Abstract
Obesity is a principal risk factor for type 2 diabetes, and elevated fatty acids reduce β-cell function and survival. An unbiased proteomic screen was used to identify targets of palmitate in β-cell death. The most significantly altered protein in both human islets and MIN6 β-cells treated with palmitate was carboxypeptidase E (CPE). Palmitate reduced CPE protein levels within 2 h, preceding endoplasmic reticulum (ER) stress and cell death, by a mechanism involving CPE translocation to Golgi and lysosomal degradation. Palmitate metabolism and Ca2+ flux were also required for CPE proteolysis and β-cell death. Chronic palmitate exposure increased the ratio of proinsulin to insulin. CPE null islets had increased apoptosis in vivo and in vitro. Reducing CPE by ≈30% using shRNA also increased ER stress and apoptosis. Conversely, overexpression of CPE partially rescued β-cells from palmitate-induced ER stress and apoptosis. Thus, carboxypeptidase E degradation contributes to palmitate-induced β-cell ER stress and apoptosis. CPE is a major link between hyperlipidemia and β-cell death pathways in diabetes.
Keywords: 2D difference gel electrophoresis proteomics, free fatty acids, hyperproinsulinemia, mechanisms of β-cell lipotoxicity, type 2 diabetes
There is a strong association between type 2 diabetes and obesity. High levels of circulating lipids, including free fatty acids, are a prominent clinical feature of type 2 diabetes and represent an important risk factor for this disease (1, 2). But exactly how elevated lipids might lead to diabetes remains unresolved. Fatty acids increase basal insulin secretion (3) and the relative levels of circulating proinsulin (4). Chronic exposure to the free fatty acid palmitate has been shown to impair glucose-stimulated insulin release (i.e., lipotoxicity) (5–10). β-Cell apoptosis can be initiated by high levels of palmitate (6, 7, 11–14), which may account in part for alterations in insulin secretory function (13). A number of studies have established palmitate targets in the β-cell, including lipid metabolism (15, 16), mitochondrial function (17–23), and prosurvival transcription factors such as Pdx1 (24, 25). Recently, a role for endoplasmic reticulum (ER) stress in lipotoxicity has been demonstrated in multiple cell types, including β-cells (11, 26, 27). The effects of palmitate on β-cell survival are likely mediated by a number of mechanisms.
In the present study, we conducted unbiased proteomic screens using human islets and MIN6 β-cells to elucidate targets of palmitate. Carboxypeptidase E (CPE) was the most significantly changed protein in both screens. Mice lacking CPE develop hyperproinsulinemia and hyperglycemia (28), but the involvement of this protein in β-cell apoptosis has not been reported. Palmitate caused the rapid intracellular redistribution and degradation of CPE via mechanisms that required palmitate metabolism, KATP channel closure, Ca2+ influx, and protease activity. We further showed that CPE levels control β-cell ER stress and apoptosis. Thus, CPE is a critical link between saturated free fatty acids, a major type 2 diabetes risk factor, and β-cell dysfunction.
Results
Analysis of the β-Cell Proteome During Palmitate-Induced Apoptosis.
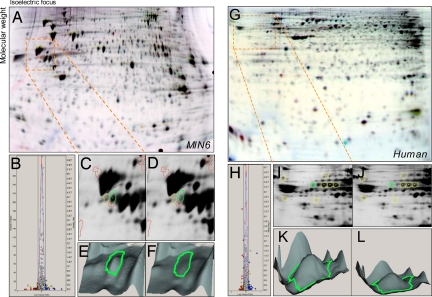
Palmitate, but not oleate, induced dose-dependent apoptosis, illustrated by DNA laddering [supporting information (SI) Fig. S1A]. A high-throughput, real-time assay of β-cell death demonstrated that palmitate-induced propidium iodide incorporation was significant at ≈12 h. Neither the rate nor the amplitude of palmitate-induced death differed between 5 mM glucose and 25 mM glucose (Fig. S1B). Palmitate did not require high glucose to induce ER stress in MIN6 cells (Fig. S1C) or human islets (Fig. S1D; see also Fig. S4B). We have shown previously that palmitate could induce apoptosis in mouse islets cultured in 5 mM glucose (29). To better understand the mechanism of β-cell lipotoxicity, we undertook proteomic analyses of MIN6 cells and human pancreatic islets exposed to palmitate. Strikingly, the gel features most reduced by palmitate in both MIN6 cells and human islets were identified as CPE (Fig. 1 and Figs. S2 and S3). Significant changes were also found in protein spots identified as key metabolic enzymes (NADH dehydrogenase, phosphoglycerate mutase, ATP citrate lyase), and components of the proteasome (UCHL1, proteosome subunit α3) (Tables S1 and S2). Because of its known role in insulin processing and diabetes, we focused primarily on CPE in this study.
Fig. 1.
Fluorescence-based analysis of palmitate-induced changes in the MIN6 and human islet cell proteomes under conditions of β-cell apoptosis and ER stress. (A) In 2D gels of MIN6 cells treated with palmitate for 24 h, red spots represent decreased protein species, whereas blue spots show increased proteins. (B) Normalization of all spot volumes across treatments revealed significantly different spots (>2 SD). (C–F) C is a close-up of orange box in D and 3D rendering (E and F) of the most altered gel feature between control (Left) and palmitate-treated (Right) cells. (G–L) The identical proteomics screen performed on human islets also identified CPE as the most changed gel feature.
Palmitate Causes Rapid CPE Degradation.
