Abstract
The expression of Ig-like transcript (ILT) inhibitory receptors is a characteristic of tolerogenic dendritic cells (DCs). However, the mechanisms of modulation of DCs via ILT receptors remain poorly defined. HLA-G is a preferential ligand for several ILTs. Recently, we demonstrated that triggering of ILT4 by HLA-G1 inhibits maturation of human monocyte-derived conventional DCs and murine DCs from ILT4 transgenic mice, resulting in diminished expression of MHC class II molecules, CD80 and CD86 costimulatory molecules, and prolongation of skin allograft survival. Different isoforms of HLA-G have diverse effects on the efficiency to induce ILT-mediated signaling. In this work, we show that HLA-G1 tetrameric complex and HLA-G5 dimer, but not HLA-G5 monomer, induce strong ILT-mediated signaling. We determined that the arrest of maturation of ILT4-positive DCs by HLA-G ligands involves the IL-6 signaling pathway and STAT3 activation. Ligation of ILT4 with HLA-G on DCs results in recruitment of SHP-1 and SHP-2 protein tyrosine phosphatases. We propose a model where SHP-2 and the IL-6–STAT3 signaling pathway play critical roles in the modulation of DC differentiation by ILT4 and HLA-G.
Keywords: immunomodulation, inhibitory receptor
Dendritic cell (DC) maturation and activation are pivotal events in the control of innate and adaptive immunity. Myeloid DCs develop from CD11c+ precursor cells and undergo maturation and activation in response to several stimuli, such as pathogen-associated products [e.g., lipopolysaccharide (LPS)], endogenous “danger” signals (e.g., TNF-α), and activated T cells. DC maturation typically is characterized by up-regulation of MHC class II and costimulatory molecules and by production of IL-12 (1–3). Ig-like transcripts (ILTs, also referred to as LILR, LIR, and CD85) represent an Ig type of activating and inhibitory receptors that are involved in regulation of immune cell activation and control the function of immune cells. ILT2 and ILT4 receptors, the most characterized immune inhibitory receptors, are expressed predominantly on myeloid DCs (4–6). The expression of ILTs on DCs is tightly regulated by inflammatory stimuli, cytokines, and growth factors and is down-regulated after DC activation (7). The preferential ligand for most of the ILTs is HLA-G. HLA-G is characterized by tissue-restricted expression and limited polymorphisms and is found in seven isoforms (HLA-G1 to HLA-G7, of which HLA-G5, HLA-G6, and HLA-G7 are soluble isoforms) (8–11). Recently, HLA-G dimers were discovered when recombinant HLA-G protein was generated in vitro (12). Dimers were then observed on the surface of transfected cells (12, 13), choriocarcinoma JEG-3 cells (13, 14), and normal trophoblast cells (15). The role of these trophoblastic HLA-G dimers in the modulation of maternal immunocompetent cells, especially DCs, remains unknown. Therefore, evaluation of the efficiency of the different forms of HLA-G to induce inhibitory signaling will provide crucial information to understand the molecular and cellular mechanisms of modulation of DCs and to design better strategies for targeting immune responses. Ligation or cross-linking of inhibitory ILT receptors on transfected RBL cells resulted in tyrosine phosphorylation of receptors and recruitment of the SH2-containing tyrosine phosphatase SHP-1 (16). The existence of three potential consensus sequences for phosphorylation by Src family tyrosine kinases in the cytoplasmic tail of ILT4 suggested that molecules different from SHP-1 might interact with this receptor in a phosphotyrosine-dependent manner, especially in additional signaling pathways such as activation/differentiation of DCs. SHPs are involved in regulation of cytokine production and the cytokine-receptor signaling pathway. Generally, SHP-1 and SHP-2 perform opposing roles in signaling processes; SHP-1 acts as a negative regulator of transduction in hematopoietic cells, whereas SHP-2 acts as a positive regulator. It has been recently discovered that SHP-2 acts to promote IL-6 production via the NF-κB pathway in a mitogen-activated protein (MAP) kinase-independent manner (17). The maintenance of immature or tolerogenic DCs is mediated by several factors, and IL-6 plays a major role in the control of DC differentiation. It has been shown that this maintenance correlates with IL-6-induced STAT3 activation (18, 19). Naïve IL-6-deficient mice have decreased numbers of immature DCs and increased numbers of mature DCs in the lymph nodes compared with their wild-type littermates (18). These mice also have a decreased number of activated/mature DCs in the spleen after LPS stimulation. Here, we present evidence that triggering of ILT4 in vitro and in vivo by certain isoforms of HLA-G phosphorylates ILT4 receptor, recruits SHP-1 and SHP-2, up-regulates the expression of IL-6, and down-regulates the differentiation of DCs via the IL-6–STAT3 pathway.
