTORC1 signaling, we probed the requirement of PLD in Rheb-mediated S6K1 activation by using 1-butanol, an inhibitor of PA production through PLD. We reported previously (4) that 1-butanol at low concentrations (e.g., 0.5%) inhibits serum-stimulated S6K1 activation by ≈60%. As shown in Fig. 1A, Rheb-stimulated S6K1 activation, as well as T389-S6K1 phosphorylation, in HEK293 cells was also inhibited by 1-butanol, the degree of which was comparable to that of inhibition of serum stimulation. As a negative control, 2-butanol had minimal effect. This observation suggested that PLD might be involved in the activation of S6K1 by Rheb. However, the relatively nonspecific nature of 1-butanol as an inhibitor called for more definitive evidence for the involvement of PLD.
Fig. 1.
PLD1 is required for Rheb activation of S6K1. (A) HEK293 cells were transfected with Myc-S6K1 with or without FLAG-Rheb for 24 h and serum-starved overnight. Where indicated, cells were pretreated with 0.5% 1-butanol (1-BtOH) or 2-butanol (2-BtOH) for 30 min before lysis. Myc-S6K1 was immunoprecipitated from cell lysates and subjected to S6 kinase assays in vitro. (B and C) HEK293 cells were infected with lentiviruses expressing two independent shRNAs for PLD1 (B), PLD2 (C), or a scrambled sequence (Scram) as a negative control. The infected cells were selected with puromycin, transfected with Flag-Rheb or pCDNA3 (empty vector), serum-starved, and PA-stimulated. The cell lysates were analyzed by Western blotting. Quantitative RT-PCR was performed to quantify knockdown efficiency of PLD2.
Of the two known mammalian PLD isoforms, we previously showed that PLD1 is required for serum-stimulated S6K1 activation in HEK293 cells (6), although PLD2's role in mTOR signaling is also possible (7, 19). To test whether either PLD mediates Rheb activation of S6K1, we knocked down PLD1 and PLD2 separately in HEK293 cells via lentivirus-mediated shRNA expression. As shown in Fig. 1B, knockdown of PLD1 led to a reduction of S6K1 activation (as measured by T389 phosphorylation) in response to Rheb overexpression. The different levels of PLD1 knockdown resulting from the two independent shRNAs correlated closely with the degrees of reduction of S6K1 activation upon Rheb overexpression, confirming the knockdown specificity. Exogenous PA, the product of PLD, rescued T389 phosphorylation in the presence of PLD1 knockdown (Fig. 1B, last two lanes), suggesting that the function of PLD1 in Rheb activation of S6K1 is most likely dependent on the phospholipase activity. In contrast, knockdown of PLD2 by two independent shRNAs had no effect on Rheb activation of S6K1 (Fig. 1C). Although we could not directly assess the degree of PLD2 protein knockdown due to the lack of an effective antibody and/or low abundance of PLD2 in these cells, the mRNA levels of PLD2 were reduced by 90% and 60%, making it highly likely that the protein would be dramatically reduced based on our experience with knockdown of many other genes. Taken together, these results strongly suggest that PLD1, but not PLD2, is required for Rheb-mediated S6K1 activation through the production of PA. Of note, we have found that PLD1, and not PLD2, also is required for serum-stimulated mTORC1 activation in HEK293 cells (data not shown), as well as the activation of a myogenic mTOR pathway in C2C12 myoblasts (20).
Rheb Activates PLD1 in Cells.
At least two alternative possibilities existed to explain the dependence of Rheb-S6K1 activation on PLD1: Either Rheb was an upstream activator of PLD1 or PLD1 acted in parallel to Rheb in the mTOR signaling network. To probe the possibility that Rheb might be an upstream regulator of PLD1, we asked whether the overexpression of Rheb could activate PLD1. A recombinant HA-tagged PLD1 was transiently expressed in HEK293 cells together with various small G proteins, including WT Rheb, WT Cdc42, and constitutively active Cdc42 (L61), Rap1 (V12), and Rab5A (L79). As we reported previously (6), the constitutively active Cdc42-L61, but not WT Cdc42, activated PLD1 by ≈2-fold in these cells in the absence of any stimulus (Fig. 2A). Significantly, the overexpression of WT Rheb activated PLD1 by ≈2.5-fold. Rap1-V12 and Rab5A-L79, previously shown to be unable to activate S6K1 when overexpressed in cells (21), did not have any effect on PLD1. Thus, the ability of the small G proteins to activate PLD1 correlated well with their capacity to activate S6K1.