The reduced protein spots identified as CPE in the 2-D gels were the approximate size of full-length CPE, rather than fragments. To exclude the possibility that the observed decrease in relative abundance of the gel features was due solely to posttranslational modifications that alter the isoelectric point, we performed Western blot analysis. These experiments demonstrated that palmitate significantly and dose dependently decreased total CPE protein in both MIN6 cells and human islets (Fig. 2A and Fig. S4A and B), with the strongest inhibition seen at a 6:1 palmitate-to-albumin ratio, a dose similar to what would be expected in obese or diabetic patients (30, 31). Similar results were seen when GAPDH or tubulin were used as loading controls (not shown). A loss of CPE was also detected with antibodies to the C-terminal and N-terminal regions (Fig. S4C), suggesting loss of the entire protein. A related enzyme, carboxypeptidase D, was not reduced by palmitate in low glucose (Fig. S4 D and F). Prohormone convertase 1/3 and prohormone convertase 2 were not significantly altered by 24-h culture in palmitate or glucose (Fig. S4 D, G, and H). Together, these findings indicate that palmitate specifically reduces the total levels of CPE protein.
Fig. 2.
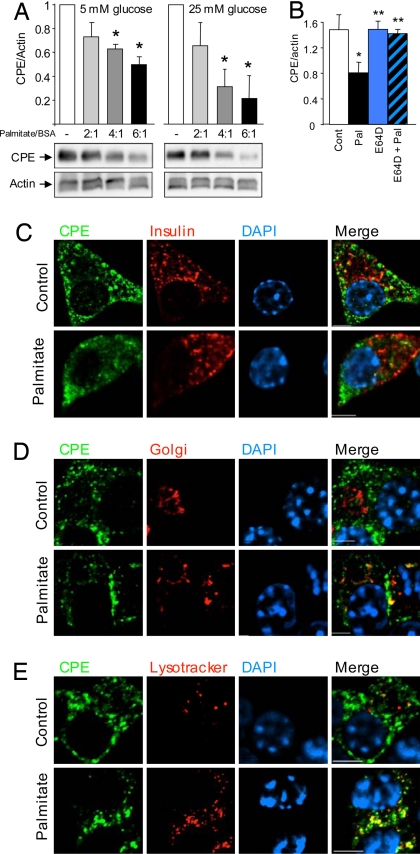
Palmitate causes CPE degradation. (A) CPE levels in MIN6 cells treated for 24 h with different ratios of palmitate to BSA, in 5 mM or 25 mM glucose. The asterisk denotes a significant difference from the control (n = 4). (B) Quantification of Western blot of MIN6 cells treated with palmitate (6:1) and/or 25 μM E64D for 24 h at 25 mM glucose. The asterisk denotes significant difference from the control; the double asterisk denotes significant difference from palmitate treatment (n = 3). (C–E) Representative images of the colocalization of CPE with respect to insulin, Golgi marker (GM130), and lysosomes are shown. (Scale bars, 5 μm.)
We tested whether altered CPE translation or transcription were involved in the loss of CPE. As expected, cycloheximide alone decreased CPE protein synthesis, indicating that a large fraction of CPE is degraded in less than 24 h. The co-incubation of cells with palmitate and cycloheximide resulted in a further decrease in CPE expression compared to the effects of cycloheximide alone (Fig. S4I), suggesting that palmitate caused CPE degradation. RT-PCR of palmitate-treated MIN6 cells suggested that palmitate did not affect steady-state CPE mRNA (Fig. S4 J and K). CPE levels were not significantly altered by high glucose, indicating that CPE was not lost via exocytosis (Fig. S4 A–D, and I). In our hands, palmitate is a weak insulin secretagogue compared to glucose (Fig. S5). We used a protease inhibitor to directly test the hypothesis that CPE was degraded in response to palmitate. Indeed, the cysteine protease inhibitor E64D prevented the palmitate-induced reduction in CPE protein (Fig. 2B). These data strongly support the concept that palmitate caused CPE proteolysis.
Soluble and membrane-bound forms of CPE reside in secretory granules, Golgi, and ER (32, 33). In the present study, we examined the subcellular location of CPE in the presence and absence of palmitate. In control conditions, CPE was found both within and outside of secretory granules. Palmitate caused CPE to be lost from insulin granules (Fig. 2C). In the presence of palmitate, there was a dramatic accumulation of CPE in Golgi and lysosomes (Fig. 2 D and E). Experiments were repeated with similar results with other CPE antibodies (not shown). Thus, the loss of CPE protein is associated with its redistribution to Golgi and lysosomes, where it is presumably degraded by E64D-sensitive proteases. Taken together with the rapidity of the CPE loss (see below), these results suggest that CPE is targeted for degradation in palmitate-treated β-cells.
Mechanisms of CPE Degradation, ER stress, and β-Cell Death.
Because of the apoptotic effects of palmitate, it was essential to determine whether the decrease in CPE protein was secondary to an increase in ER stress and cell death. The palmitate-induced loss of CPE, already maximal at 2 h, preceded CHOP induction (6 h; Fig. 3 A and B) and the beginning of widespread cell death (12 h, Fig. S1B). In addition, CPE was unaffected by thapsigargin (Fig. 3C), a drug known to induce β-cell ER stress and death. Together, these results further show that neither ER stress nor apoptosis caused the decrease in CPE. These findings support the idea that the palmitate-induced decrease in CPE lies upstream of ER stress and apoptosis.
Fig. 3.
