Abstract
A subset of gastrointestinal stromal tumors (GISTs) lack gain-of-function mutations in c-KIT and PDGFRα. These so-called wild-type (WT) GISTs tend to be less responsive to imatinib-based therapies and have a poor prognosis. We identified amplification of IGF1R in a SNP analysis of GIST and thus studied its potential as a therapeutic target in WT and mutant GIST. Expression of IGF1R and downstream effectors in clinical GIST samples was examined by using immunoblots and immunohistochemistry. The roles of IGF1R signaling in GIST and viability were analyzed by using NVP-AEW541, an inhibitor of IGF1R, alone and in combination with imatinib, or via siRNA silencing of IGF1R. IGF1R was strongly overexpressed, and IGF1R amplification was detected at a significantly higher frequency in WT GISTs, including a pediatric WT GIST, compared with mutant GISTs (P = 0.0173 and P = 0.0163, respectively). Inhibition of IGF1R activity in vitro with NVP-AEW541 or down-regulation of expression with siIGF1R led to cytotoxicity and induced apoptosis in GIST cell lines via AKT and MAPK signaling. Combination of NVP-AEW541 and imatinib in GIST cell lines induced a strong cytotoxicity response. Our results reveal that IGF1R is amplified and the protein is overexpressed in WT and pediatric GISTs. We also demonstrate that the aberrant expression of IGF1R may be associated with oncogenesis in WT GISTs and suggest an alternative and/or complementary therapeutic regimen in the clinical management of all GISTs, especially in a subset of tumors that respond less favorably to imatinib-based therapy.
Keywords: pediatric GIST, tyrosine kinase inhibitors, imatinib mesylase, adult wild-type GIST, NYP-AEW541
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the digestive tract. Mazur and Clark originally described these tumors in 1983, noting that they contained smooth muscle and neural elements (1). Clinically, diagnosis of GIST is typically confirmed by immunohistochemical staining of a 145-kDa transmembrane glycoprotein, KIT, referred to as CD117. Molecular genetic studies have shown that the vast majority of primary GISTs (≈70%) possess gain-of-function mutations of c-KIT in exon 9, 11, 13, or 17, and that a subset of GISTs (≈10%) possess gain-of-function mutations of PDGFRα in exon 12, 14, or 18 (2–4).
Imatinib mesylate is an oral 2-phenylaminopyrimidine derivative that acts as a selective inhibitor against type III tyrosine kinases such as KIT, PDGFRα and BCR-ABL (the causative chimeric fusion protein in chronic myelogenous leukemia) (5). Since its approval by the FDA in 2002, it has been successfully administered to treat patients with metastatic and/or unresectable GISTs (6, 7). Response to imatinib is correlated with the type of c-KIT and PDGFRα mutation present in a given tumor. GIST patients with exon 11 c-KIT mutations have the best response and disease-free survival, whereas GIST with non-exon 11 mutations or wild-type (WT) have a poorer disease-free survival and overall survival (8, 9). The small but significant portion of GIST patients (10–20%) whose tumors lack mutations in either c-KIT or PDGFRα or who possess “imatinib-resistant” mutations (such as exon 17 mutations in KIT and exon 18 mutations in PDGFRα) have lower response rates and shorter disease-free progression compared with patients whose tumors have exon 11 mutations.
A subset of GISTs arises in the pediatric age group. A typical pediatric patient presents with anemia secondary to gastrointestinal bleeding, has a median age of 12, and is female (10). Only 15% of tumors will have evidence of a c-KIT or PDGFRα mutation (11). In general, the growth of these tumors is more indolent, and surgery is a mainstay of therapy; however, tumors can metastasize. There is limited benefit of available drug therapies, including imatinib in pediatric patients. Therefore, identifying additional genetic factors that contribute to the pathogenesis of GIST, independent of KIT and PDGFRα, may be helpful in developing additional, individually tailored anti-GIST therapies.
Insulin-like growth factor (IGF) signaling plays a critical role in the growth and development of many tissues and regulates overall cell growth (12). The IGF system is composed of the IGF ligands (IGF-1 and IGF-2), receptors (IGFR), the insulin receptor (IR) and six regulatory IGF-binding proteins (IGFBPs). IGF1R is a transmembrane receptor that interacts with both IGF-1 and IGF-2. IGF1R is activated via autophosphorylation after ligand binding, thereby leading to activation of the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) cascades (13). Because overexpression of IGF1R has been identified in several tumor types (14–17) and because of its role in metabolism, which potentially has relevance to the survival of malignant cells, IGF1R has become a target for anticancer therapy.
Although aberrant expression of IGF1R is detected in many different types of cancer, little is known regarding the molecular mechanism behind it. It was first reported in 1994 that chromosome 15q26, where IGF1R is located, was amplified in <10% of breast cancers (18). Recently, others have reported IGF1R amplification at low levels in pancreatic adenocarcinoma xenografts and in two gastric cancer cell lines and in a small percentage of Wilms' tumors (19, 20). In this work, we have found that IGF1R is highly expressed in adult and pediatric WT GISTs compared with GISTs with c-KIT or PDGFRα mutations. Using genomic real-time PCR and interphase fluorescence in situ hybridization (FISH), we have determined that a significant portion of WT GISTs and in a pediatric case have IGF1R gene amplification. We also show that a tyrosine kinase inhibitor, NVP-AEW541, which targets IGF1R (21), has significant inhibitory effects on IGF1R phosphorylation and on GIST cell proliferation in vitro, regardless of the KIT mutational status and IGF1R expression levels. Furthermore, knocking down IGF1R expression alone by siRNA silencing could induce cytotoxicity, even in the presence of activated KIT. Our findings support the conclusion that IGF1R is driving GIST pathogenesis in tumors lacking c-KIT and PDGFRα activating mutations. Therefore, IGF1R should be considered a therapeutic target in all GIST patients, especially adult and pediatric GISTs that express high levels of IGF1R.