Fig. 2.
Rheb activates PLD1 in cells. (A) HEK293 cells were cotransfected with HA-PLD1 and various epitope-tagged GTPases as indicated, followed by incubation in serum-free medium containing 3H-palmitic acid for 1 day, and in vivo PLD assays were performed as described in Materials and Methods. Expression of the recombinant proteins was confirmed by Western blotting using anti-epitope tag antibodies. (B) FLAG-Rheb (WT C181S or D60K mutant) was cotransfected with HA-PLD1 or Myc-S6K1 into HEK293 cells, followed by in vivo PLD assays and in vitro S6 kinase assays, respectively. Expression of the recombinant proteins is shown by Western blots. (C) HeLa cells were infected with lentiviruses expressing two independent shRNAs for Rheb or a scrambled sequence (Scram). The infected and puromycin-selected cells were subjected to in vivo PLD assays as described in A with or without serum stimulation for 30 min. In parallel experiments, the cell lysates were subjected to Western blot analyses. *, Student's t tests were performed to compare PLD activity to that of Scram+FBS (P < 0.01).
Two Rheb mutants, C181S and D60K, were examined for their ability to activate PLD1 when overexpressed. Mutation of the CAAX motif (C181S) presumably eliminates farnesylation of Rheb, and this mutant has been reported to have diminished capacity to activate S6K1 (22, 23). Another mutant, D60K, has been reported to be inactive toward S6K1 (24, 25). As shown in Fig. 2B, C181S significantly diminished, whereas D60K abolished Rheb's ability to activate PLD1 in cells, and the activities of these two mutants toward PLD1 correlated well with their abilities to activate S6K1. These observations are consistent with the notion that Rheb activates S6K1 via PLD1.
To further validate Rheb's role in the activation of PLD in the cell, we knocked down Rheb in HeLa cells and assessed its effect on serum activation of endogenous PLD. As shown in Fig. 2C, knockdown of Rheb by two independent shRNAs led to diminished PLD activation, accompanied by reduced T389 phosphorylation of S6K1. The effect of Rheb knockdown on S6K1 phosphorylation in HeLa cells is consistent with observations by others (26). These data suggest that the mitogenic activation of PLD in cells depends on Rheb.
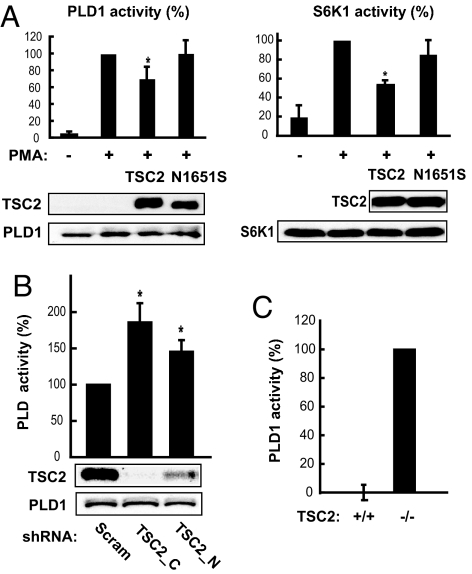
TSC2 negatively regulates mTOR signaling by acting as a GAP for Rheb. The ability of Rheb to activate PLD1 prompted us to ask whether the overexpression of TSC2 would have a negative effect on PLD1 activation. As shown in Fig. 3A, the overexpression of TSC2 diminished phorbol 12-myristate 13-acetate (PMA)-stimulated recombinant PLD1 activity and S6K1 activation. A TSC patient-derived, GAP-inactive mutant of TSC2, N1651S (23), had no inhibitory effect on PLD1, suggesting that the GAP activity of TSC2 is involved in its regulation of PLD1. The effect of TSC2 overexpression was modest probably because TSC1 is required for the stabilization and maximal function of TSC2 in cells. However, we were unable to reliably assess the effect of TSC1/TSC2 coexpression on PLD1 activation due to technical difficulties in coexpressing those three recombinant proteins. Alternatively, PMA as a stimulus may activate a significant pool of PLD1 (e.g., through cPKC) in the cells independent of the Rheb-mTOR pathway. It should be noted that we used PMA in these experiments because of its robust activation of PLD1, which allowed for the detection of the modest effect of TSC2 overexpression. Serum stimulation would be performed for all other experiments described later because serum (like insulin) is a well established mitogen for the activation of the PI3K-TSC-mTOR pathway.
