Abstract
The CCN family of genes consists presently of six members in human (CCN1-6) also known as Cyr61 (Cystein rich 61), CTGF (Connective Tissue Growth Factor), NOV (Nephroblastoma Overexpressed gene), WISP-1, 2 and 3 (Wnt-1 Induced Secreted Proteins). Results obtained over the past decade have indicated that CCN proteins are matricellular proteins, which are involved in the regulation of various cellular functions, such as proliferation, differentiation, survival, adhesion and migration. The CCN proteins have recently emerged as regulatory factors involved in both internal and external cell signaling. CCN3 was reported to physically interact with fibulin-1C, integrins, Notch and S100A4. Considering that, the conformation and biological activity of these proteins are dependent upon calcium binding, we hypothesized that CCN3 might be involved in signaling pathways mediated by calcium ions.
In this article, we review the data showing that CCN3 regulates the levels of intracellular calcium and discuss potential models that may account for the biological effects of CCN3.
Review
Introduction
The control of normal cell biology, from life to death involves an extremely complex array of interconnected signaling pathways, which govern inward and outward communication. Over the two past decades, a plethora of proteins have been found to participate in these fundamental regulatory circuits. In most cases, alterations of signaling result in pathological conditions. For example, many of the proteins discovered as oncogenes and tumor suppressors in cancer cells were shown to be key signaling molecules.
Signaling is pivotal to the coordinated response of cells in tissues and organs within the whole body. It is often considered that cell populations function as « societies » and that intercellular communication is pivotal to harmonious development during life and to intracellular biological modifications leading to cell death. Efficient coordination is an absolute requirement to safe functioning
It is critical to study and understand cross talking in cell population and to identify messengers that allow integrated responses. Unfortunately, very little is known presently about the processes that coordinate the various cellular signaling pathways.
An increasing amount of data points to matricellular proteins as major players in global control. Members of the CCN family of proteins have recently emerged as important matricellular regulatory factors involved in both internal and external cell signaling.
The CCN family of proteins
Bork coined the CCN acronym in 1993 [1], soon after our discovery of a new gene –nov– presently designated CCN3 [2] whose expression was enhanced in all myeloblastosis associated virus (MAV)-induced nephroblastomas [3], which represent a unique animal model of the Wilms' tumor in human [4].
Analysis of the predicted primary structure of CCN3 indicated that it was structurally related to two other proteins (CYR61/CCN1 and CTGF/CCN2) that had been previously described as « immediate early » proteins showing mitogenic activities. The concept of a protein family was sustained by the fact that these three proteins were sharing a common mosaic organization with four structural modules sharing identity with Insulin like growth factor (IGF)-binding proteins (IGFBPs), Von Willebrand factor type C repeat, thrombospondin type 1 repeat, and secreted regulatory factors containing a cystin knot motif involved in dimerization.
The discovery of CCN3 provided the first evidence for a member of the CCN family of proteins showing antiproliferative activity on chicken embryo fibroblasts (CEF). Interestingly, the aminotruncated version of CCN3 expressed in one MAV-induced nephroblastoma was stimulating growth and inducing morphological transformation of the CEF [3].
Since then, three new members of the CCN family have been identified. CCN4 and CCN5, which were originally designated elm1 and rCOP1 respectively [5,6], were also described as rodent proteins devoted of an antiproliferative activity. Their human counterpart were identified a few years later as WINT-induced secreted proteins (WISP), with a new member (CCN6) originally identified from EST libraries and designated WISP3.
All CCN proteins except CCN5 – which lacks the CT module – show a similar multimodular organization.
The ccn genes are expressed in a wide variety of tissues. Expression of CCN proteins has been associated to several fundamental biological processes such as angiogenesis, chondrogenesis, wound healing [7-9]; their expression is detected during muscular differentiation, nervous system development and bone remodeling with an enhancement of CTGF correlated to several fibrotic situations [7-9]. Aberrant ccn gene expression is also associated to tumorigenesis [10], and has been shown to provide useful markers for tumor typing and prognosis.
Considering the variety of sometimes opposite biological functions attributed to the CCN proteins and the tight spatio-temporal regulation of their expression in many tissues of different origins, we proposed that the CCN proteins interact with several different partners and that the resulting combinatorial events are responsible for the variety of their functions [7].