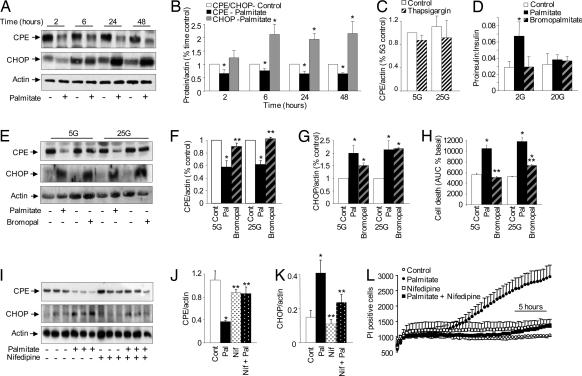
Ca2+-dependent mechanisms of palmitate-induced CPE degradation, ER stress, and β-cell death and dysfunction. (A and B) MIN6 cells incubated with palmitate at 25 mM glucose at specific time points (n = 3). (C) Twenty-four-hour incubation of MIN6 cells with 1 μM thapsigargin did not affect CPE expression (n = 3). (D) The molar ratio of proinsulin to insulin secreted from human islets treated with palmitate and 2-bromopalmitate over 48 h (n = 5). (E–G) CPE and CHOP expression in MIN6 cells treated for 24 h with palmitate or 2-bromopalmitate at low or high glucose (n = 3). (H) Propidium iodide incorporation in MIN6 cells treated with palmitate or 2-bromopalmitate over 24 h (n = 3). (I–K) Western blot and quantification of CPE and CHOP in MIN6 cells treated with palmitate and 10 μM nifedipine for 24 h at 25 mM glucose (n = 3). (L) Propidium iodide incorporation in MIN6 cells at 25 mM glucose with palmitate and 10 μM nifedipine (n = 3). The asterisk denotes a significant difference from control; the double asterisk denotes a significant difference from palmitate treatments.
Next, the role of cellular palmitate metabolism in the loss of CPE was investigated by using the nonmetabolizable palmitate homolog 2-bromopalmitate. Incubation of both MIN6 cells (Fig. 3 E and F) and human islets (not shown) with bromopalmitate at low or high glucose did not significantly decrease CPE protein. CHOP expression was induced by palmitate and, to a lesser extent, bromopalmitate (Fig. 3G). This indicated that some ER stress can occur under conditions where CPE is not significantly altered, especially in high glucose conditions. Cell death in the presence of bromopalmitate was significantly retarded compared with palmitate-treated cells at both low and high glucose (Fig. 3H). Taken together, these data illustrate that palmitate metabolism is important for the loss of CPE and maximal β-cell death.
Palmitate is known to increase Ca2+ influx into β-cells (17), and we have confirmed this in MIN6 cells and human β-cells (J.D.J., unpublished data). It would be expected that palmitate metabolism, but not activation of the FFA receptor GPR40, would increase the β-cell ATP to ADP ratio, close KATP channels, and activate voltage-gated Ca2+ channels in the plasma membrane. Diazoxide, a KATP channel activator, blocked palmitate-induced MIN6 cell death (Fig. S4 L and M), further implicating metabolism in the toxic effects of palmitate. Incubation of MIN6 cells with a dose of nifedipine known to completely block β-cell voltage-gated Ca2+ currents (34) prevented the loss of CPE protein, attenuated CHOP induction (Fig. 3 I–K), and prevented palmitate-induced cell death (Fig. 3L and Fig. S4 N). These results suggest that the effects of palmitate on CPE, as well as its effects on ER stress and cell death, are Ca2+-dependent.
Hyperproinsulinemia is found in type 2 diabetics, and some studies have suggested that this condition can predict the onset of diabetes (35, 36). Mice lacking functional CPE also exhibit elevated plasma proinsulin levels (28). After 24 h, palmitate, but not bromopalmitate, significantly increased the ratio of proinsulin to insulin that had been secreted into the medium by human islets (Fig. 3D), suggesting a defect in insulin processing. Acute palmitate treatment did not alter the secreted proinsulin-to-insulin ratio (Fig. S5 B–D). These findings are consistent with the idea that a chronic reduction in CPE accounts for the defect in proinsulin processing.
CPE Regulates Islet Survival in Vivo and in Vitro.
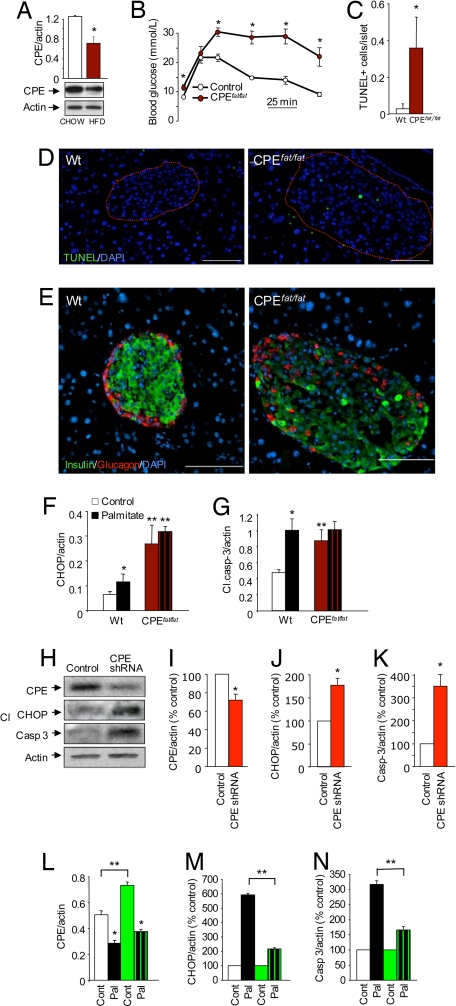
The effects of lipids on CPE levels in vivo were assessed by subjecting C57BL/6J mice and their littermate controls to a high-fat diet. Analysis of islets isolated from these mice demonstrated that hyperlipidemia decreased CPE protein expression in vivo (Fig. 4A). The ratio of CPE-processed mature insulin to total insulin was dramatically decreased in islets from high-fat-fed mice and in MIN6 cells treated with palmitate. This decrease in mature insulin was comparable to that seen in mice lacking CPE (Fig. S6). The studies described above show that CPE protein is decreased under conditions in which FFA induces ER stress and β-cell death. We next used both loss-of-function and gain-of-function experiments to test the hypothesis that CPE is a causal factor in β-cell apoptosis. We took advantage of mice deficient in CPE (Fig. 4B) to determine whether CPE plays a role in β-cell survival in vivo and in vitro. Pancreatic sections from CPEfat/fat mice showed significantly more TUNEL-positive cells in their islets compared to those of littermate controls (Fig. 4 C and D). Moreover, although CPEfat/fat islets generally covered a larger area, there was a striking loss of normal architecture, with marked cell loss within the islet (Fig. 