Fig. 3.
TSC2 lies upstream of PLD1. (A) FLAG-TSC2 or the N1651S mutant was cotransfected with HA-PLD1 or Myc-S6K1 into HEK293 cells, followed by in vivo PLD assays and in vitro S6 kinase assays, respectively. Expression of the recombinant proteins is shown by Western blots. ∗, Student's t tests were performed to compare these data to that of PMA-stimulated cells without recombinant TSC2 expression (P < 0.01). (B) HEK293 cells were infected with lentiviruses expressing two shRNAs against TSC2, drug-selected, serum-starved, and then subjected to in vivo PLD assay. TSC2 knockdown efficiency was assessed by Western blotting. ∗, Student's t tests were performed to compare the PLD activity to that in scramble cells (P < 0.01). (C) TSC2+/+ and TSC2−/− EEF cells were transiently transfected with HA-PLD1, serum-starved, and subjected to in vivo PLD assays. The recombinant PLD1 activity was obtained by subtracting the activity of empty vector-transfected cells from that of HA-PLD1-transfected cells and then normalized against HA-PLD1 band intensity on Western blots.
To better assess the role of TSC in the potential activation of PLD by Rheb, we knocked down TSC2 in HEK293 cells and examined the cellular PLD activity. As shown in Fig. 3B, TSC2 knockdown led to a significant elevation in PLD activity in serum-starved cells, and the degree of increase was correlated with the knockdown efficiency by two independent shRNAs. Because of technical hurdles involved in quantifying recombinant PLD1 activity in TSC2 knockdown and PLD1 overexpressed cells, we took advantage of the TSC2−/− or TSC2+/+ Eker rat embryonic fibroblasts (EEFs) (27), in which we transiently expressed HA-PLD1. Under serum-starvation conditions, the recombinant PLD1 activity was undetectable in TSC2+/+ cells and was significantly activated in TSC2−/− cells (Fig. 3C). In contrast, the recombinant PLD2 activity was comparable in TSC2+/+ and TSC2−/− cells [supporting information (SI) Fig. S1]. Collectively, these observations cogently state the model that Rheb activates PLD1 in cells.
Regulation of PLD by Signals Upstream of mTOR Signaling.
Several upstream signals regulate mTORC1 by impinging on the TSC/Rheb axis, including cellular energy levels through AMPK and growth factor signaling via the PI3K/Akt pathway (3). To examine the potential roles of PI3K and the cellular energy levels on PLD activation, we performed in vivo PLD assays in HEK293 cells to test the effects of the specific PI3K inhibitor wortmannin and the AMPK activator 2-deoxyglucose on serum stimulation of PLD. As shown in Fig. 4A, serum stimulation activated cellular PLD by ≈1.5-fold, comparable to reported serum activation of PLD in other types of cells (28). This activation was dampened by wortmannin and drastically inhibited by 2-deoxyglucose; similar inhibition of S6K1 T389 phosphorylation was also observed (Fig. 4A). As expected, rapamycin had no effect on PLD activity, although it abolished S6K1 activation. These observations are consistent with PLD lying downstream of TSC/Rheb and upstream of mTOR.
Fig. 4.
PLD activity is regulated by upstream signals in the mTOR pathway. (A) HEK293 cells were serum-starved overnight, followed by in vivo PLD assays as described in Materials and Methods. The cells were stimulated by 20% FBS before lipid extraction. Some cells were pretreated with 100 nM wortmannin (Wort), 100 mM 2-deoxyglucose (2-DG), or 100 nM rapamycin (Rap) for 30 min before serum stimulation. (B) Serum-starved HEK293 cells were subjected to amino acids withdrawal for 60 min (−AA) where indicated, stimulated by 20% dialyzed FBS for 30 min in the presence (+AA) or absence of amino acids, and then subjected to in vivo PLD assays. (C) HEK293 cells expressing an shRNA for TSC2 was treated as above and subjected to in vivo PLD assays. All data shown are the average results of three to five independent experiments, with error bars representing SD. ∗, Student's t tests were performed to compare the data sets as indicated (P ≤ 0.01).