Results
IGF1R Is Highly Expressed in WT GISTs.
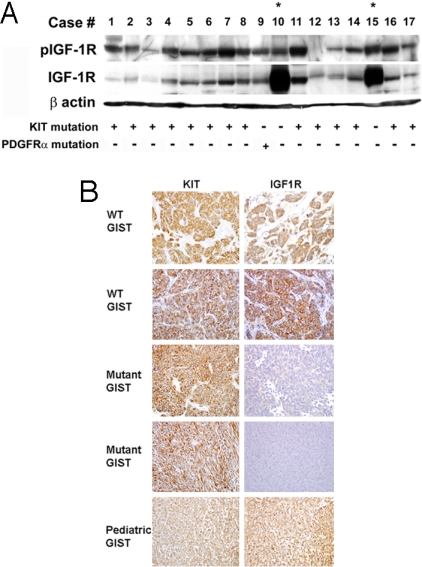
High-density single-nucleotide polymorphism (SNP) arrays were used to evaluate changes in gene copy number in GISTs. We subjected 24 GISTs and two GIST cell lines, i.e., GIST 882 and GIST-T1 cells, to copy number analysis by using the Affymetrix 50K Xba array. We found frequent loss of chromosomal regions, such as 1p, and monosomy for 9, 14, 15, and 22 that are common to most GISTs (refs. 22 and 23 and data not shown). In addition, we uncovered focal regions of amplification, including the IGF1R locus [supporting information (SI) Table S1 and Y. Skorogabotko, M. Belinsky, and A.K.G., unpublished data]. Based on these observations, immunoblotting was done on fresh-frozen GIST biopsies collected from Fox Chase Cancer Center for phospho-IGF1R and total IGF1R expression. All tumors samples were found to express KIT by standard immunohistochemical approaches. Of the 17 tumors examined, 14 possessed a c-KIT mutation, 1 possessed two individual PDGFRα missense mutations within the same allele, and 2 lacked detectable mutations in either gene. Mutation analysis was carried out as described in ref. 4. Immunoblotting revealed that IGF1R was expressed and activated in all GISTs but was markedly overexpressed (10- to 30-fold) in WT GISTs compared with mutant GISTs (Fig. 1A, lanes with asterisk signs). However, phospho-IGF1R levels did not correlate well with overall IGF1R levels. Because there is no other known oncogenic driving force in WT GISTs, we initially concluded that overexpression of IGF1R may be a key tumorigenic event for this subset of GISTs.
Fig. 1.
IGF1R expression in GIST biopsies. (A) Immunoblot assays of 17 consecutive fresh-frozen GIST biopsies with anti-phospho-IGF1R and anti-IGF1R antibodies. One hundred micrograms of WCE from each sample was subjected to immunoblotting. The c-KIT or PDGFRα genotype for each GIST is listed below (+ or −). Asterisks indicate WT GISTs. (B) Immunohistochemistry of WT, mutant, and pediatric GISTs for IGF1R expression. A score of 3 is considered marked expression (all tumor cells express high levels of IGF1R).
IGF1R Mutational and Gene Amplification Analyses.
We next sought to determine whether IGF1R is mutated in WT GISTs. We were able to isolate DNA from 10 fresh-frozen WT GISTs collected by needle biopsy. We examined the tumor DNA for potential gain-of-function mutations in IGF1R and performed mutational analyses of the exons encoding the juxtamembrane domain and the entire kinase domain of the receptor. No mutations in IGF1R were found in the WT GISTs. We detected a polymorphism (IGF1R-c.3129A > G; p.E1013E) in exon 16 of IGF1R in 30% of the WT GIST samples (3 of 10 samples) that was also found in 40% of an age/race/gender-matched disease-free control population (data not shown).
To validate the SNP array results and determine whether enhanced expression of IGF1R might be associated with gene amplification, we developed a genomic-based quantitative PCR assay to evaluate IGF1R gene copy number in mutant and WT GISTs. When tested on WT GISTs, we demonstrated that 7 of the 10 WT GISTs possessed amplified IGF1R (copy number range, 2.5–4 copies), compared with only 5 of 18 mutant GISTs showing amplification (P = 0.04) (Fig. S1). IGF1R gene amplification was also confirmed by FISH (Fig. S2 and Table S2). These results confirm that enhanced expression of IGF1R in a subset of GISTs is in part associated with gene amplification.
After demonstrating by Western blot analysis that IGF1R is abundantly expressed in WT GISTs (Fig. 1A and data not shown), we tested whether immunohistochemistry (IHC) could be used to evaluate IGF1R levels in clinical samples rapidly. We accessed 8 paraffin-embedded WT GISTs, a pediatric GIST, and 16 mutant GIST samples. Slides were stained for IGF1R and KIT expression by IHC and scored according to the criteria described in Materials and Methods. Fig. 1B shows representative examples of IGF1R expression for WT, mutant GISTs, and pediatric GISTs. For the 16 mutant GISTs, the majority showed low or no detectable levels of IGF1R, and none of these tumors was found to express very high levels (overall score of >2) (Table S1). In comparison, all of the WT GISTs, including the pediatric GIST, showed intense IGF1R staining throughout the tumor (overall score of ≥2) (P = 0.023, two-sided Fisher's exact test). These data further confirm the Western blotting results and indicate that abundant IGF1R expression is much more prominent in WT GISTs (both adult and pediatric) than in mutant GISTs. We also evaluated expression of a number of signaling molecules downstream of IGF1R and KIT, i.e., phospho-AKT (pAKT), phospho-mTOR (pmTOR), and phospho-S6 ribosomal protein (pS6), a protein target for activated mTOR. Interestingly, we observed that the majority of mutant GISTs had higher levels of pAKT and pmTOR compared with WT GISTs (Table S3), suggesting that both WT and mutant GISTs are under oncogenic stimulation albeit driven by different “oncogenic forces.”