4E and Fig. S6). A caveat with these experiments is that the CPEfat/fat mice exhibit hyperglycemia (Fig. 4B) and it is possible that chronic exposure to high glucose could augment β-cell death in this model. To determine whether islets of the CPEfat/fat mice were more susceptible to apoptosis in a controlled in vitro setting, we exposed isolated islets to palmitate for 24 h. Even in the absence of palmitate, islets from CPEfat/fat mice showed significantly higher CHOP and caspase-3 activation (Fig. 4 F and G), consistent with the in vivo findings described above. Palmitate increased CHOP expression and cleaved caspase-3 levels in wild-type islets, but the effects of palmitate were not additive to the caspase-3 activation induced by CPE deficiency (Fig. 4 F and G). In preliminary experiments, we also observed an increase in cleaved caspase-3 in freshly isolated CPEfat/fat mouse islets (data not shown). These results suggested that the islets of mice lacking CPE have higher basal levels of ER stress and apoptosis, both in vivo and in vitro. The observation that palmitate did not induce further caspase-3-dependent apoptosis in CPEfat/fat islets suggests that the suppression of CPE may play an essential role in palmitate-induced β-cell death.
Fig. 4.
In vivo and in vitro role of CPE in β-cell death. The ratio of CPE-processed mature insulin to total insulin was dramatically decreased in islets from high-fat-fed mice and in MIN6 cells treated with palmitate. This decrease in mature insulin was comparable to that seen in mice lacking CPE (Fig. S6). (A) Reduced CPE protein expression in isolated islets from C57BL/6J mice fed high-fat diet (40% calories from fat) for 6 months (n = 3). (B) Homozygous null CPEfat/fat mice were significantly heavier (28 ± 2 g vs. 41 ± 3 g) and exhibited significantly impaired i.p. glucose tolerance compared with littermate controls (n = 4). (C and D) Increased TUNEL staining in islets of pancreatic sections of CPEfat/fat mice compared with wild-type controls (n = 3). (Scale bar, 100 μm.) (E) Representative insulin (green), glucagon (red), and DAPI (blue) staining in the islets of CPEfat/fat mice and wild-type controls. Islets from mutant mice had weaker and more heterogeneous insulin staining, as well as disrupted architecture. (F and G) CHOP and cleaved caspase-3 protein were quantified in isolated islets from control and CPEfat/fat mice treated as indicated for 24 h in 20 mM glucose (n = 3). (H–K) Significant small hairpin RNA-mediated reduction in CPE protein increases CHOP expression and caspase-3 cleavage, compared with MIN6 cells transfected with a scrambed shRNA control (n = 3). (L–N) MIN6 cells were transfected with a CPE plasmid (green bars) with palmitate for 24 h at 25 mM glucose (n = 3). The asterisk denotes significance between palmitate and control; the double asterisk denotes significance between vectors within the same treatment.
Next, we sought to further verify the causal link between CPE deficiency and increased β-cell ER stress and apoptosis. Using a combination of plasmid-based RNA interference and fluorescence-activated cell sorting (to enrich for shRNA-GFP expressing cells), we were able reduce CPE protein levels in MIN6 cells by ≈30%. This modest, but significant, decrease in CPE expression was sufficient to significantly increase levels of CHOP and cleaved caspase-3, relative to control cells transfected with scrambled shRNA-GFP (Fig. 4 H–K). Together, with the results presented above, this experiment demonstrates that a reduction in CPE similar to that caused by palmitate significantly increases β-cell apoptosis.
Finally, we determined whether an increase in CPE might be able to rescue β-cells from palmitate-induced death, by exploring the effects of overexpressing CPE in palmitate-treated MIN6 cells. Using a vector with a CMV promoter, we were able to transiently overexpress CPE by 1.5 fold (Figs. 4L and S7A), whereas only minor over-expression was observed with the rat insulin promoter (data not shown). Palmitate treatment caused a similar relative reduction of CPE in CPE overexpressing and untransfected cells. However, the absolute level of CPE remained higher in the palmitate-treated CPE overexpressing cells compared to the empty-vector transfected cells. CPE overexpression itself caused an increase in ER stress, as might be expected with the increased ER protein load. For this reason, the results of palmitate treatment were normalized to their respective controls. When the effects of CPE over-expression alone were controlled for, palmitate-induced CHOP and cleaved caspase-3 activation were significantly reduced in these cells (Fig. 4 M and N). Together, these findings suggest that increased CPE levels can limit the deleterious effects of palmitate on β-cells and suggest a role for CPE in the control of β-cell ER stress and apoptosis.