Because amino acid sufficiency is essential for mTORC1 signaling, although the exact sensing mechanism is unclear, we examined the requirement of amino acids for PLD activation. A short-term amino acid withdrawal using amino acid-free DMEM significantly suppressed serum stimulation of PLD activation in HEK293 cells, and replenishing amino acids in the deprived cells restored PLD activation (Fig. 4B). Furthermore, the enhancement of PLD activity by TSC2 knockdown also was inhibited by amino acid deprivation (Fig. 4C). These results reveal regulation of PLD by amino acid availability and suggest that PLD may partly mediate amino acid sensing in the mTOR pathway. Because exogenous PA is not able to override the amino acid requirement for S6K1 activation (4), amino acid signals likely impinge on other targets in the mTORC1 pathway in addition to PLD.
Rheb Binds PLD1.
Taken together, our observations placed PLD1 downstream of Rheb on a linear pathway. To dissect the mechanisms by which Rheb may regulate PLD1, we asked whether Rheb and PLD1 physically interact. GST pulldown assays were performed with lysates of HEK293 cells transiently expressing GST-Rheb or GST. As shown in Fig. 5A, GST-Rheb, but not GST, associated with endogenous PLD1. GST-Rheb also interacted with mTOR, as reported by others previously (14, 18).
Fig. 5.
Rheb interacts with PLD1. (A) HEK293 cells were transfected with GST or GST-Rheb for 24 h. The cell lysates were incubated with glutathione beads and analyzed by Western blotting for GST proteins and endogenous PLD1. (B) Lysates of CHO cells expressing HA-PLD1 were incubated with purified GST, GST-Cdc42, or GST-Rheb in the presence of GTPγS or GDPβS, followed by GST pulldown using glutathione beads and Western blot analyses of HA-PLD1, endogenous mTOR, and Coomassie blue staining of GST proteins. The band intensity for PLD1 was quantified by densitometry. The average results of four independent experiments are shown, with error bars representing SD. ∗, P < 0.01 by Student's t test.
To address whether GTP loading of Rheb is required for the Rheb–PLD1 interaction, in vitro GST pulldown assays were performed with bacterially expressed and purified GST fusion proteins and lysates of CHO cells expressing HA-PLD1. The GST proteins were loaded with GTPγS or GDPβS before incubation with HA-PLD1-containing lysates and subsequent glutathione pulldown. GST-Cdc42, a well characterized PLD1-binding protein, was used as a positive control. As shown in Fig. 5B, GST-Rheb pulled down PLD1 in a GTP-dependent manner. Binding of mTOR to GST-Rheb also was observed, and it was independent of GTP loading, which is consistent with previous reports (14, 18).
Rheb Activates PLD1 in Vitro.
The physical interaction between Rheb and PLD1 prompted us to ask whether Rheb could directly activate PLD1 in vitro. Stably expressed HA-PLD1 was immunoprecipitated from serum-starved cells and incubated with bacterially purified GST-Rheb in the presence or absence of GTPγS. As shown in Fig. 6A, PLD1 was markedly activated by GST-Rheb, and this activation was absolutely dependent on the presence of GTPγS. The degree of PLD1 activation by Rheb was somewhat higher than that by GST-Cdc42. Neither H-Ras nor RalA was able to activate PLD1. The lack of activation by RalA, despite its reported interaction with PLD1, is consistent with observations by others (29). Taken together, Rheb is likely a direct activator of PLD1 and is at least as potent as Cdc42.
Fig. 6.
Rheb activates PLD1 in vitro. (A) HA-PLD1 was immunoprecipitated from serum-starved CHO cells induced to express HA-PLD1. Purified GST fusion of GTPases, either unloaded or loaded with GTPγS, were added to the immunocomplex, and in vitro PLD assays were carried out as described in Materials and Methods. The relative activities are shown with that of GST-added reaction as 1; background activities (“No PLD1”) are shown and have not been subtracted from the data. The average results of three to six experiments are shown, with error bars representing SD. ∗, These data are significantly higher than that of GST when analyzed by Student's t tests (P < 0.01). (B) A proposed model for PLD1 involvement in Rheb-mTOR signaling.
Discussion
As an essential growth-regulating signaling network and an attractive target of anticancer drug development in recent years, the molecular wiring of the rapamycin-sensitive pathways is of tremendous interest and remains an intriguing puzzle. Our study described here has revealed a surprising connection between two previously established signaling pathways in this intricate network. We have demonstrated that PLD1 is required for Rheb activation of mTOR signaling to S6K1 and that the TSC/Rheb pathway controls PLD1 activation in cells. We have further provided biochemical evidence that Rheb binds and activates PLD1 in a GTP-dependent manner. In conclusion, our findings strongly suggest that PLD1 is a bona fide effector for Rheb (Fig. 6B), filling a sizable gap in our knowledge of the signal transduction by the nutrient-sensing and growth-promoting mTOR network.