Results
HLA-G5 Dimer and HLA-G1 Tetramer Induce Strong ILT-Mediated Signaling in Vitro.
We have analyzed the formation and induction of efficiency of ILT-mediated signaling by HLA-G5 monomer (HLA-G5m) and HLA-G5 dimer (HLA-G5d) by using supernatant from an HLA-negative cell line transfected with HLA-G5. HLA-G5 monomer was purified, and the gel filtration chromatogram showed a single peak corresponding to the expected molecular mass of 49 kDa (data not shown). It has been demonstrated that several factors, including the concentration of monomer, low temperature, or DTT, affect the dimerization of HLA-G (12–14, 20). Size exclusion chromatography of HLA-G5m incubated at 4°C for 7 days showed the presence of two peaks, one corresponding to the molecular mass of 49 kDa and the other corresponding to approximately twice that (Fig. 1A). Using an HLA-G-specific mAb we detected the presence of two bands under nonreducing conditions, one of ≈37 kDa, the expected molecular mass of the heavy chain of the soluble form of HLA-G5, and the other ≈74 kDa, the expected molecular mass of dimerized HLA-G5 heavy chain (Fig. 1B). However, only a small fraction (<10%) of the entire pool of HLA-G5 heavy chains exists in the dimerized form. The analysis of avidity of the different isoforms of HLA-G on ILT2-mediated signaling by using an NFAT-GFP reporter system showed minor stimulation of the reporter cells by HLA-G5m (Fig. 1C). Even at a high concentration of 100 ng/ml HLA-G5m, only 5.6% ± 0.86%, mean ± SD, of total cells were GFP-positive, with a mean fluorescent intensity (MFI) of 7.8 ± 0.9 (Fig. 1C). In contrast, HLA-G5d remarkably enhanced ILT2-mediated signaling at a much lower concentration (2 ng/ml), and at 100 ng/ml 56.8% ± 7.2% cells were GFP-positive (MFI = 9.9 ± 1.1), suggesting that the HLA-G5d form has more efficient signaling and similar efficacy to HLA-G1 tetrameric complexes (HLA-G1t) (Fig. 1C). Our results indicate that the dimer form of HLA-G5 and the tetrameric complexes of HLA-G1 are potent to induce the most efficient ILT-mediated inhibitory signaling.
Fig. 1.
Formation of HLA-G5 dimer in vitro and efficiency of different isoforms of HLA-G to induce ILT-mediated signaling. (A) HLA-G5 dimerizes in vitro. HLA-G5 monomer was incubated at 4°C for 7 days. A gel filtration chromatogram of HLA-G5 was analyzed by size exclusion chromatography using a Superdex 200 10/30 column. Arrows show molecular mass calibration standards. (B) Purified HLA-G5 proteins were electrophoresed under nonreducing and reducing conditions and blotted to nitrocellulose. HLA-G heavy chains were detected by using the HLA-G-specific mAb MEM-G/9. Approximate molecular masses of HLA-G5m and HLA-G5d were 37 and 74 kDa, respectively. (C) Efficiency of different isoforms of HLA-G to induce ILT2-mediated signaling. NFAT-GFP reporter cells expressing the ILT2-PILRβ chimera were stimulated with the indicated concentration of immobilized HLA-G5m, HLA-G5d, or HLA-G1t for 18 h. GFP expression on reporter cells was analyzed by flow cytometry. Numbers indicate the percentage of GFP-positive cells (Upper) and MFI of GFP (Lower). Data shown are from one of four independent experiments.
Arrest of Maturation/Activation of ILT4-Positive DCs in Vivo by Different Isoforms of HLA-G.