Overall, we found that although IGF1R amplification is not seen exclusively in WT GISTs, it is clearly more prevalent in this subset of GISTs (70% in WT GISTs vs. 27.7% in mutant GISTs, P = 0.01632, two-sided Fisher's exact test). Furthermore, although only a single case was available, it is intriguing to demonstrate that IGF1R is amplified and overexpressed in pediatric GIST, which provides evidence that abnormal regulation of IGF1R may be driving GIST pathogenesis in tumors lacking c-KIT and PDGFRα activating mutations. We concluded that IGF1R could be considered a therapeutic target for WT GISTs, including pediatric cases, if not for all GISTs, because the protein is constitutively activated in the vast majority of tumors.
Inhibition of IGF1R Signaling with NVP-AEW541.
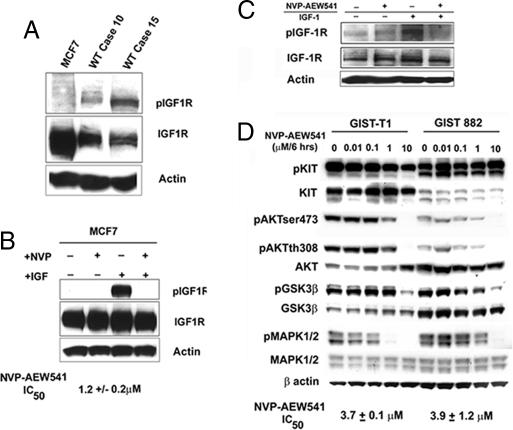
To test the efficiency of the small molecule inhibitor of IGF1R, NVP-AEW541 (Novartis) (24), we first examined MCF7 cells, which are known to have high IGF1R levels (25), and compared the expression of both pIGF1R and total IGF1R levels in MCF7 cells vs. two of the WT GISTs (Fig. 2A). As indicated, no cell lines have yet to be established from WT GISTs; therefore, we used MCF7 cells as a surrogate to evaluate the activity of NVP-AEW541 initially. As shown in Fig. 2A, MCF7 cells have ≈2- to 10-fold higher levels of IGF1R than the WT tumors, but they lack phosphorylation in the absence of IGF-1 stimulation. Stimulation with IGF-1 after serum starvation significantly enhanced pIGF1R in MCF7 cells, which could be completely inhibited by NVP-AEW541 (Fig. 2B).
Fig. 2.
NVP-AEW541 treatment inhibits constitutive activation of effectors downstream of IGF1R. (A) Immunoblot assays of MCF7 cells and two fresh-frozen WT GIST biopsies with specific anti-phospho-IGF1R and anti-IGF1R antibodies. Fifty micrograms of WCE from each sample was subjected to immunoblotting. (B) MCF7 cells were serum-starved for 12 h followed by either NVP-AEW541 treatment for 12 h and/or IGF-1 stimulation for 30 min. Immunoblot assays of phospho- and total IGF1R were performed. IC50 for NVP-AEW541 was quantitated based on four independent viability assays listed. (C) Immunoblot assays of phospho- and total IGF1R. GIST 882 cells were serum-starved for 12 h followed by either NVP-AEW541 treatment for 12 h and/or IGF-1 stimulation for 30 min. (D) GIST-T1 cells and GIST 882 cells were treated with NVP-AEW541 for 6 h at the indicated concentrations. Equal amounts (40 μg) of WCE from each sample were subjected to immunoblotting with specific antibodies as indicated. In all cases, actin served as a loading control. IC50 for NVP-AEW541 was quantitated based on four independent viability assays for each cell line.
Given that IGF1R is expressed and constitutively activated in GISTs, we next examined the effect of targeting IGF1R by using two GIST cell lines (26). Both GIST cell lines express IGF1R, with higher constitutive levels detected in GIST 882 cells. Importantly, IGF1R is autophosphorylated after serum starvation, and stimulation with IGF-1 increased phosphorylated levels of IGF1R (Fig. 2C and data not shown). As with MCF7 cells, NVP-AEW541 could inhibit IGF1R phosphorylation after ligand stimulation (Fig. 2C).
We next evaluated the consequence of inhibition of IGF1R by using pAKT and pGSK3β, and pMAPK1/2 as molecular surrogates of downstream IGF1R signaling. We have evaluated GIST 882 and GIST-T1 cells for response to imatinib and have shown them to be highly reliable models that represent the clinical experience, in that T1 cells that possess an exon 11 c-KIT mutation are more sensitive to imatinib than 882 cells that have an exon 13 mutation (26, 27). Using standard cytotoxicity assays, we determined the IC50 of NVP-AEW541 for GIST 882 cells and for GIST-T1 cells to be ≈3.9 ± 1.2 μM and ≈3.7 ± 0.1 μM, respectively. We had shown that GIST 882 cells were significantly more resistant to imatinib mesylate than to GIST-T1 cells (26), but importantly, both were equally sensitive to NVP-AEW541. Interestingly, the dose of NVP-AEW541 required to achieve an IC50 in MCF7 cells was ≈3-fold lower (Fig. 2B) than in the GIST-T1 and 882 cells, further suggesting that GIST with high levels of IGF1R may be more sensitive to IGF1R-based therapies. As expected, NVP-AEW541 did not have any effect on pKIT levels. However, pAKT and pGSK3β were inhibited at 10 μM NVP-AEW541 in both GIST cell lines (Fig. 2D). Interestingly, the effect of IGF1R inhibition on MAPK1/2 signaling was more cell type-dependent, perhaps associated with type of c-KIT mutation, i.e., phosphorylation of MAPK1/2 was inhibited at 10 μM NVP-AEW541 in GIST 882 cells, whereas only 1 μM was required in GIST-T1 cells (Fig. 2D).