Previously found to mediate mitogenic signals in the mTOR pathway (4, 6), PLD1 may also receive nutrient signals as suggested by the sensitivity of PLD1 activity to amino acid deprivation (Fig. 4 B and C). Amino acid signals appear to impinge on the mTORC1 pathway at least at two distinct levels. On the one hand, depletion of amino acids abolishes insulin-induced GTP-loading of Rheb (15). On the other hand, in the presence of activated Rheb, as found in TSC2 knockout cells, amino acid deprivation can still block mTOR signaling likely via inhibition of the hVps34 pathway (13, 17). This dual level of regulation is fully consistent with our current and previous observations: PLD activation by serum, as well as by TSC2 knockdown, requires amino acid sufficiency, yet exogenous PA cannot override the requirement for amino acids in the activation of S6K. These situations should be discriminated from experiments in which Rheb is overexpressed. Under such conditions, Rheb is fully GTP loaded and activates mTOR even in the absence of amino acids or mitogens (21, 23, 26, 30, 31). Therefore, it is not surprising that the interaction between overexpressed GST-Rheb and endogenous PLD1 (Fig. 5A) in the GST pulldown assays performed with HEK293 cell lysates was not affected by amino acid deprivation or serum starvation of the cells (Fig. S2). How exactly PLD1 receives amino acid signals is of obvious interest. Although regulation of PLD1 binding to and/or activation by the GTP loading of Rheb in cells remains a strong possibility to be further examined, a potential relationship between PLD1 and hVps34 also merits future investigation (Fig. 6B).
While this article was in its final stage of preparation, a report appeared, revealing another direct target of Rheb-FKBP38, an endogenous inhibitor of mTOR (32). Rheb is shown to bind FKBP38 in a GTP-dependent and amino acid-sensitive manner, and it activates mTOR by displacing FKBP38. Interestingly, both PA, the product of PLD, and FKBP38 bind a region of mTOR that contains the rapamycin-FKBP12-binding (FRB) domain (4, 32). It is tempting to speculate that PA also may function to displace FKBP38 from mTOR and that Rheb activation of PLD1 and binding of FKBP38 synergize to achieve complete removal of FKBP38 and maximal activation of mTOR. Alternatively, PA may be necessary for mTOR activation after FKBP38 is removed; Rheb may play a dual role in eliminating the inhibitor and supplying the activator, and the two events may take place independently, but concurrently, to activate mTOR.
Three classes of proteins directly interact with and activate PLD1: cPKC, ARF, and Rho. We have now identified Rheb as a new regulator of PLD1. PLD1 appears indispensable for Rheb activation of the mTOR pathway (Fig. 1B) and presumably cell size regulation. We found that Rheb activated PLD1 expressed and immunoprecipitated from mammalian cells, but not PLD1 purified from insect cells (data not shown), implying that a yet-to-be-identified cofactor or certain mammalian cell-specific posttranslational modification of PLD1 is required for the activation of PLD1 by Rheb. Thus, several intriguing questions remain to be addressed: Does Rheb compete for or synergize with the other known regulators in binding and activating PLD1? How does Rheb cross-talk with the other regulators in a cell to regulate PLD1 and mTOR signaling? Is Rheb involved in PLD1 functions other than cell size control, such as vesicle trafficking and cytoskeleton reorganization? These questions will guide future research efforts to reveal a clearer picture of the TSC-Rheb-PLD regulatory network.
Materials and Methods
Antibodies and Other Reagents.
The antibodies were obtained from the following sources: anti-FLAG M2 from Sigma; anti-HA (16B12) and anti-Myc (9E10.2) from Covance; anti-phospho-T389-S6K1, anti-PLD1, and anti-Rheb from Cell Signaling; anti-tubulin from Abcam; and anti-mTOR (FRB domain) was generated at the University of Illinois at Urbana–Champaign Biotechnology Center. Rapamycin and wortmannin were purchased from Calbiochem. Insulin, PMA, 2-deoxyglucose, 1-butanol, 2-butanol, polybrene, and puromycin were from Sigma. GTPγS and GDPβS trilithium salts were from Roche. [Choline-methyl-3H] dipalmitoyl-phosphatidylcholine (3H-DPPC) was from Amersham. [9,10-3H]-palmitic acid was from PerkinElmer. All other lipids were from Avanti.
Plasmids.