To examine the effect of different isoforms of HLA-G on the activation/maturation of ILT4-positive DCs in vivo, we analyzed the number of activated/mature and immature DCs in draining lymph nodes and in spleens from recipient ILT4 transgenic mice after allogeneic skin transplantation from the MHC class II-disparate mutant B6.C-H-2bm12 (bm12) donor mice at different time points. Transgenic mice expressing ILT4 receptor exclusively on DCs have been described in ref. 21. In addition, analysis of the key cytokines (IL-6, IL-10, and IL-12) involved in maturation/activation of DCs was evaluated by intracellular staining with cytokine-specific mAbs and flow cytometry. The number of mature/activated DCs that expressed high levels of MHC class II molecules and CD86 with elevated levels of IL-12 was decreased in lymph nodes from ILT4 mice targeted with HLA-G5d and HLA-G1t [supporting information (SI) Fig. S1]. In contrast, ILT4 mice treated with HLA-G5m had more activated/mature DCs and a phenotype similar to the DCs from ILT4 mice not treated with HLA-G (22). Moreover, we observed that immature DCs from mice targeted with HLA-G1t and with HLA-G5d have enhanced expression of IL-6, demonstrated by the increased numbers of cells and capacity to secrete IL-6 (Fig. S1B). These results further strengthen the hypothesis that different isoforms of HLA-G have distinct roles in suppression of maturation/activation of DCs through the ILT4 receptor.
Engagement of ILT4 by HLA-G Ligands Increases Transcriptional Levels of IL-6 in DCs.
To investigate the role of certain isoforms of HLA-G on the regulation of gene expression in ILT4-positive bone marrow-derived DCs (BMDCs), we analyzed the gene expression profiles of cells exposed to HLA-G ligands at different times by using the mouse dendritic and antigen-presenting cell Gene Array System (SuperArray Bioscience).
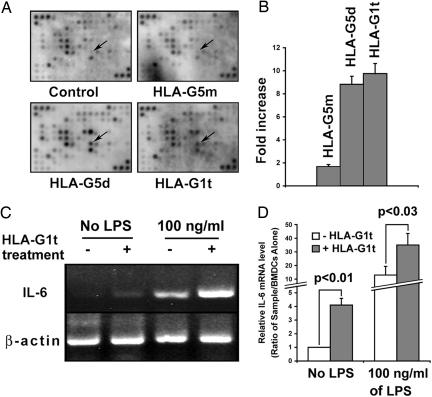
The comparison between HLA-G5m-treated and nontreated ILT4-positive DCs shows that the expression levels of most transcripts were similar (Fig. 2A). However, treatment of DCs with HLA-G5d or HLA-G1t affected several genes, the majority of which were down-regulated. These genes include chemokine ligand 3 (CCL3), myristoylated alanine-rich protein kinase C substrate (MARCKS), IFN-induced with tetratricopeptide repeats 1 (IFIT1), and histocompatibility class II locus DMa (H2-DMa). However, the expression levels of a very limited number of genes were up-regulated. These genes include guanylate nucleotide-binding protein 3 (GBP3) and CD36. The most frequent transcript was identified as IL-6 (8.9-fold and 9.3-fold increase by HLA-G5d and HLA-G1t, respectively; Fig. 2 A and B). RT-PCR analysis of ILT4-positive BMDCs revealed that IL-6 transcription can be detected in HLA-G1t-treated cells without stimulation with LPS but was considerably increased in the treated cells after maturation/activation with LPS (Fig. 2 C and D). The same results were obtained with ILT4 BMDCs treated with HLA-G5d (data not shown). These data suggest that the transcriptional level of IL-6 on ILT4-positive DCs is mediated by particular isoforms of HLA-G and can be modified by LPS signaling.
Fig. 2.
Elevated level of IL-6 transcription in BMDCs treated with HLA-G5 dimer and HLA-G1 tetramer. (A) Hybridization intensity of genes in HLA-G-treated and untreated ILT4-positive BMDCs on GEArray. BMDCs (2.5 × 105) were plated onto wells coated with 50 ng/ml HLA-G5m, HLA-G5d, or HLA-G1t. After a 3-h incubation, cells were stimulated with 100 ng/ml LPS for an additional 18 h. Total RNA was used for cDNA probe synthesis after hybridization to gene-specific cDNA fragments were spotted onto GEArray membranes. Arrows indicate the most differentially expressed gene, IL-6. Data were analyzed by ImageQuant 1.2 software (Amersham Bioscience) with STORM 840 gel and blot imaging system. The signal for each transcript was normalized by comparison with the housekeeping gene GAPDH. (B) The levels of IL-6 transcription in HLA-G-treated cells were compared with untreated cells in LPS-stimulated, ILT4-positive BMDCs. (C and D) RT-PCR analysis of ILT4-positive BMDCs treated with HLA-G1t and untreated cells after stimulation with 100 ng/ml LPS. Data represent three independent experiments.
IL-6 Neutralization Abolishes Inhibition of the Expression of MHC Class II Molecules on DCs Mediated by HLA-G and ILT4.