NVP-AEW541 and Imatinib Have Additive Effects on GIST Cell Growth.
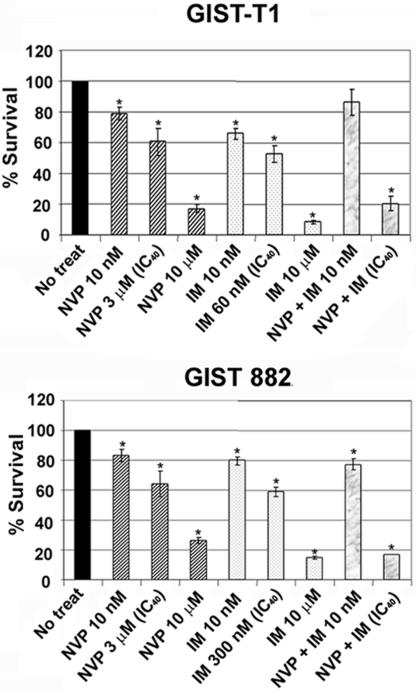
Growing evidence suggests that monotherapies may not be sufficient to eradicate tumor cells effectively and that targeting multiple pathways may be beneficial (28). Therefore, we examined the consequences of single and combined inhibition of IGF1R and KIT/PDGFRα in GIST cells. As shown in Fig. 3, NVP-AEW541 or imatinib alone (at 10 nM) had little effect on cell viability. However, when GIST-T1 and GIST 882 cells were treated with the combination at doses that induce ≈40% cytotoxicity, the decrease in cell viability was similar to or even greater than with NVP-AEW541 or imatinib at the highest doses (10 μM). However, inhibiting either KIT or IGF1R activity with these high doses of imatinib or NVP-AEW541 alone appears to be sufficient to induce cytotoxicity and growth inhibition in GIST cells in vitro, indicating little synergistic effect of these two drugs when various concentrations of imatinib are combined with 10 nM NVP-AEW541 and when increasing concentrations of NVP-AEW541 are combined with 10 nM imatinib (Fig. S3).
Fig. 3.
NVP-AEW541 and imatinib have additive cytotoxicity effects on mutant GIST cells. Cell viability assays of GIST-T1 and GIST 882 cells 72 h after treatment with NVP-AEW541 alone or in combination with imatinib at indicated concentrations are shown. ∗, P values between 0.01 and 2.5 × 10−7.
Next, we determined the consequence of single and combined inhibition on the molecular surrogates of downstream RTK signaling. As expected, the 10 nM NVP-AEW541 alone or combined with 10 nM imatinib did not inhibit KIT, IGF1R (in the absence of IGF-1 stimulation) or their downstream effectors in GIST cells (Fig. S4). However, when GIST cells were treated with higher doses (10 μM) of NVP-AEW541, or with imatinib alone, or with a combination of NVP-AEW541 and imatinib at the IC40 concentrations, the phosphorylation of MAPK1/2, AKT, and GSK3β was inhibited. These results strongly demonstrate that imatinib and NVP-AEW541 show a potentially additive effect on KIT and IGF1R signaling in GIST, which could be clinically beneficial because imatinib alone is not always curative, especially in metastatic GIST. Indeed, treatment of GIST cells with combined therapy at the IC40 doses of both drugs achieved a greater inhibitory effect than did either single agent at the highest dose. Importantly, the combined IC40 doses or 10 μM NVP-AEW541 alone induced caspase 3 activity as evidenced by poly(ADP-ribose)polymerase (PARP) cleavage in GIST-T1 cells and to a lesser extent in GIST 882 cells (Fig. S4 and data not shown).
IGF1R Silencing Induces Cytotoxicity in c-KIT Mutant GIST Cells.
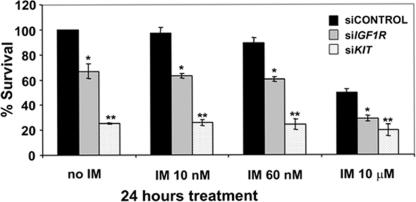
We further validated IGF1R as a therapeutic target in GIST by using RNAi approaches. We used GIST-T1 cells for these experiments because this cell line has much higher transfection efficiency than GIST 882 cells. We observed that IGF1R levels were depleted by ≈70% after electroporation with small interfering RNAs to IGF1R (siRNA-IGF1R) (Fig. S5A). Both immunoblots and densitometry quantitations showed that pKIT and pAKT levels remained similar in siRNA-control vs. siRNA-IGF1R-transfected samples; however, there was ≈50% decrease in the pMAPK1/2 levels (Fig. S5A, Lower, sicontrol vs. siIGF1R without imatinib treatment). These results suggest that in KIT mutant GIST cells, blocking IGF1R has minimal effect on AKT activation; however, IGF1R depletion does affect MAPK activation. As expected, treatment with imatinib at concentrations as low as 60 nM inhibits KIT and AKT phosphorylation. Importantly, siRNA against c-KIT resulted in KIT knockdown and inactivation of pAKT and pMAPK1/2 (Fig. S5B). Viability assays revealed that siRNA-IGF1R alone resulted in significant cell death (≈40%), whereas siRNA-KIT caused nearly 80% cell death compared with siRNA-control (Fig. 4). These data confirm that in these mutant GIST cells, KIT activation is the primary oncogenic event but that IGF1R activation, even in the presence of mutant KIT, may also be contributing to tumorigenesis. Our results also suggest that NVP-AEW541 may possess some unknown off-target activities or that the level of siRNA suppression of IGF1R was not sufficient to elicit complete inhibition of the IGF1R signaling pathway.
Fig. 4.
The cytotoxic effect of KIT and IGF1R silencing in GIST. Cells were treated with imatinib at the indicated doses 24 h before 48-h harvest time followed by cytotoxicity assays. ∗, P values between 0.003 and 5.1 × 10−5; ∗∗, P values between 3 × 10−4 and 5.4 × 10−6.