D60K mutation was introduced into FLAG-Rheb (see below) by using QuikChangeII Kit (Stratagene). pGEX-Rheb was generated by inserting the PCR product of Rheb into pGEX-4T. pRK7-GST-Rheb was made by inserting the PCR product of GST via XbaI and BamHI sites into pRK7-FLAG-Rheb. Myc-S6K1 and HA-PLD1 were described previously (6). The following plasmids were generous gifts from various laboratories: plasmids for mammalian expression of FLAG-tagged Rheb, Rheb-C181S, TSC1, TSC2, TSC2-N1651S, and bacterial expression vector for GST-Cdc42 were from J. Blenis (Harvard Medical School, Boston) (33). FLAG-Rab5A-L79 and FLAG-Rap1-V12 were from K. L. Guan (University of Michigan School of Medicine, Ann Arbor, MI) (21). Bacterial expression plasmids for GST-H-Ras and GST-RalA were from L. A. Quilliam (Indiana University School of Medicine, Indianapolis) (34).
Cell Culture and Transfection.
HEK293 cells were grown in DMEM containing 10% FBS at 37°C with 5% CO2. All transient transfections were carried out with PolyFect (Qiagen) when the cells were 60–70% confluent. The amount of DNA transfected was between 0.5 and 1.5 μg for each plasmid, adjusted to ensure equal expression of the recombinant protein to be compared within each experiment. Various treatments were applied to the cells 24 h after transfection. Serum starvation of cells was carried out in serum-free DMEM overnight. Amino acid deprivation was achieved by incubating cells in amino acid-free DMEM (HyClone) for 1 h. For PA stimulation of the cells, 42 μl of 1-palmitoyl 2-oleoyl phosphatidic acid in chloroform (25 mg/ml) was dried under nitrogen, resuspended in 250 μl of DPBS, and sonicated in a water bath sonicator (80 watts) for 5 min. Freshly prepared PA vesicles were added to the cell medium to a final concentration of 100 μM. CHO cells stably expressing HA-PLD1 under the control of the tetracycline promoter (a generous gift from M. Frohman, State University of New York, Stony Brook, NY) (35) were grown in F-12 medium containing 10% FBS, 10 μg/ml blasticidin, and 300 μg/ml zeocin at 37°C with 5% CO2. At the cell density of 60–70%, 1 μg/ml doxycycline was added in the medium to induce HA-PLD1 expression. TSC2+/+ and TSC2−/− EEF cells (a generous gift from R. S. Yeung, University of Washington, Seattle) (27) were cultured in DME/F-12 medium with 10% FBS under 5% CO2. Nucleofection was used to introduce DNA into the EEF cells (Amaxa nucleofector, program D23, Kit L).
Lentivirus-Mediated RNAi.
All shRNA was purchased from Sigma–Aldrich (Mission shRNA). Lentivirus packaging and infection were performed as described previously (36). See SI Materials and Methods for target sequences.
Quantitative RT-PCR.
Quantitative RT-PCR was performed as described previously (36). The primers used were: hPLD2 forward, 5′-TCCATCCAGGCCATTCTGCAC; hPLD2 reverse, 5′-CGTTGCTCTCAGCCATGTCTTG; β-actin forward, 5′-GCACTCTTCCAGCCTT CCT; and β-actin reverse, 5′-AGGTCTTTGCGGATGTCCAC.
S6 Kinase Assay.
Myc-S6K1 was immunoprecipitated from transfected cells and subjected to in vitro S6 kinase assay by using a peptide substrate as described previously (6).
In Vivo PLD Assay.
In vivo PLD activity was measured in a transphosphatidylation assay adopted from methods published previously (37, 38) and modified as described previously (20). To calculate recombinant PLD1 activity, the PLD activity in cells transfected with empty vector (pCDNA3) was subtracted from the activity in transfected cells under the same conditions. For EEF cell nucleofection, 1 × 106 cells were electroporated with 5 μg pCDNA3 or HA-PLD1, seeded into a six-well plate, and cultured for 2 days, followed by serum starvation in DME/F-12 with 0.1% FBS overnight. PLD1 activity was measured as above and normalized against HA-PLD1 band intensity on Western blots (quantified by using Image J).
GST Pulldown Assays.