Bone marrow-derived immature myeloid ILT4-positive DCs were subjected to LPS stimulation for 18 h, which resulted in increased expression of MHC class II molecules (increased number of MHC class II-positive cells from 23.3% ± 2.6% to 61.3% ± 8.9%, mean ± SD, P < 0.002; Fig. S2 Top). Treatment of ILT4-positive DCs with HLA-G after stimulation with LPS decreases cell surface expression of MHC class II molecules (number of MHC class II-positive cells decreases after treatment with HLA-G1t from 61.3% ± 8.9% to 31.0% ± 4.5%, P < 0.006, Fig. S2). Additional treatment of ILT4-positive DCs with anti-IL-6 neutralizing antibody increased the number of MHC II-positive cells from 31.0% ± 4.5% to 44.1% ± 6.5%, P < 0.02, Fig. S2) and abolished the HLA-G1t-mediated inhibition of expression of MHC class II molecules on ILT4-positive DCs. A similar abolishing effect of neutralizing anti-IL-6 antibody was found on ILT4-positive DCs treated with HLA-G5d (data not shown). The treatment of DCs with neutralizing anti-IL-6 antibody has no effect on the number of cells that are positive for CD86 (Fig. S2 Middle). These data suggested that IL-6 plays a major role in the down-regulation of expression of MHC class II molecules by HLA-G and ILT4 on DCs.
The Arrest of Maturation/Activation of ILT-4-Positive DCs by HLA-G Is Correlated with an Increase in STAT3 Activation, but Not in STAT1.
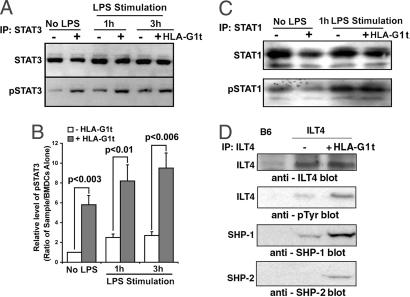
It has been reported that IL-6 induces an inhibitory signal for DC maturation by triggering the activation of STAT3 molecules (18, 23). To investigate the relationship between an increased level of IL-6 and STAT3 activation, we analyzed the phosphorylation of STAT3 molecules in ILT4-positive HLA-G-treated DCs. First, we observed that the treatment of ILT4-positive DCs with HLA-G1t induces phosphorylation of STAT3 molecules (Fig. 3A, first 2 lanes, and B). Additional stimulation of DCs with LPS increases the levels of STAT3 protein and enhances phosphorylation of STAT3 molecules (Fig. 3 A, next 4 lanes, and B). No activation of STAT1 was detected in HLA-G1t-treated DCs without LPS stimulation and stimulated with LPS (Fig. 3C). Similar data were observed in ILT4 DCs treated with HLA-G5d (data not shown). Together, these results demonstrate direct involvement of STAT3 activation in HLA-G-mediated arrest of maturation/activation of ILT4 DCs.
Fig. 3.
Engagement of ILT4 receptors by HLA-G1t increased STAT3 activation, phosphorylation of ILT4, and recruitment of SHP-1 and SHP-2. (A and B) Levels of STAT3 activation in ILT4-positive BMDCs treated with HLA-G1t for 3 h with and without stimulation with 100 ng/ml LPS for the indicated time. ILT4-positive BMDCs (5 × 106) were treated for 3 h with 50 ng/ml HLA-G1t or were left untreated. DCs were stimulated for an additional 1 h with 100 ng/ml LPS or left unstimulated. Cells were lysed, and proteins were immunoprecipitated (IP) with anti-STAT3 or anti-STAT1 antibodies. Immunoprecipitates were analyzed on Western blot for STAT proteins (A, Upper) and reprobed with anti-phosphotyrosine antibody 4G10 (A, Lower). Proteins were visualized by enhanced chemiluminescence. (C) Levels of STAT1 activation in ILT4-positive BMDCs treated with HLA-G1t for 3 h with and without stimulation with 100 ng/ml LPS. (D) Phosphorylated ILT4 is associated with SHP-1 and SHP-2. BMDCs were generated from ILT4 transgenic and B6 (control) mice, and the receptors were immunoprecipitated from cell extracts by using an anti-ILT4 mAb (42D1). Immunoblotting was performed by using anti-ILT4 mAb or 4G10, an anti-phosphotyrosine antibody as indicated. Immunoblots were reprobed sequentially with anti-SHP-1 and anti-SHP-2 antibodies as indicated. Proteins were visualized by enhanced chemiluminescence. Approximate molecular masses of the ILT4, SHP-1, and SHP-2 proteins were 95, 68, and 72 kDa, respectively. Data are representative of four independent experiments.