Discussion
IGF1R is activated and signals its downstream pathways after binding of its ligands, IGF1 and IGF-II. IGF1R signaling has been implicated in many different human cancers based on experimental model systems and population studies (29). Drugs inhibiting this pathway are actively being tested in phase I and II clinical trials (30–33). Although it has been shown by others that IGF1R is expressed in a number of hematological neoplasias and solid tumors, the molecular mechanism by which overexpression of IGF1R contributes to cancer is still not clear. In addition, the role of IGFBP-3, a regulator of circulating IGF1 levels, has recently been studied in GISTs. Interestingly, Trent et al. (34) reported that IGFBP-3 expression is regulated by imatinib in GISTs and may play a role in the antitumor activity of imatinib. We have shown that IGF1R expression is enhanced greatly in numerous GISTs and that this enhanced expression is found primarily in GISTs that lack c-KIT and PDGFRα mutations (Fig. 1). Importantly, our results also demonstrated that IGF1R is overexpressed and that the gene may be amplified, in pediatric GIST, a subset of GISTs that commonly lack known oncogenic mechanisms and demonstrate few genomic alterations (11). The finding of high expression of IGF1R in the pediatric GIST sample is in accordance with a previous publication (35). Furthermore, our studies suggest that IGF1R may be a relevant target for both mutant and WT GISTs (including adult and pediatric cases), although an in vitro WT GIST model does not currently exist. Importantly, these studies provide insights into the pathogenesis of this disease, namely that IGF1R overexpression may be an important oncogenic event in the pathogenesis of GISTs that lack c-KIT and PDGFRα mutations, including pediatric GISTs.
It was demonstrated that imatinib at equivalent drug concentrations has equal efficacy in decreasing the phosphorylation of both WT induced with steel factor and a mutated c-KIT (36). This finding is in contrast with the clinical experience where the WT GISTs are less likely to have responses to imatinib therapy and have shorter event-free and overall survival compared with GISTs containing exon 11 mutations (8, 9). WT GISTs have an increased relative risk of disease progression and death from GIST of 108% and 76%, respectively, compared with GISTs containing an exon 11 mutation. Therefore, metastatic GIST patients with WT tumors would benefit from an alternative treatment to imatinib alone, and blockade of the IGF1R pathway may be one such approach.
Our results strongly suggest, because IGF1R is commonly overexpressed in most WT GISTs, that IHC analysis of IGF1R could serve as a rapid means for prescreening GIST, before c-KIT and PDGFRα genotyping. Furthermore, we have shown that GISTs lack mutations in the IGF1R juxtamembrane and kinase domains. However, we have demonstrated by genomic qPCR, and we confirmed by FISH, that the majority of WT GISTs, including a pediatric GIST, possess IGF1R amplification, a genomic abnormality that could contribute in part, but not necessarily totally, to the high levels observed. Although IGF1R amplification is not confined to WT GISTs, it is clearly more prevalent compared with mutant GISTs (P = 0.01632). We have also determined that treatment of GIST-T1 and GIST 882 cell lines, with a demethylation agent, 5-aza-2′-deoxycytidine, did not result in increased levels of IGF1R (data not shown). This result suggests that transcriptional factors, rather than DNA methylation, may contribute to the regulation of IGF1R in GISTs, although additional studies will be necessary to identify the mechanism. Furthermore, downstream signaling effectors, such as AKT and mTOR, remain active in both WT and mutant tumors (Table S3), although this pathway seems to be more prominently activated in mutant tumors. This result indicates that both WT and mutant GISTs are under oncogenic stimulation albeit driven by different oncogenic forces. This concept is similar to those reported in other types of cancer in which multiple receptor tyrosine kinases (RTKs) are coactivated and thereby limit the efficacy of therapies targeting a single RTK (37). Our results suggest that aberrant IGF1R expression may be one of several oncogenic forces in this subset of GISTs and thus, represents a therapeutic target to consider in combination with clearly effective, but not always curative, therapies. Even in mutant GISTs, concomitant activation of multiple RTKs, e.g., IGF1R, may serve to reduce dependence on only KIT for the maintenance of critical downstream signaling, thus rendering some tumors refractory to imatinib or related RTK inhibitors. Furthermore, because we demonstrated that the vast majority of GISTs have constitutive activation of IGF1R in the absence of gain-of-function mutations, we examined an IGF1R ligand, IGF-1, in GIST tissue samples and blood from GIST patients for the potential existence of an IGF-1 autocrine loop. Overall, we found comparable levels of IGF-1 in WT and mutant GISTs (data not shown), suggesting similar levels of autophosphorylation of IGF1R via an autocrine loop.
There are now multiple agents available for the inhibition of IGF1R signaling in cancer cells (38, 39), including IGF-1- and IGF-2-neutralizing antibodies as well as monoclonal antibodies and small tyrosine kinase inhibitors of the receptor itself (29). We examined the drug NVP-AEW541, which has specific activity against IGF1R, and showed inhibitory effect of cell signaling and growth in mutant GIST cells, which was independent of KIT signaling. Unlike published data that suggest that inhibition of IGF1R enhances the therapeutic effect of or sensitizes tumor cells to standard drug treatment (40, 41), our results do not show a synergistic effect of NVP-AEW541 and imatinib on GIST cell viability.