CCN3 interacts with several different proteins
To identify the potential partners of CCN3, we first used the yeast two-hybrid strategy [11,12]. Two types of considerations dictated our choice of the full length CCN3 protein as bait. Firstly, each individual module was potentially interacting with several factors whose participation is essential to the biological functions of the families of proteins that contain these modules. Therefore, interaction with many of the potential interactions taking place with individual modules might not be directly relevant to the functions of CCN3. Secondly, and more importantly, some interactions critical for the functions of CCN3 might require the tertiary structure of the full-length protein.
Several different proteins were found to physically interact with CCN3 in the yeast assay especially fibulin-1C, integrins, Notch and S100A4 [7]. The physical interaction of CCN3 with these proteins has been checked either by GST-pull down assay, co-immunprecipitation, co-immunolocalization, or functional interdependence. The proteins interacting with CCN3 were either secreted, or localized in the cytosol, or localized in the nucleus of various cell types.
In as much as the conformation and biological activity of fibulin-1C, integrins, Notch and S100A4 were dependent upon calcium, we hypothesized that CCN3 might be involved in signaling pathways involving calcium ions.
Considering the considerable importance of calcium as a second messenger, these observations open new avenues for understanding the role of CCN proteins in cell growth regulation.
CCN3-Fibulin-1C and calcium
Yeast two-hybrid screens indicated that CCN3 interacted with the calcium-binding extracellular matrix glycoprotein fibulin-1C [11]. Fibulin-1C is associated with various connective tissues, basement membranes and blood; it was also reported to interact with extracellular matrix proteins such as fibronectin, laminin, and fibrinogen [13]. Although a distinct function for fibulin-1C has not yet been described, evidence has emerged to indicate that it may regulate cell adhesion and migration along protein fibers within the extracellular matrix and play a role in homeostasis and thrombosis [14]. Moreover, fibulin-1C exhibits calcium-dependent binding and its functions were reported to dependent upon the calcium binding [11].
The identification of an interaction between fibulin-1C and CCN3 provides clue for possible interaction of CCN in signaling pathways involving extracellular matrix, cytoskeleton proteins and calcium. The C-terminal part of CCN3 is involved in the interaction with fibulin-1C. Truncated recombinant protein that represented only the C-terminal portion of CCN3 was able to interact with fibulin-1C. Similar to CCN3, fibulin-1C has a multimodular structure and contains a portion of nine calcium-binding type II EGF-like modules [13]. Using these modules, fibulin-1C is able to interact with the extracellular domain of the heparin-binding EGF-like growth factor precursor suggesting an important role during extracellular matrix formation in wound healing [15]. Binding of CCN3 to fibulin-1C involves this EGF-like module as previously reported for fibronectin [16]. This interaction might induce modification in calcium levels in the neighborhood of the cells.
CCN3 – Integrins and calcium
Integrins are cell surface receptors that are involved in adhesive interactions. They interact with several extracellular matrix proteins and other cell surface receptors. Integrins are heterodimeric transmembrane proteins that are composed of non-covalently associated α and β subunits [17]. The activation of integrins has been associated with conformational changes in the extracellular domains, which leads to an increased affinity for the ligand binding. Integrin-ligand interactions are dependent upon divalent cations. Some cations can enhance the affinity for the ligand, while others can suppress or lower it. For many integrins, Mg2+ and Mn2+ can induce conformational changes associated with a higher affinity for the ligand. On the contrary, Ca2+ often has an inhibitory effect on ligand binding. For instance, for integrin αvβ3, the increase in affinity for the ligand mediated by Mg2+ needs the chelation of Ca2+ [18].
It has been shown that extracellular Ca2+ can modulate the function of integrins but the Ca2+ binding sites on integrins are poorly characterized. To date, two distinct classes of Ca2+ binding sites were identified on integrins: a low and a high affinity-binding site for Ca2+. For integrins α5β1 and β3, a high affinity site for Ca2+ promotes the binding to the ligand, and a low affinity site seems to compete with a Mg2+ site [19,20]. Studies on integrin αIIbβ3 suggested that a high affinity Ca2+ site is involved in the heterodimerization of the two subunits. Since several integrins exhibit a high affinity Ca2+ binding site, this suggests a possible common role in heterodimerization of the two subunits. Furthermore, the binding of Ca2+ to a high affinity site seems to be necessary for ligand binding.
It was recently shown that CCN3 physically interacts with integrins αVβ3 and α5β1 [21]. CCN3 acts on endothelial cells to stimulate pro-angiogenic activities, and supports cell migration and adhesion through different cell surface receptors including integrins αVβ3, α6β1 and α5β1. Extracellular Ca2+ could modulate CCN3-integrins interactions and thus, play a role in the functions of CCN3 in angiogenesis.