Engagement of ILT4 Receptors by HLA-G on DCs Results in Phosphorylation of ILT4 and Recruitment of SHP-1 and SHP-2 Protein Tyrosine Phosphatases.
Using different isoforms of HLA-G, we determined that ILT4 becomes phosphorylated after engagement of ILT4 receptor with HLA-G5d or HLA-G1t and recruits both SHP-1 and SHP-2 phosphatases (Fig. 3D). Recently, it has been shown that C-terminal Src kinase (Csk) binds ILT2 and potentially regulates its function (24). In addition, it has been shown that Csk plays an important role in LPS-induced signal transduction and IL-6 production in myeloid cells (25). However, we did not detect the presence of Csk in ILT4 immunoprecipitates from DCs (data not shown). No phosphorylation of ILT4 receptor was determined on ILT4-positive DCs treated with HLA-G5m (data not shown).
Down-Regulation of SHP-2 Gene Expression, but Not SHP-1, Diminishes Inhibitory Effect of HLA-G and ILT4 in DCs.
To determine the role of SHPs recruited to phosphorylated ILT4 receptor after engagement with HLA-G on the level of IL-6 and on the differentiation of ILT4-positive DCs, we performed analyses with down-regulated gene expression of these molecules by using short hairpin RNA (shRNA) knockdown technology. Using lentiviral transduction particles containing shRNAs for the target genes, we were able to down-regulate ≈70% of SHP-1 and ≈80% of SHP-2 gene expression in DCs, as detected by immunoblot analyses (Fig. 4 A and B). Knockdown of SHP-1 slightly reduces mRNA levels of IL-6 compared with nontarget control shRNA on ILT4-positive DCs treated with HLA-G1t (Fig. 4C). In contrast, knockdown of SHP-2 resulted in 65% reduction of mRNA levels of IL-6 in cells treated with HLA-G1t, suggesting that SHP-2 plays a critical role for up-regulation of IL-6 in ILT4-positive DCs. In addition, we determined that down-regulation of SHP-1 has very little effect on alteration of the reduced level of expression of MHC class II molecules mediated by HLA-G1t treatment of ILT4-positive DCs (Fig. 4D). However, down-regulation of SHP-2 increases the level of MHC class II molecules on ILT4-positive DCs treated with HLA-G1t, suggesting that SHP-2 is a key molecule in diminishing the inhibitory effect of HLA-G and ILT4 in DCs. Based on these data, we propose a model of the potential role of SHP-2 and the IL-6–STAT3 pathway in down-regulation of expression of MHC class II molecules and control of DC differentiation by HLA-G and ILT4 (Fig. 5).
Fig. 4.
SHP-2 is a central mediator of HLA-G–ILT4 inhibitory effect in DCs. (A and B) ILT4-positive BMDCs were infected with lentiviral particles expressing SHP-1 shRNA or SHP-2 shRNA. Cellular lysates were analyzed by Western blotting with antibodies against SHP-1, SHP-2, and actin as indicated. Actin was detected as a loading control. Bars represent a percentage of the maximum signal per nontarget control band measured by semiquantitative densitometry. Densities were calculated as an average of five measurements per band. (C) RNA was isolated 48 h after infection with the indicated lentiviral particles, and RT-PCR was performed with primers to detect β-actin (internal control) and IL-6. (D) Flow cytometry analysis of the expression of MHC class II molecules on ILT4-positive BMDCs. Cells were stimulated with 100 ng/ml LPS for 18 h or left unstimulated (left two panels). After transduction with the indicated lentiviral particles, cells were treated with HLA-G1t for 3 h and then stimulated with 100 ng/ml LPS for 18 h (right three panels). Cells were stained with APC-conjugated anti-CD11c and FITC-conjugated anti-MHC class II (I-Ab) mAbs. Histograms shown were gated on a CD11c+ population. Blue lines represent isotype control. Numbers indicate percentage of positive cells of total gated cells. The results are from one representative experiment of four performed.
Fig. 5.
Proposed model of arrest of maturation/activation of DCs via ILT4 receptor and HLA-G ligand. HLA-G induces phosphorylation of ILT4 receptor and recruitment of SHP-1 and SHP-2 phosphatases. SHP-2 enhances activation of NF-κB and downstream IL-6 production. IL-6 induces STAT3 activation, which decreases cystatin C level, the endogenous inhibitor of cathepsins, and enhanced cathepsin S activities. Cathepsin S decreased intracellular MHC class II αβ dimer levels, invariant chain (Ii), and H2-DM molecule levels in DCs. The moderate signal generated through TLR4 leads to modest induction of IL-12 and IL-6, therefore additionally enhancing IL-6 production.