Discussion
Pathways involved in fatty acid-induced ER stress and β-cell apoptosis were investigated in this study. An unbiased proteomics screen elucidated several unexpected targets for the saturated fatty acid palmitate. CPE was the most decreased protein in two independent screens using MIN6 cells and human islets. CPE is a key enzyme in the insulin secretory pathway, and disruptions in this pathway are known to alter the function and survival of pancreatic β-cells, and to cause diabetes in humans and animals (37, 38). In the CPEfat/fat mouse strain, a single point mutation in CPE is sufficient to produce an animal with multiple disorders including obesity and diabetes (28, 39). Importantly, one study found CPE polymorphisms associated with type 2 diabetes in humans (40). The finding that changes in CPE protein levels may mediate the adverse effects of the saturated fatty acid palmitate on β-cell function and may contribute to the pathogenesis of diabetes is an important advancement in our understanding of the molecular pathways involved in the progression of this disease.
The rapid loss of CPE in palmitate-treated cells, and the insensitivity of CPE levels to thapsigargin treatment, placed CPE upstream of ER stress and apoptosis in the β-cell. The findings that the loss of functional CPE leads to apoptosis in vivo and in vitro, and that CPE overexpression partially rescues β-cells from palmitate-induced ER stress and death, demonstrate a previously unappreciated role for CPE in programmed cell death. FFAs have been shown to induce ER stress (i.e., expression of CHOP, spliced XBP-1, ATF4, and eIF2α) in multiple studies (11, 26, 27, 41–43), and ER stress is known to be induced under situations of protein overload in the secretory pathway (44, 45). Our results suggest that a component of palmitate-induced β-cell death can be explained by the loss of CPE, an event that is sufficient to cause ER stress potentially because of a backlog of unprocessed proinsulin in the secretory pathway.
Our studies also defined, to a large extent, the mechanism by which palmitate reduces CPE levels. The results point to a rapid CPE degradation that requires palmitate metabolism, ATP synthesis, plasma membrane depolarization, and Ca2+ influx. We and others (46, 47) have found that palmitate is more cytotoxic than 2-bromopalmitate. These results argue against a prominent role in β-cell death for the G protein-coupled FFA receptor GPR40, which nevertheless appears to be involved in β-cell Ca2+ signaling and insulin secretion (48, 49). This conclusion is supported by studies of GPR40 transgenic and knockout mice. Overexpression of GPR40 in mice led to overt diabetes, but morphological analysis of the pancreas showed no evidence of reduced β-cell mass (49). Although GPR40 knockout mice were protected from developing diabetes following a high-fat diet in vivo, islets of these mice were not rescued from palmitate-induced apoptosis in vitro (49, 50).
We and others have established that palmitate increases cytosolic Ca2+ levels in primary β-cells and β-cell lines (17, 51, 52). In our experiments, blocking Ca2+ influx with nifedipine abolished the palmitate-associated decrease in CPE and the induction of ER stress, and prevented palmitate-induced death in MIN6 cells. Cell death was also prevented by co-incubation of cells with diazoxide, a KATP channel activator that prevents ATP-dependent membrane depolarization and subsequent Ca2+ influx through l-type Ca2+ channels. Our results show that nifedipine can prevent the palmitate-associated reduction in CPE, and other studies have also investigated the potential link between Ca2+ and CPE. Notably, local Ca2+ has been implicated in the control of CPE stability in the trans-Golgi network (53, 54). Together, these results strongly suggest that increased β-cell Ca2+ flux is a requirement for palmitate-induced apoptosis, and that altered Ca2+ flux may play a role in the degradation of CPE.
Efforts to pinpoint the exact protease(s) responsible for the loss of CPE were only partially successful. E64D is a general inhibitor of cysteine proteases, and its targets include both lysosomal proteases and calpains. This drug prevented the palmitate-induced loss of CPE, a finding that agrees well with our data showing CPE translocation to lysosomes. However, both E64D and another protease inhibitor (ALLM; data not shown) caused ER stress on their own, likely because of a backlog of undegraded proteins, making interpretation of their effects on palmitate-induced β-cell death difficult. An inhibitor of UCHL1, a component of the ubiquitin system and the 2-D gel feature most increased by palmitate in the proteomic analysis, prevented the loss of CPE in response to palmitate in high glucose, but not low glucose (Fig. S7B). In our previous study, ALLM and specific deletion of calpain-10 partially reduced apoptosis in primary mouse islets in long-term cultures containing palmitate and 5 mM glucose (29). However, the link between the calpain-10 pathway and the ER stress-associated cascade that CPE participates in remains unclear.
Our results demonstrated that palmitate, but not bromopalmitate, increased the proinsulin-to-insulin ratio at physiological glucose concentrations. This indicates another important functional consequence of CPE degradation. An elevated proinsulin-to-insulin ratio is a well established clinical finding in diabetes and it has also been suggested to play a possible role in prediabetic states (35, 36). Our results provide a plausible mechanism for this defect. While our work demonstrates effects of FFAs on CPE, a previous study found reduced PC2 and PC1/3 posttranslational processing and increased proinsulin-to-insulin ratio in MIN6 cells treated with FFA for 7 days (4). Interestingly, CPE is required for the processing of prohormone convertases, and hyperproinsulinemic mice lacking CPE have reduced prohormone convertase (PC) 1/3 and 2 activity (55). CPE also reduces the ability of prohormone convertase products to inhibit PC1/3 and 2 (56). Thus, CPE may regulate PC1/3 and PC2 at multiple levels. Because we did not observe significant effects of palmitate on PC1/3 or PC2 at 24 h, it is likely that the loss of CPE precedes the loss of the prohormone convertases. Thus, palmitate may target multiple insulin-processing enzymes, perhaps CPE first, ultimately leading to a relative increase in the secretion of proinsulin, which has only 10% of the biological activity of insulin (57).