GST fusions of small GTPases were purified from bacteria by using glutathione Sepharose affinity chromatography. The purified GST-GTPase proteins (5 μg each) were first stripped of nucleotides by incubation in 5 mM EDTA (in PBS) on ice for 15 min, followed by incubating with 20 mM MgCl2and 100 μM guanine nucleotide (GTPγS or GDPβS) in 30 μl for 30 min at 4°C and then for 10 min at 30°C. The nucleotide-loaded protein was then mixed with 20 μl of CHO cell lysates [lysis buffer: 40 mM Tris·Cl (pH 7.2), 1 mM Na3VO4, 25 mM NaF, 25 mM b-glycerophosphate, 2 mM EDTA, 2 mM EGTA, 1 mM DTT, 1 mM PMSF, and 0.3% Triton X-100] containing HA-PLD1 (see above) and incubated at 30°C for 30 min. Glutathione Sepharose beads were added to the mixture and gently rocked at 4°C for 1 h, followed by washing with lysis buffer and eluting in SDS sample buffer containing 8 M urea. For in vivo pulldown, GST- or GST-Rheb-transfected HEK293 cells were lysed, and the lysates were incubated with glutathione Sepharose beads, followed by washing with PBS.
In Vitro PLD Assay.
In vitro PLD assays were performed by measuring the release of [3H]choline modified from previously reported methods (39, 40). HA-PLD1 was immunoprecipitated from doxycycline-treated and serum-starved CHO cells described earlier and washed in lysis buffer with 1% Nonidet P-40. The immunocomplex was resuspended in 50 μl of reaction buffer [50 mM Hepes (pH 7.5), 3 mM EGTA, 80 mM KCl, 1 mM DTT, 2 mM CaCl2, and 3 mM MgCl2], to which 5 μg of GST fusion protein with or without GTPγS (final concentration 30 μM) were added and incubated at 37°C for 10 min. The PLD reaction was initiated by adding the substrate in the form of phospholipid vesicles composed of 1,2-dipalmitoyl-sn-glycero-3-phosphatidylethanomine, PIP2, and dipalmitoyl PC (DPPC) in a molar ratio of 16:1.4:1. [3H]DPPC was included at 20,000 cpm per assay. After incubation at 37°C for 30 min, the reaction was stopped by the addition of 200 μl of 10% trichloroacetic acid and 100 μl of 10 mg/ml BSA, followed by centrifugation at 3,000 × g for 10 min at 4°C. A 0.3-ml aliquot of the supernatant was removed and analyzed by liquid scintillation counting.
Supplementary Material
Acknowledgments.
We thank Dr. Nissim Hay for helpful discussions, Dr. Lee Henage for performing in vitro PLD assays, Dr. Troy Hornberger for helpful suggestions on PA stimulation, and Drs. John Blenis, Kun-Liang Guan, Lawrence Quilliam, and Michael Frohman for generously providing various reagents. This work was supported by grants from the National Institutes of Health and American Cancer Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712268105/DCSupplemental.
References
- 1.Fingar DC, Blenis J. Target of rapamycin (TOR): An integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 2.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 3.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 5.Foster DA. Regulation of mTOR by phosphatidic acid? Cancer Res. 2007;67:1–4. doi: 10.1158/0008-5472.CAN-06-3016. [DOI] [PubMed] [Google Scholar]
- 6.Fang Y, et al. PLD1 regulates mTOR signaling and mediates Cdc42 activation of S6K1. Curr Biol. 2003;13:2037–2044. doi: 10.1016/j.cub.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Zheng Y, Foster DA. Phospholipase D confers rapamycin resistance in human breast cancer cells. Oncogene. 2003;22:3937–3942. doi: 10.1038/sj.onc.1206565. [DOI] [PubMed] [Google Scholar]
- 8.Hornberger TA, et al. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci USA. 2006;103:4741–4746. doi: 10.1073/pnas.0600678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Exton JH. Regulation of phospholipase D. Biochim Biophys Acta. 1999;1439:121–133. doi: 10.1016/s1388-1981(99)00089-x. [DOI] [PubMed] [Google Scholar]
- 10.Frohman MA, Sung TC, Morris AJ. Mammalian phospholipase D structure and regulation. Biochim Biophys Acta. 1999;1439:175–186. doi: 10.1016/s1388-1981(99)00093-1. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Corradetti MN, Inoki K, Guan KL. TSC2: Filling the GAP in the mTOR signaling pathway. Trends Biochem Sci. 2004;29:32–38. doi: 10.1016/j.tibs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci. 2003;28:573–576. doi: 10.1016/j.tibs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Nobukuni T, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci USA. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem. 2005;280:18717–18727. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- 15.Roccio M, Bos JL, Zwartkruis FJ. Regulation of the small GTPase Rheb by amino acids. Oncogene. 2006;25:657–664. doi: 10.1038/sj.onc.1209106. [DOI] [PubMed] [Google Scholar]