Discussion
All DCs have a capacity for initiating tolerance or immunity; the distinction depends on the maturation or activation state of the DCs (26, 27). The presence of powerful inhibitory receptors on DCs is indicative of their potential to control the activation or maturation of DCs and confer tolerogenic capacity. In this work, we used the β2-microglobulin-associated forms of HLA-G ligands, which are the most important forms for ILT recognition (13, 28). Our results demonstrate that HLA-G5 dimer and HLA-G1 tetrameric complexes have a similar capacity to induce an ILT-mediated inhibitory signal and modulation of DC activation and maturation. In contrast, HLA-G5 monomer neither triggers an ILT inhibitory signal nor modulates ILT4-positive DCs in vitro and in vivo. The precise role of different isoforms of HLA-G in the modulation of DCs depends on their concentration and conformation, affecting binding to a specific receptor. At least in vitro, the increasing concentration of HLA-G5 monomeric form significantly enhanced the formation of HLA-G5 dimer. Thus, in this situation, the ILT-mediated inhibitory signal occurs via the HLA-G5 dimer form because a high concentration of purified HLA-G5 monomer did not trigger the ILT-mediated signal. It is most likely that the monomeric form of HLA-G5 plays an important function involving the control of angiogenesis. Monomeric HLA-G5 has been shown to bind an activating receptor, KIR2DL4, on human resting NK cells and trigger the expression of a set of chemokines and cytokines driving a proinflammatory/proangiogenic response (29).
Signaling events downstream of the ILT4 receptor and their functional impact on DC maturation/activation are incompletely understood. The present work provides evidence that engagement of ILT4 by HLA-G ligand results in recruitment of both SHP-1 and SHP-2 phosphatases and involves the IL-6–STAT3 pathway. Our previous data on the analysis of human DCs (21) and recent experiments with murine ILT4-positive DCs suggest that one of the major targets of the HLA-G and ILT4 interaction on DCs is MHC class II molecules. During maturation, DCs increase their surface expression of MHC class II molecules by severalfold. This increase is accompanied by a dramatic change in localization of MHC class II molecules, which are abundant in endosomal structures in immature DCs but are located mostly on the plasma membrane in mature DCs (30). The control of the synthesis and degradation, trafficking, and peptide loading of MHC class II molecules, represent key mechanisms in antigen presentation by DCs. Recently, it was discovered that the IL-6–STAT3 pathway controls the intracellular MHC class II αβ dimer level through cathepsin S activity in DCs (31).
Although IL-6 is involved in the development of mature T and B cell responses, it does not have only proinflammatory properties. It was recently demonstrated that forced activation of cytokine IL-6 in DCs resulted in the development of immune suppressive tolerogenic DCs. IL-6 knockout mice had an increased number of mature DCs, indicating that IL-6 blocks DC maturation in vivo (18). This observation corresponds to our findings that engagement of ILT4 on DCs by certain isoforms of HLA-G results in increasing the transcriptional and protein levels of IL-6 and conferring DCs with tolerogenic properties. The signaling pathway of IL-6 leads to the activation of STAT3 and STAT1. IL-6 activates STAT3 exclusively; when IL-6 levels were raised 10- to 100-fold, STAT1 activation was noted as well (19, 32). In our work, treatment of ILT4-positive DCs with HLA-G1 tetramer and HLA-G5 dimer induced phosphorylation of STAT3. Additional stimulation of DCs with LPS increases the levels of STAT3 protein and enhances its phosphorylation. In contrast, no activation of STAT1 was detected. The experiments with knockdown of tyrosine phosphatases enable us to determine that SHP-2 is a key molecule involved in the increase of IL-6 by the HLA-G–ILT4 interaction on DCs during the maturation process that is mediated by LPS signaling. Because the HLA-G–ILT4 interaction on DCs especially targets MHC class II genes and does not affect the expression of MHC class I molecules, it is most likely that in ILT4-positive DCs, SHP-2 modulates the NF-κB pathway in a MAP kinase-independent fashion in induction of IL-6. The exact molecular mechanisms of the induction of negative regulators of TLR4 signaling remain to be determined.
The mechanism proposed in Fig. 5 can be applied to control the maturation/activation of DCs via HLA-G–ILT4 in the absence of a strong inflammatory response and during a moderate signal through TLR4. This situation could be similar or equivalent to a normal pregnancy or surgical procedure with tissue or organ transplantation. However, upon receiving a strong activated signal associated with a pathogen or inflammation, ILT4-positive DCs will most likely force a robust rise in IL-6 levels, which will result in activation of STAT3 and STAT1, conferring DCs with immune-stimulating properties.