Intracellular Ca2+ is also involved in the intracellular integrin-pathways leading to integrin-mediated adhesion (for review [22]). The Ca2+ increase induced by the activation of integrins by their ligands [23] can activate the protease calpain. Studies on migrating CHO cells transfected with β1 and β3 integrins suggest that the Ca2+-activated calpain could be involved in the dissociation between integrins and cytoskeleton, and thereby could influence the binding of integrins to their ligands. Since CCN3 modulates the Ca2+ influx in some cells, CCN3 could also acts on integrins function in an indirect manner by activating the calpain protease via Ca2+ entry. It would be of prime interest to determine if CCN3 modifies the Ca2+ influx in endothelial cells that could be linked to the migration of these cells.
CCN3 – Notch and calcium
CCN3 has been found to interact with Notch1 and to activate downstream effectors of the Notch pathway [24]. Notch1 is a member of a family of highly conserved transmembrane receptors, which are involved in fundamental biological processes during embryonic development such as differentiation, proliferation and apoptosis [25].
Notch receptors are synthesized in the form of an approximatively 300 to 350 kDa transmembrane precursor. This single protein is cleaved in the trans-Golgi network to form a heterodimeric receptor, consisting of a N-terminal extracellular subunit and a C-terminal transmembrane domain [25]. The extracellular subunit of various Notch receptors contains several tandemly repeated EGF (Epidermal growth Factor) modules. The N-terminal part of the receptor acts to restrain receptor activation in absence of ligand, and the structural integrity of the EGF repeats is dependent upon the presence of Ca2+. The N- and C-terminal parts of the Notch heterodimer are non-covalently associated [26]. The association is stabilized in the presence of Ca2+ and destabilized by EDTA, resulting in the activation of Notch target genes [26]. These results suggest a regulation of the Notch activity implying a Ca2+-dependent interaction between the extracellular and the transmembrane parts of the Notch receptor, prior to activation.
Although CCN3 physically interacts with the EGF-like repeats of Notch1, this binding is Ca2+-independent, since co-immnunoprecipitation of CCN3 and Notch1 was maintained in buffer without Ca2+ and in buffer containing EGTA [24].
CCN3-S100A4 and calcium
Our recent studies demonstrated that CCN3 physically interacts with the calcium binding protein S100A4 [27]. This provides a clue for the detection of CCN3 at sites where S100A4 was reported to be expressed in normal conditions and where calcium is known to play critical roles.
S100A4 belongs to the group of S100 proteins, one of the largest subfamilies of the EF-hand proteins, which bind calcium selectively and with high affinity [28,29]. The S100 proteins are thus thought to modulate the propagation of calcium signals [30]. The human S100A4 gene was reported to be frequently rearranged through deletions, duplications and translocations, and is altered in several cancers [31]. Recently, interest has focused on S100A4 due to its implication in tumor progression and metastasis.
Binding of calcium to S100A4 proteins induces conformational changes, which result in exposure of new binding sites at their surface, and consequently allows for interaction with target proteins [32]. Thus, the interaction of CCN3 with S100A4 might be dependent upon local intracellular calcium concentration. The interaction of CCN3 with S100A4, as revealed with the yeast two hybrid system and GST pull down assay in vitro, strongly suggests that these two proteins can interact in vivo [27]. In support to this hypothesis, studies performed with CCN3 and S100A4 indicated that their site of expression showed significant overlap [7,33-36].
Involvement of CCN3 and S100A4 in tumorigenesis
CCN3 is expressed in many different types of tumors and shows positive or negative effects on tumorigenesis and metastasis [10]. On the other hand, the elevated expression of S100A4 in tumor metastasis suggests a role in tumor progression [37]. Since S100A4 itself is not able to initiate tumors, it was proposed that it might act in cooperation with other oncogenes [38]. The interaction of CCN3 with S100A4 makes it interesting to check whether these two proteins act in synergy or antagonize during tumorigenesis.
In one hand, CCN3 proteins were detected in the extracellular matrix, conditioned cell culture medium, cytoplasm and nucleus [7,10,12], in the other hand, S100A4 has been essentially described as a cytoplasmic protein, although it was also found to be secreted [39]. These results therefore suggest that CCN3-S100A4 could interact intra and extra cellularly, and that this interaction might be critical during development, both in physiological and pathological conditions.
Biological effects of CCN3-S100A4 interaction
S100A4 protein is now known to be involved in the regulation of cell motility and cell cycle progression, intercellular adhesion, angiogenesis, and metastatic properties of cancer cells. The interaction of CCN3 with S100A4 should therefore be helpful in deciphering the biological function of CCN3 and extends the roles of CCN proteins to S100 family.