In conclusion, it was established in this study that in vitro treatment of both MIN6 cells and human islets with palmitate and in vivo exposure to a high-fat diet led to a reduction in CPE protein. Previously, CPE has been considered to be a “housekeeping” enzyme; here we demonstrate that CPE can be degraded in response to an extracellular signal in β-cells. Both loss-of-function and gain-of-function approaches suggested that CPE positively controls β-cell survival, via effects on ER stress. We propose a model for β-cell lipoxicity, enabled by our unbiased proteomic screens (Fig. S8). Together, these results provide evidence that CPE is a key link between hyperlipidemia/FFAs, insulin processing, and β-cell apoptosis pathways in type 2 diabetes
Experimental Procedures
Reagents and Animals.
A detailed list of reagents can be found in the SI Text. CPEfat/fat mice on the C57BL/6J background were from Jackson Laboratories. Intraperitoneal glucose tolerance tests (IPGTT; 2 g glucose/kg body weight) were performed on littermate males after a 12-h fast. In some studies, male C57BL/6J mice were fed a high-fat diet (40% of calories from fat, TD88137; Harlan Teklad) for 6 months and compared to littermates fed normal chow. Baseline phenotypes of these mice are detailed in the Supplement. All studies were approved by the University of British Columbia Animal Care Committee.
Cell Culture.
Our human islet, mouse islet, and MIN6 cell culture methods have been described (25, 29, 58) and are detailed in the SI Text. Hormone release was assessed by static incubation or perifusion (25). Insulin and proinsulin were assayed with Linco RIA or ELISA, respectively. Immunofluorescent staining is described in SI Text.
Cell Death and Apoptosis Assays.
The incorporation of propidium iodide was monitored in the incubated chamber of a KineticScan Reader (Cellomics). Propidium iodide fluoresces brightly once it passes through the compromised plasma membrane of dying cells. Additional details on this assay and the TUNEL assay are outlined in the SI Text.
Immunoblot and Proteomics.
We used standard methods for immunoblots, and our approach to 2-d-DIGE proteomics of human islets has been described (see SI Text for expanded details). Briefly, lysates from control and palmitate-treated cells were labeled with Cy dyes and analyzed in the same 2-D gel. Individual “spots” were quantified and considered significantly different if they were >2 standard deviations outside of the normalized distribution of gel feature intensities. Significantly different spots were sequenced by using mass spectroscopy.
shRNA-Mediated CPE Knockdown and CPE Overexpression.
CPE levels were reduced in MIN6 cells by using a vector expressing shRNA sequences designed against CPE (see SI Text) transfected with Lipofectamine 2000 (Invitrogen). GFP-positive cells were sorted by fluorescence-activated cell sorting (BD FACS Vantage SE/DIVA). CPE plasmids, one under the control of the CMV promoter, and another under the control of the rat insulin promoter, were kindly supplied by Dr. L. D. Fricker. CPE DNA (1 μg) or empty vector, was transfected into MIN6 cells by using Lipofectamine 2000 (Invitrogen).
Statistics.
Statistical analysis was performed with SigmaStat (Systat). ANOVA (Student–Newman–Keuls) or t test was used where appropriate. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments.
We thank Ting Yang and Ali Asadi for technical assistance, Dr. Michael Underhill for access to the KineticScan instrument, and Dr. Tim Kieffer and Bruce Verchere for advice. This work was supported by operating grants from the Canadian Diabetes Association (CDA) and the Canadian Institutes for Health Research (CIHR) (to J.D.J.). Proteomic studies were funded by National Institutes of Health Grant DK31842 (to K.S.P.), an institutional grant to the Washington University (WU) Proteomics Center, and WU Digestive Diseases Research Core Center Grant DK52574 and the National Center for Research Resources (P41RR00954) (to R.R.T.). J.D.J was supported by awards from the Juvenile Diabetes Research Foundation, the Michael Smith Foundation for Health Research, CIHR, and CDA.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711232105/DCSupplemental.