Finally, we further examined the role of IGF1R in GIST cell viability by using siRNA silencing. We show that, as with NVP-AEW541 treatment at a high dose (10 μM), IGF1R knockdown induces cytotoxic effect in GIST cells, albeit to a lesser degree compared with KIT knockdown (Fig. 4). This result suggests that in GIST cell lines that harbor mutant c-KIT, the gain-of-function of mutant KIT plays a dominant role in GIST survival. However, unlike NVP-AEW541 treatment at a lethal dose, IGF1R knockdown does not lead to AKT inactivation, indicating that there may be some off-target effects of NVP-AEW541. As with many newer molecularly targeted agents, few have single target specificity. In fact, it is thought that “dirtier” drugs, such as sunitinib or AMG706, may be more effective in the clinical setting as long as toxicities are manageable and long-term administration is feasible (42, 43).
After we submitted this article, Braconi et al. (44) reported that IGF1 and IGF2 expression correlated with relapse in GIST patients, whereas IGF1R expression was strong in all cases. Although different methods were used, taken together with our current and previous observations it appears that abnormal IGF1R signaling may indeed be involved in the pathogenesis of GIST via KIT independent pathways (26).
In conclusion, we have shown that IGF1R is amplified and overexpressed in the majority of GISTs that lack c-KIT or PDGFRα mutations. More importantly, we have shown that imatinib-sensitive and -resistant GIST cells respond equally well to a small molecular inhibitor of IGF1R, suggesting an alternative and/or complementary therapeutic regimen in the clinical management of GIST, especially in tumors that respond less favorably to imatinib-based therapy, including pediatric cases. These findings are particularly exciting given the number of agents targeting IGF1R that are currently being tested in clinical trials. It is feasible in the near future to initiate clinical trials by using IGF1R-targeted therapies for imatinib-refractory GIST patients, initially focusing on adult and pediatric GIST patients lacking c-KIT or PDGFRα mutations and expanding possibly to all GIST patients.
Materials and Methods
Cell Cultures.
GIST 882 cells that possess a homozygous mutation in KIT exon 13 and GIST-T1 cells possessing a heterozygous mutation in KIT exon 11 were grown as described in ref. 26. MCF7 cells were cultured in DMEM with 10% FBS and insulin (0.3 unit/ml). For drug treatment, drugs were added directly to the cell medium at the indicated final concentration for the specified period. Imatinib mesylate (Gleevec) was dissolved in sterile PBS and stored at −20°C. NVP-AEW541 was provided by Novartis and made into a 10 mM stock with DMSO. All antibodies used in this work were purchased from Cell Signaling Technologies, except β-actin, purchased from Sigma, and used according to the manufacturer's instructions. Lyophilized recombinant human IGF-1 was purchased from R&D Systems, resuspended in PBS, and stored at −20°C. The secondary antibody and detection system used for immunohistochemical staining was super-sensitive link-label (biotin-based) IHC detection systems from Biogenex. For siRNA studies, a smart pool of double-stranded siRNA against IGF1R (IGF1R-NM-000875) as well as nonspecific siRNA (D-001206-01) were obtained from Dharmacon Tech. Cell line Nucleofector kit V for electroporation was purchased from Amaxa Biosystems.
Preparation of Whole-Cell Extract (WCE) from Cells and Immunoblotting Assays.
The WCEs were prepared as described in ref. 26.
DNA Extraction and Mutational Analysis.
Genomic DNA isolation.
Frozen tumor samples ≈2 mm in diameter were homogenized in 200 μl of lysis buffer [50 mM Tris·HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA (pH 8.0), 0.5% Nonidet P-40]. After homogenization, genomic DNA was isolated by using Easy-DNA kit (Invitrogen) following the manufacturer's instructions. The purified DNA was resuspended in 50 μl of TE buffer.
Mutational analysis.
Primer pairs for IGF1R exons 15–20 are listed in Table S4. Each PCR was performed in a 50-μl volume containing 10 ng of genomic DNA, a 15 μM concentration of each primer, 0.2 mM dNTP, 5 μl of 10× reaction buffer, 2.5 mM MgCl2, and 1 unit of AmpliTaq Gold DNA polymerase (Applied Biosystems). The PCR conditions were: 30 s at 94°C, 30 s at 52°C, 1 min at 68°C for 36 cycles followed by a 10-min extension at 68°C. PCR products were analyzed in 2% agarose gel electrophoresis and purified by a PCR purification kit (Qiagen). Direct sequencing was carried out from both directions by using BigDye Terminator V3.1 cycle sequencing kit on an ABI PRISM 3100 genetic analyzer (Applied Biosystems).
IHC Analysis.
IHC staining was performed on 5-μm slides. After deparaffinization and rehydration, sections were subjected to heat-induced epitope retrieval by immersion in a 0.01 M citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked for 15 min in 3% hydrogen peroxide in methanol. Nonspecific binding was blocked by treatment with a blocking reagent (protein block serum-free; DAKO) for 30 min at room temperature. The slides were then incubated overnight with primary antibody at 4°C in a humidified chamber. All primary antibodies were diluted to a concentration of 1:50. Immunodetection was performed by using the super-sensitive link-label (biotin-based) IHC detection systems.
Histological scoring and analysis.
All IHC evaluation was performed in a blinded manner by one author (D.F.). The following criteria were used to assess distribution and intensity of positive tumor cell staining.
Distribution.
Absent tumor cell staining was scored as 0, <10% of positive tumor cells staining was scored 1, 10–50% of cells staining was scored as 2, 50–90% of cells staining was scored as 3, and >90 of cells staining was scored as 4.
Intensity.
Absent staining in tumor cells was scored as 0, equivocal was scored as 1, clearly positive was scored as 2, and strong positive staining was scored as 3.
Scoring.
The results for intensity and distribution were summed, and a “score” assigned as follows: sum of 0, no staining (score 0); sum of 1–3, slight staining (score 1); sum of 4–5, moderate staining (score 2); and sum of 6–7, marked staining (score 3).
Cell Proliferation/Viability Assay.