In conclusion, our results demonstrate a HLA-G-based strategy used by different isoforms of HLA-G to down-regulate DC activation and function. The identification of a powerful HLA-G ligand that specifically targets ILT4 in DCs might be useful for certain clinical applications for the development of tolerogenic vaccines for the treatment of autoimmune diseases or induction and maintenance of transplantation tolerance.
Materials and Methods
Mice.
C57BL/6 and B6.C-H-2bm12 (bm12) were purchased from Jackson Laboratory. ILT4-transgenic mice have been described in ref. 21. The use of animals for this work was approved by the animal care committee of the Medical College of Georgia.
Generation of HLA-G5 Monomer, HLA-G5 Dimer, and HLA-G1 Tetrameric Complexes Coupled to Polystyrene Microspheres.
HLA-G5 was expressed and pu-rified and HLA-G1 tetrameric complexes were generated as described in SI Experimental Procedures.
Antibodies and Flow Cytometry Analysis.
DCs were stained with mAbs, and flow cytometry analysis was performed as described in SI Experimental Procedures.
RNA Isolation and DNA Microarray Analysis.
Total RNA from BMDCs treated with HLA-G ligands was isolated, and DNA microarray analysis was conducted as described in SI Experimental Procedures.
shRNA-Targeted Protein Knockdown.
pLKO.1-puro lentiviral vectors expressing nontarget control shRNA or one of five different SHP-1 shRNAs or one of five different SHP-2 shRNAs were purchased from Sigma–Aldrich. The production of lentiviral particles and the infection of BMDCs were performed as described in SI Experimental Procedures.
Immunoprecipitation and Western Blot Analysis.
DCs (5 × 106), untreated or treated with HLA-G ligands and LPS, were lysed followed by immunoprecipitation and Western blot analysis, as described in SI Experimental Procedures.
RT-PCR Analysis for Quantitation of IL-6 mRNA.
Relative amounts of IL-6 mRNA were determined by semiquantitative RT-PCR followed by Southern blot analysis as described in SI Experimental Procedures.
Statistical Analysis.
Where applicable, values were compared by the Mann–Whitney U test with Stata 7.0 software.
Supplementary Material
Acknowledgments.
We are grateful to Drs. Rhea-Beth Markowitz, Nahid Mivechi, and Dimitrios Moskofidis for helpful discussions and critical reading of the manuscript. This work was supported by National Institutes of Health Grant R01 AI055923 (to A.H.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803341105/DCSupplemental.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 3.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 4.Colonna M, et al. Human myelomonocytic cells express an inhibitory receptor for classical and nonclassical MHC class I molecules. J Immunol. 1998;160:3096–3100. [PubMed] [Google Scholar]
- 5.Allan DS, et al. Tetrameric complexes of human histocompatibility leukocyte antigen (HLA)-G bind to peripheral blood myelomonocytic cells. J Exp Med. 1999;189:1149–1156. doi: 10.1084/jem.189.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borges L, Cosman D. LIRs/ILTs/MIRs, inhibitory and stimulatory Ig-superfamily receptors expressed in myeloid and lymphoid cells. Cytokine Growth Factor Rev. 2000;11:209–217. doi: 10.1016/s1359-6101(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 7.Ju XS, et al. Immunoglobulin-like transcripts ILT2, ILT3, and ILT7 are expressed by human dendritic cells and down-regulated following activation. Gene. 2004;331:159–164. doi: 10.1016/j.gene.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Kirszenbaum M, Moreau P, Gluckman E, Dausset J, Carosella ED. An alternatively spliced form of HLA-G mRNA in human trophoblasts and evidence for the presence of HLA-G transcript in adult lymphocytes. Proc Natl Acad Sci USA. 1994;91:4209–4213. doi: 10.1073/pnas.91.10.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreau P, et al. HLA-G protein processing and transport to the cell surface. Cell Mol Life Sci. 2002;59:1460–1466. doi: 10.1007/s00018-002-8521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carosella ED, et al. HLA-G molecules: From maternal–fetal tolerance to tissue acceptance. Adv Immunol. 2003;81:199–252. doi: 10.1016/s0065-2776(03)81006-4. [DOI] [PubMed] [Google Scholar]