References
- 1.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 2.Paolisso G, et al. A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia. 1995;38:1213–1217. doi: 10.1007/BF00422371. [DOI] [PubMed] [Google Scholar]
- 3.Bollheimer LC, Skelly RH, Chester MW, McGarry JD, Rhodes CJ. Chronic exposure to free fatty acid reduces pancreatic beta cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. J Clin Invest. 1998;101:1094–1101. doi: 10.1172/JCI420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furukawa H, Carroll RJ, Swift HH, Steiner DF. Long-term elevation of free fatty acids leads to delayed processing of proinsulin and prohormone convertases 2 and 3 in the pancreatic beta-cell line MIN6. Diabetes. 1999;48:1395–1401. doi: 10.2337/diabetes.48.7.1395. [DOI] [PubMed] [Google Scholar]
- 5.Paolisso G, et al. Opposite effects of short- and long-term fatty acid infusion on insulin secretion in healthy subjects. Diabetologia. 1995;38:1295–1299. doi: 10.1007/BF00401761. [DOI] [PubMed] [Google Scholar]
- 6.Biden TJ, Robinson D, Cordery D, Hughes WE, Busch AK. Chronic effects of fatty acids on pancreatic beta-cell function: New insights from functional genomics. Diabetes. 2004;53(Suppl 1):S159–S165. doi: 10.2337/diabetes.53.2007.s159. [DOI] [PubMed] [Google Scholar]
- 7.Maedler K, et al. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 2001;50:69–76. doi: 10.2337/diabetes.50.1.69. [DOI] [PubMed] [Google Scholar]
- 8.Lupi R, et al. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: Evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes. 2002;51:1437–1442. doi: 10.2337/diabetes.51.5.1437. [DOI] [PubMed] [Google Scholar]
- 9.Zhou YP, Grill V. Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of Langerhans. J Clin Endocrinol Metab. 1995;80:1584–1590. doi: 10.1210/jcem.80.5.7745004. [DOI] [PubMed] [Google Scholar]
- 10.Unger RH, Zhou YT. Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes. 2001;50(Suppl 1):S118–S121. doi: 10.2337/diabetes.50.2007.s118. [DOI] [PubMed] [Google Scholar]
- 11.Kharroubi I, et al. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145:5087–5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 12.Eitel K, et al. Different role of saturated and unsaturated fatty acids in beta-cell apoptosis. Biochem Biophys Res Commun. 2002;299:853–856. doi: 10.1016/s0006-291x(02)02752-3. [DOI] [PubMed] [Google Scholar]
- 13.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: A link between obesity and diabetes. Proc Natl Acad Sci USA. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003;52:726–733. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- 15.Listenberger LL, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cnop M, Hannaert JC, Hoorens A, Eizirik DL, Pipeleers DG. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes. 2001;50:1771–1777. doi: 10.2337/diabetes.50.8.1771. [DOI] [PubMed] [Google Scholar]
- 17.Remizov O, et al. Palmitate-induced Ca2+-signaling in pancreatic beta-cells. Mol Cell Endocrinol. 2003;212:1–9. doi: 10.1016/j.mce.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 18.Maestre I, et al. Mitochondrial dysfunction is involved in apoptosis induced by serum withdrawal and fatty acids in the beta-cell line INS-1. Endocrinology. 2003;144:335–345. doi: 10.1210/en.2001-211282. [DOI] [PubMed] [Google Scholar]
- 19.Carlsson C, Borg LA, Welsh N. Sodium palmitate induces partial mitochondrial uncoupling and reactive oxygen species in rat pancreatic islets in vitro. Endocrinology. 1999;140:3422–3428. doi: 10.1210/endo.140.8.6908. [DOI] [PubMed] [Google Scholar]
- 20.Zhou YP, Grill VE. Palmitate-induced beta-cell insensitivity to glucose is coupled to decreased pyruvate dehydrogenase activity and enhanced kinase activity in rat pancreatic islets. Diabetes. 1995;44:394–399. doi: 10.2337/diab.44.4.394. [DOI] [PubMed] [Google Scholar]
- 21.Zhou YP, Berggren PO, Grill V. A fatty acid-induced decrease in pyruvate dehydrogenase activity is an important determinant of beta-cell dysfunction in the obese diabetic db/db mouse. Diabetes. 1996;45:580–586. doi: 10.2337/diab.45.5.580. [DOI] [PubMed] [Google Scholar]
- 22.Assimacopoulos-Jeannet F, et al. Fatty acids rapidly induce the carnitine palmitoyltransferase I gene in the pancreatic beta-cell line INS-1. J Biol Chem. 1997;272:1659–1664. doi: 10.1074/jbc.272.3.1659. [DOI] [PubMed] [Google Scholar]
- 23.Joseph JW, et al. Free fatty acid-induced beta-cell defects are dependent on uncoupling protein 2 expression. J Biol Chem. 2004;279:51049–51056. doi: 10.1074/jbc.M409189200. [DOI] [PubMed] [Google Scholar]
- 24.Yoshikawa H, et al. Effects of free fatty acids on beta-cell functions: A possible involvement of peroxisome proliferator-activated receptors alpha or pancreatic/duodenal homeobox. Metabolism. 2001;50:613–618. doi: 10.1053/meta.2001.22565. [DOI] [PubMed] [Google Scholar]
- 25.Johnson JD, et al. Increased islet apoptosis in Pdx1+/− mice. J Clin Invest. 2003;111:1147–1160. doi: 10.1172/JCI16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 27.Karaskov E, et al. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology. 2006;147:3398–3407. doi: 10.1210/en.2005-1494. [DOI] [PubMed] [Google Scholar]
- 28.Naggert JK, et al. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet. 1995;10:135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- 29.Johnson JD, et al. RyR2 and calpain-10 delineate a novel apoptosis pathway in pancreatic islets. J Biol Chem. 2004;279:24794–24802. doi: 10.1074/jbc.M401216200. [DOI] [PubMed] [Google Scholar]
- 30.Kleinfeld AM, et al. Increases in serum unbound free fatty acid levels following coronary angioplasty. Am J Cardiol. 1996;78:1350–1354. doi: 10.1016/s0002-9149(96)00651-0. [DOI] [PubMed] [Google Scholar]