Proliferation and viability were assessed with an assay based on the cleavage of the tetrazolium salt WST-1 to formazan by cellular mitochondrial dehydrogenases (Roche). GIST 882 and GIST-T1 cells were seeded in 96-well plates at a density of 1.5 × 105 cells per well. Twenty-four hours later, cells were treated with varying doses of NVP-AEW541 and/or imatinib mesylate. Cell proliferation and viability were measured 72 h after treatment with WST-1 reagent. The metabolic activity of viable cells was quantified 4 h later with an EnVision microplate reader (PerkinElmer).
siRNA Transfection.
GIST-T1 cells were trypsin-treated and resuspended in Amaxa Nucleofector solution V at a concentration of 1 × 106 cells per 100 μl. Nucleofection was done by using the T20 program on the Nucleofector II device. Five microliters of each 20 μM siRNA was used for electroporation. After electroporation, cells were washed with 3 ml of growth medium, counted, and seeded according to experimental designs.
Statistical Analysis.
All reported values are the means ± SEM. Statistical comparisons were determined with either one-sided (comparing tumor with normal control) or two-sided (comparing WT GISTs with mutant GISTs) Fisher's exact test. Results were considered statistically significant if the P value was <0.05.
Supplementary Material
Acknowledgments.
We acknowledge the valuable input of Ms. Yuliya Skorogabotko and Drs. Chong Xu, Samuel Litwin, and Alfred Knudson in this work. We acknowledge the support of Ms. Tania Stutman and the GIST Cancer Research Fund. This work was supported in part by National Institutes of Health Grant CA106588 (to A.K.G.), FCCC Translational Research Committee Grant 5P30CA06927-44 (to C.T, M.v.M., and A.K.G.), and by National Institutes of Health Training Institutional National Research Service Award CA009035-31 (to L.R.). J.R.T. was supported by National Institutes of Health Grants CA77429, and J.R.T., M.V.M., and A.K.G. are supported by P30 CA06927. L.R. was supported by a GIST Cancer Research Fund fellowship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803383105/DCSupplemental.
References
- 1.Mazur MT, Clark HB. Gastric stromal tumors: Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507–519. doi: 10.1097/00000478-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Heinrich MC, Corless CL. Gastric GI stromal tumors (GISTs): The role of surgery in the era of targeted therapy. J Surg Oncol. 2005;90:195–207. doi: 10.1002/jso.20230. [DOI] [PubMed] [Google Scholar]
- 3.Subramanian S, et al. Gastrointestinal stromal tumors (GISTs) with KIT and PDGFRA mutations have distinct gene expression profiles. Oncogene. 2004;23:7780–7790. doi: 10.1038/sj.onc.1208056. [DOI] [PubMed] [Google Scholar]
- 4.Tarn C, et al. Analysis of KIT mutations in sporadic and familial gastrointestinal stromal tumors: Therapeutic implications through protein modeling. Clin Cancer Res. 2005;11:3668–3677. doi: 10.1158/1078-0432.CCR-04-2515. [DOI] [PubMed] [Google Scholar]
- 5.Buchdunger E, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000;295:139–145. [PubMed] [Google Scholar]
- 6.Demetri GD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 7.Verweij J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: Randomised trial. Lancet. 2004;364:1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 8.Debiec-Rychter M, et al. Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2004;40:689–695. doi: 10.1016/j.ejca.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 9.Heinrich MC, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 10.Price VE, Zielenska M, Chilton-MacNeill S, Smith CR, Pappo AS. Clinical and molecular characteristics of pediatric gastrointestinal stromal tumors (GISTs) Pediatr Blood Cancer. 2005;45:20–24. doi: 10.1002/pbc.20377. [DOI] [PubMed] [Google Scholar]
- 11.Janeway KA, et al. Pediatric KIT wild-type and platelet-derived growth factor receptor α-wild-type gastrointestinal stromal tumors share KIT activation but not mechanisms of genetic progression with adult gastrointestinal stromal tumors. Cancer Res. 2007;67:9084–9088. doi: 10.1158/0008-5472.CAN-07-1938. [DOI] [PubMed] [Google Scholar]
- 12.LeRoith D, Roberts CT., Jr The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 13.Garrouste F, et al. Prevention of cytokine-induced apoptosis by insulin-like growth factor-I is independent of cell adhesion molecules in HT29–D4 colon carcinoma cells: Evidence for a NF-κB-dependent survival mechanism. Cell Death Differ. 2002;9:768–779. doi: 10.1038/sj.cdd.4401022. [DOI] [PubMed] [Google Scholar]
- 14.Gotlieb WH, et al. Insulin-like growth factor receptor I targeting in epithelial ovarian cancer. Gynecol Oncol. 2006;100:389–396. doi: 10.1016/j.ygyno.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 15.Sarfstein R, Maor S, Reizner N, Abramovitch S, Werner H. Transcriptional regulation of the insulin-like growth factor-I receptor gene in breast cancer. Mol Cell Endocrinol. 2006;252:241–246. doi: 10.1016/j.mce.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Wu JD, et al. Interaction of IGF signaling and the androgen receptor in prostate cancer progression. J Cell Biochem. 2006;99:392–401. doi: 10.1002/jcb.20929. [DOI] [PubMed] [Google Scholar]
- 17.Yanochko GM, Eckhart W. Type I insulin-like growth factor receptor overexpression induces proliferation and antiapoptotic signaling in a three-dimensional culture model of breast epithelial cells. Breast Cancer Res. 2006;8:R18. doi: 10.1186/bcr1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almeida A, Muleris M, Dutrillaux B, Malfoy B. The insulin-like growth factor I receptor gene is the target for the 15q26 amplicon in breast cancer. Genes Chromosomes Cancer. 1994;11:63–65. doi: 10.1002/gcc.2870110110. [DOI] [PubMed] [Google Scholar]