- 11.Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005;19:681–693. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 12.Boyson JE, et al. Disulfide bond-mediated dimerization of HLA-G on the cell surface. Proc Natl Acad Sci USA. 2002;99:16180–16185. doi: 10.1073/pnas.212643199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonen-Gross T, et al. The CD85J/leukocyte inhibitory receptor-1 distinguishes between conformed and β2-microglobulin-free HLA-G molecules. J Immunol. 2005;175:4866–4874. doi: 10.4049/jimmunol.175.8.4866. [DOI] [PubMed] [Google Scholar]
- 14.Shiroishi M, et al. Efficient leukocyte Ig-like receptor signaling and crystal structure of disulfide-linked HLA-G dimer. J Biol Chem. 2006;281:10439–10447. doi: 10.1074/jbc.M512305200. [DOI] [PubMed] [Google Scholar]
- 15.Apps R, Gardner L, Sharkey AM, Holmes N, Moffett A. A homodimeric complex of HLA-G on normal trophoblast cells modulates antigen-presenting cells via LILRB1. Eur J Immunol. 2007;37:1924–1937. doi: 10.1002/eji.200737089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colonna M, et al. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med. 1997;186:1809–1818. doi: 10.1084/jem.186.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You M, Flick LM, Yu D, Feng GS. Modulation of the nuclear factor κB pathway by Shp-2 tyrosine phosphatase in mediating the induction of interleukin (IL)-6 by IL-1 or tumor necrosis factor. J Exp Med. 2001;193:101–110. doi: 10.1084/jem.193.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SJ, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173:3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 19.Barton BE. STAT3: A potential therapeutic target in dendritic cells for the induction of transplant tolerance. Expert Opin Ther Targets. 2006;10:459–470. doi: 10.1517/14728222.10.3.459. [DOI] [PubMed] [Google Scholar]
- 20.Gonen-Gross T, et al. Complexes of HLA-G protein on the cell surface are important for leukocyte Ig-like receptor-1 function. J Immunol. 2003;171:1343–1351. doi: 10.4049/jimmunol.171.3.1343. [DOI] [PubMed] [Google Scholar]
- 21.Ristich V, Liang S, Zhang W, Wu J, Horuzsko A. Tolerization of dendritic cells by HLA-G. Eur J Immunol. 2005;35:1133–1142. doi: 10.1002/eji.200425741. [DOI] [PubMed] [Google Scholar]
- 22.Ristich V, Zhang W, Liang S, Horuzsko A. Mechanisms of prolongation of allograft survival by HLA-G/ILT4-modified dendritic cells. Hum Immunol. 2007;68:264–271. doi: 10.1016/j.humimm.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Ohtani T, et al. Dissection of signaling cascades through gp130 in vivo: Reciprocal roles for STAT3- and SHP2-mediated signals in immune responses. Immunity. 2000;12:95–105. doi: 10.1016/s1074-7613(00)80162-4. [DOI] [PubMed] [Google Scholar]
- 24.Sayos J, Martinez-Barriocanal A, Kitzig F, Bellon T, Lopez-Botet M. Recruitment of C-terminal Src kinase by the leukocyte inhibitory receptor CD85j. Biochem Biophys Res Commun. 2004;324:640–647. doi: 10.1016/j.bbrc.2004.09.097. [DOI] [PubMed] [Google Scholar]
- 25.Aki D, et al. Modulation of TLR signalling by the C-terminal Src kinase (Csk) in macrophages. Genes Cells. 2005;10:357–368. doi: 10.1111/j.1365-2443.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 26.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 27.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 28.Shiroishi M, et al. Structural basis for recognition of the nonclassical MHC molecule HLA-G by the leukocyte Ig-like receptor B2 (LILRB2/LIR2/ILT4/CD85d) Proc Natl Acad Sci USA. 2006;103:16412–16417. doi: 10.1073/pnas.0605228103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajagopalan S, et al. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol. 2006;4:70–86. doi: 10.1371/journal.pbio.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villadangos JA, Schnorrer P, Wilson NS. Control of MHC class II antigen presentation in dendritic cells: A balance between creative and destructive forces. Immunol Rev. 2005;207:191–205. doi: 10.1111/j.0105-2896.2005.00317.x. [DOI] [PubMed] [Google Scholar]
- 31.Kitamura H, et al. IL-6–STAT3 controls intracellular MHC class II αβ dimer level through cathepsin S activity in dendritic cells. Immunity. 2005;23:491–502. doi: 10.1016/j.immuni.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Darnell JE., Jr Reflections on STAT3, STAT5, and STAT6 as fat STATs. Proc Natl Acad Sci USA. 1996;93:6221–6224. doi: 10.1073/pnas.93.13.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.