- 31.Cistola DP, Small DM. Fatty acid distribution in systems modeling the normal and diabetic human circulation. A 13C nuclear magnetic resonance study. J Clin Invest. 1991;87:1431–1441. doi: 10.1172/JCI115149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fricker LD, Snyder SH. Enkephalin convertase: purification and characterization of a specific enkephalin-synthesizing carboxypeptidase localized to adrenal chromaffin granules. Proc Natl Acad Sci USA. 1982;79:3886–3890. doi: 10.1073/pnas.79.12.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guest PC, Ravazzola M, Davidson HW, Orci L, Hutton JC. Molecular heterogeneity and cellular localization of carboxypeptidase H in the islets of Langerhans. Endocrinology. 1991;129:734–740. doi: 10.1210/endo-129-2-734. [DOI] [PubMed] [Google Scholar]
- 34.Larsson-Nyren G, Sehlin J. Interaction between perchlorate and nifedipine on insulin secretion from mouse pancreatic islets. Biosci Rep. 1993;13:107–117. doi: 10.1007/BF01145963. [DOI] [PubMed] [Google Scholar]
- 35.Kahn SE, et al. Proinsulin levels predict the development of non-insulin-dependent diabetes mellitus (NIDDM) in Japanese–American men. Diabet Med. 1996;13:S63–S66. [PubMed] [Google Scholar]
- 36.Nijpels G, Popp-Snijders C, Kostense PJ, Bouter LM, Heine RJ. Fasting proinsulin and 2-h post-load glucose levels predict the conversion to NIDDM in subjects with impaired glucose tolerance: The Hoorn Study. Diabetologia. 1996;39:113–118. doi: 10.1007/BF00400421. [DOI] [PubMed] [Google Scholar]
- 37.Støy J, et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci USA. 2007;104:15040–15044. doi: 10.1073/pnas.0707291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harding HP, Ron D. Endoplasmic reticulum stress and the development of diabetes: A review. Diabetes. 2002;51(Suppl 3):S455–S461. doi: 10.2337/diabetes.51.2007.s455. [DOI] [PubMed] [Google Scholar]
- 39.Fricker LD, Berman YL, Leiter EH, Devi LA. Carboxypeptidase E activity is deficient in mice with the fat mutation. Effect on peptide processing. J Biol Chem. 1996;271:30619–30624. doi: 10.1074/jbc.271.48.30619. [DOI] [PubMed] [Google Scholar]
- 40.Chen H, et al. Missense polymorphism in the human carboxypeptidase E gene alters enzymatic activity. Hum Mutat. 2001;18:120–131. doi: 10.1002/humu.1161. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Kouri G, Wollheim CB. ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. J Cell Sci. 2005;118:3905–3915. doi: 10.1242/jcs.02513. [DOI] [PubMed] [Google Scholar]
- 42.Oyadomari S, Araki E, Mori M. Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta-cells. Apoptosis. 2002;7:335–345. doi: 10.1023/a:1016175429877. [DOI] [PubMed] [Google Scholar]
- 43.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol. 2006;291:E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 44.Ramos-Castaneda J, et al. Deficiency of ATP2C1, a Golgi ion pump, induces secretory pathway defects in endoplasmic reticulum (ER)-associated degradation and sensitivity to ER stress. J Biol Chem. 2005;280:9467–9473. doi: 10.1074/jbc.M413243200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oyadomari S, et al. Cotranslocational degradation protects the stressed endoplasmic reticulum from protein overload. Cell. 2006;126:727–739. doi: 10.1016/j.cell.2006.06.051. [DOI] [PubMed] [Google Scholar]
- 46.Roche E, et al. Palmitate and oleate induce the immediate-early response genes c-fos and nur-77 in the pancreatic beta-cell line INS-1. Diabetes. 1999;48:2007–2014. doi: 10.2337/diabetes.48.10.2007. [DOI] [PubMed] [Google Scholar]
- 47.Hardy S, et al. Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells. A role for cardiolipin. J Biol Chem. 2003;278:31861–31870. doi: 10.1074/jbc.M300190200. [DOI] [PubMed] [Google Scholar]
- 48.Itoh Y, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 49.Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab. 2005;1:245–258. doi: 10.1016/j.cmet.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Treinies I, Shrake C, Koester A. GPR40 knock-out mice do not develop obesity-induced glucose intolerance. Diabetologia. 2006;49:87–88. [Google Scholar]
- 51.Olofsson CS, Salehi A, Holm C, Rorsman P. Palmitate increases l-type Ca2+ currents and the size of the readily releasable granule pool in mouse pancreatic beta-cells. J Physiol. 2004;557:935–948. doi: 10.1113/jphysiol.2004.066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warnotte C, Gilon P, Nenquin M, Henquin JC. Mechanisms of the stimulation of insulin release by saturated fatty acids. A study of palmitate effects in mouse beta-cells. Diabetes. 1994;43:703–711. doi: 10.2337/diab.43.5.703. [DOI] [PubMed] [Google Scholar]
- 53.Nalamachu SR, Song L, Fricker LD. Regulation of carboxypeptidase E. Effect of Ca2+ on enzyme activity and stability. J Biol Chem. 1994;269:11192–11195. [PubMed] [Google Scholar]
- 54.Song L, Fricker LD. Tissue distribution and characterization of soluble and membrane-bound forms of metallocarboxypeptidase D. J Biol Chem. 1996;271:28884–28889. doi: 10.1074/jbc.271.46.28884. [DOI] [PubMed] [Google Scholar]
- 55.Berman Y, Mzhavia N, Polonskaia A, Devi LA. Impaired prohormone convertases in Cpe(fat)/Cpe(fat) mice. J Biol Chem. 2001;276:1466–1473. doi: 10.1074/jbc.M008499200. [DOI] [PubMed] [Google Scholar]
- 56.Day R, et al. Prodynorphin processing by proprotein convertase 2. Cleavage at single basic residues and enhanced processing in the presence of carboxypeptidase activity. J Biol Chem. 1998;273:829–836. doi: 10.1074/jbc.273.2.829. [DOI] [PubMed] [Google Scholar]
- 57.Steiner DF, et al. Isolation and characterization of proinsulin C-peptide from bovine pancreas. J Biol Chem. 1971;246:1365–1374. [PubMed] [Google Scholar]
- 58.Johnson JD, Misler S. Nicotinic acid–adenine dinucleotide phosphate-sensitive calcium stores initiate insulin signaling in human β-cells. Proc Natl Acad Sci USA. 2002;99:14566–14571. doi: 10.1073/pnas.222099799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.