- 19.Armengol G, et al. DNA copy number changes and evaluation of MYC, IGF1R, and FES amplification in xenografts of pancreatic adenocarcinoma. Cancer Genet Cytogenet. 2000;116:133–141. doi: 10.1016/s0165-4608(99)00118-1. [DOI] [PubMed] [Google Scholar]
- 20.Natrajan R, et al. Blastemal expression of type I insulin-like growth factor receptor in Wilms' tumors is driven by increased copy number and correlates with relapse. Cancer Res. 2006;66:11148–11155. doi: 10.1158/0008-5472.CAN-06-1931. [DOI] [PubMed] [Google Scholar]
- 21.Hopfner M, et al. Blockade of IGF-1 receptor tyrosine kinase has antineoplastic effects in hepatocellular carcinoma cells. Biochem Pharmacol. 2006;71:1435–1448. doi: 10.1016/j.bcp.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 22.El-Rifai W, Sarlomo-Rikala M, Andersson LC, Knuutila S, Miettinen M. DNA sequence copy number changes in gastrointestinal stromal tumors: Tumor progression and prognostic significance. Cancer Res. 2000;60:3899–3903. [PubMed] [Google Scholar]
- 23.Meza-Zepeda LA, et al. Array comparative genomic hybridization reveals distinct DNA copy number differences between gastrointestinal stromal tumors and leiomyosarcomas. Cancer Res. 2006;66:8984–8993. doi: 10.1158/0008-5472.CAN-06-1972. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Echeverria C, et al. In vivo antitumor activity of NVP-AEW541: A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5:231–239. doi: 10.1016/s1535-6108(04)00051-0. [DOI] [PubMed] [Google Scholar]
- 25.Hailey J, et al. Neutralizing anti-insulin-like growth factor receptor 1 antibodies inhibit receptor function and induce receptor degradation in tumor cells. Mol Cancer Ther. 2002;1:1349–1353. [PubMed] [Google Scholar]
- 26.Tarn C, et al. Therapeutic effect of imatinib in gastrointestinal stromal tumors: AKT signaling dependent and independent mechanisms. Cancer Res. 2006;66:5477–5486. doi: 10.1158/0008-5472.CAN-05-3906. [DOI] [PubMed] [Google Scholar]
- 27.Frolov A, et al. Response markers and the molecular mechanisms of action of Gleevec in gastrointestinal stromal tumors. Mol Cancer Ther. 2003;2:699–709. [PubMed] [Google Scholar]
- 28.Yanada M, Naoe T. Imatinib combined chemotherapy for Philadelphia chromosome-positive acute lymphoblastic leukemia: Major challenges in current practice. Leuk Lymphoma. 2006;47:1747–1753. doi: 10.1080/10428190600634085. [DOI] [PubMed] [Google Scholar]
- 29.Miller BS, Yee D. Type I insulin-like growth factor receptor as a therapeutic target in cancer. Cancer Res. 2005;65:10123–10127. doi: 10.1158/0008-5472.CAN-05-2752. [DOI] [PubMed] [Google Scholar]
- 30.Haluska P, et al. ASCO Annual Meeting. Chicago, IL: American Society of Clinical Oncology; 2007. p. 3586. [Google Scholar]
- 31.Karp DD, et al. ASCO Annual Meeting. Chicago, IL: American Society of Clinical Oncology; 2007. p. 7506. [Google Scholar]
- 32.Rodon J, et al. ASCO Annual Meeting. Chicago, IL: American Society of Clinical Oncology; 2007. p. 3590. [Google Scholar]
- 33.Tolcher AW, et al. ASCO Annual Meeting. Chicago, IL: American Society of Clinical Oncology; 2007. p. 3002. [Google Scholar]
- 34.Trent JC, et al. Early effects of imatinib mesylate on the expression of insulin-like growth factor binding protein-3 and positron emission tomography in patients with gastrointestinal stromal tumor. Cancer. 2006;107:1898–1908. doi: 10.1002/cncr.22214. [DOI] [PubMed] [Google Scholar]
- 35.Prakash S, et al. Gastrointestinal stromal tumors in children and young adults: A clinicopathologic, molecular, and genomic study of 15 cases and review of the literature. J Pediatr Hematol Oncol. 2005;27:179–187. doi: 10.1097/01.mph.0000157790.81329.47. [DOI] [PubMed] [Google Scholar]
- 36.Heinrich MC, et al. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96:925–932. [PubMed] [Google Scholar]
- 37.Stommel JM, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 38.Hopfner M, Baradari V, Huether A, Schofl C, Scherubl H. The insulin-like growth factor receptor 1 is a promising target for novel treatment approaches in neuroendocrine gastrointestinal tumours. Endocr Relat Cancer. 2006;13:135–149. doi: 10.1677/erc.1.01090. [DOI] [PubMed] [Google Scholar]
- 39.Sachdev D, Singh R, Fujita-Yamaguchi Y, Yee D. Down-regulation of insulin receptor by antibodies against the type I insulin-like growth factor receptor: Implications for anti-insulin-like growth factor therapy in breast cancer. Cancer Res. 2006;66:2391–2402. doi: 10.1158/0008-5472.CAN-05-3126. [DOI] [PubMed] [Google Scholar]
- 40.O'Reilly KE, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warshamana-Greene GS, et al. The insulin-like growth factor-I receptor kinase inhibitor, NVP-ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy. Clin Cancer Res. 2005;11:1563–1571. doi: 10.1158/1078-0432.CCR-04-1544. [DOI] [PubMed] [Google Scholar]
- 42.Faivre S, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 43.Morabito A, De Maio E, Di Maio M, Normanno N, Perrone F. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: Current status and future directions. Oncologist. 2006;11:753–764. doi: 10.1634/theoncologist.11-7-753. [DOI] [PubMed] [Google Scholar]
- 44.Braconi C, et al. Insulin-like growth factor (IGF) 1 and 2 help to predict disease outcome in GIST patients. Ann Oncol. 2008 doi: 10.1093/annonc/mdn040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.