- 16.Kim DH, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 17.Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem. 2005;280:33076–33082. doi: 10.1074/jbc.M507201200. [DOI] [PubMed] [Google Scholar]
- 18.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 19.Ha SH, et al. PLD2 forms a functional complex with mTOR/raptor to transduce mitogenic signals. Cell Signal. 2006;12:2283–2291. doi: 10.1016/j.cellsig.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Yoon MS, Chen J. PLD regulates myoblast differentiation through the mTOR-IGF2 pathway. J Cell Sci. 2008;121:282–289. doi: 10.1242/jcs.022566. [DOI] [PubMed] [Google Scholar]
- 21.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castro AF, Rebhun JF, Clark GJ, Quilliam LA. Rheb binds tuberous sclerosis complex 2 (TSC2) and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner. J Biol Chem. 2003;278:32493–32496. doi: 10.1074/jbc.C300226200. [DOI] [PubMed] [Google Scholar]
- 23.Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 24.Tabancay AP, Jr, et al. Identification of dominant negative mutants of Rheb GTPase and their use to implicate the involvement of human Rheb in the activation of p70S6K. J Biol Chem. 2003;278:39921–39930. doi: 10.1074/jbc.M306553200. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Inoki K, Guan KL. Biochemical and functional characterizations of small GTPase Rheb and TSC2 GAP activity. Mol Cell Biol. 2004;24:7965–7975. doi: 10.1128/MCB.24.18.7965-7975.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garami A, et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457–1466. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- 27.Jin F, et al. Suppression of tumorigenicity by the WT tuberous sclerosis 2 (Tsc2) gene and its C-terminal region. Proc Natl Acad Sci USA. 1996;93:9154–9159. doi: 10.1073/pnas.93.17.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotter K, et al. Activation of astroglial phospholipase D activity by phorbol ester involves ARF and Rho proteins. Biochim Biophys Acta. 2000;1485:153–162. doi: 10.1016/s1388-1981(00)00036-6. [DOI] [PubMed] [Google Scholar]
- 29.Luo JQ, et al. Functional association between Arf and RalA in active phospholipase D complex. Proc Natl Acad Sci USA. 1998;95:3632–3637. doi: 10.1073/pnas.95.7.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Im E, et al. Rheb is in a high activation state and inhibits B-Raf kinase in mammalian cells. Oncogene. 2002;21:6356–6365. doi: 10.1038/sj.onc.1205792. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, et al. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 32.Bai X, et al. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007;318:977–980. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- 33.Tee AR, et al. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rebhun JF, Chen H, Quilliam LA. Identification and characterization of a new family of guanine nucleotide exchange factors for the ras-related GTPase Ral. J Biol Chem. 2000;275:13406–13410. doi: 10.1074/jbc.c000085200. [DOI] [PubMed] [Google Scholar]
- 35.Xiao N, Du G, Frohman MA. Peroxiredoxin II functions as a signal terminator for H2O2-activated phospholipase D1. FEBS J. 2005;272:3929–3937. doi: 10.1111/j.1742-4658.2005.04809.x. [DOI] [PubMed] [Google Scholar]
- 36.Kim JH, Kim JE, Liu HY, Cao W, Chen J. Regulation of IL-6 induced hepatic insulin resistance by mtor through the STAT3-SOCS3 pathway. J Biol Chem. 2007;283:708–715. doi: 10.1074/jbc.M708568200. [DOI] [PubMed] [Google Scholar]
- 37.Morris AJ, Frohman MA, Engebrecht J. Measurement of phospholipase D activity. Anal Biochem. 1997;252:1–9. doi: 10.1006/abio.1997.2299. [DOI] [PubMed] [Google Scholar]
- 38.Du G, et al. Dual requirement for rho and protein kinase C in direct activation of phospholipase D1 through G protein-coupled receptor signaling. Mol Biol Cell. 2000;11:4359–4368. doi: 10.1091/mbc.11.12.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown HA, Gutowski S, Moomaw CR, Slaughter C, Sternweis PC. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- 40.Rudge SA, Morris AJ, Engebrecht J. Relocalization of phospholipase D activity mediates membrane formation during meiosis. J Cell Biol. 1998;140:81–90. doi: 10.1083/jcb.140.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.