Regarding cell motility, CCN3 was reported to decrease the adhesive capacity and increase the motility of Ewing's transfected cells [40]. It was suggested that S100A4 protein affects the assembly of the cytoskeleton [41,42]. These findings suggest that CCN3, through its interaction with S100A4, might alter cytoskeletal organization and facilitate cell motility.
The expressions of S100A4 and E-cadherin, which suppress tumor invasion, were reported to be inversely regulated in a series tumor cell lines [43]. It was suggested that the invasiveness of tumors expressing S100A4 might be at least partially induced by the abrogation of E-cadherin expression [44]. The interaction of CCN3 with S100A4 sheds new light on the potential role of CCN proteins as matricellular regulators of cell proliferation. Down-regulation of S100A4 resulted in a decrease in metalloproteinases expression and consequently in a reduction in migration process [45,46]. The interaction of CCN3 with S100A4 suggests that CCN3 might also be involved in the dysregulation of metalloproteinases expression, and may participate in a large array of connections involving several different proteins of the extracellular matrix.
Recently, an association between S100A4 and cell proliferation has been postulated. S100A4 expression was associated with elevated levels of wild-type p53. It was suggested that the physical interaction between wild-type p53 and S100A4 might result in a stimulation of the cells to enter the S phase [47,48]. The transfection of S100A4-negative cells with S100A4 constructs led to clonal death that was prevented by co-transfection with the anti-apoptotic gene bcl-2, which control calcium entry in different subcellular compartments [49]. CCN3-S100A4 interaction raises the possibility that CCN3 might promote apoptosis through its interaction with the S100A4-p53 complex. This would provide a clue (an explanation) for the dual role of CCN3 in proliferation and differentiation [6].
Recent findings established that CCN3 acts directly upon endothelial cells to stimulate pro-angiogenic activities, and induces angiogenesis in vivo [21]. Interestingly, S100A4 also promotes angiogenesis in vivo by preventing the anti-angiogenic effect of THBS1 [50]. In addition, preliminary results suggest that S100A4 protein may act directly as an angiogenic factor [51]. Whether CCN3 promotes angiogenesis directly, through its interaction with S100A4 or otherwise remains unclear.
CCN3-S100A4 interaction provides another pathway for stimulating calcium entry induced by CCN3: S100A4 could modulate cellular calcium levels via its roles in calcium uptake, transport and buffering [52]. The CCN3-S100A4 interaction might change the affinity of S100A4 for calcium and cause a release from S100A4. Because of its limited binding capacity for calcium, S100A4 might not be the main effector of calcium increase in cell upon induction by CCN3. In conclusion, the interaction of CCN3 with S100A4 might account at least in part for the association of CCN3 with tumorigenesis and metastasis.
CCN3 and calcium signaling
Because CCN3 is expressed in various tissues where the calcium metabolism and ion transport are essential [7] and that it physically interacts with proteins whose activity depended on calcium binding, we hypothesized that CCN3 might have a direct action on intracellular calcium mobilization. We used dynamic intracellular calcium fluorimetry [53] to examine the potential role of CCN3 in affecting intracellular calcium level in human tumoral cells (fig. 1).
Figure 1.
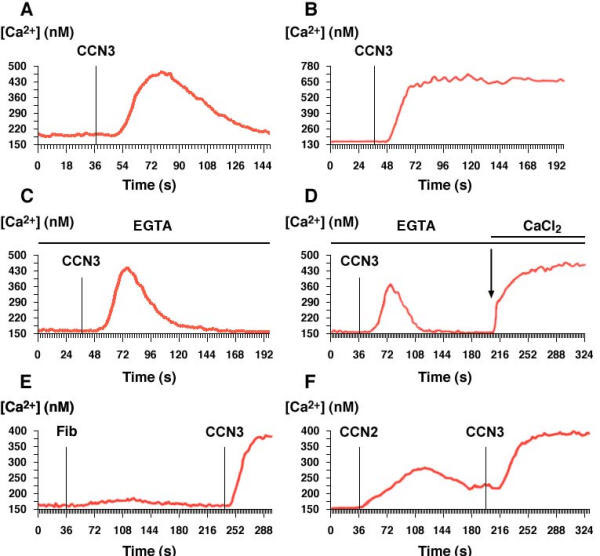
Effect of CCN proteins on intracellular calcium levels in G59 glioblastoma cells. GST-CCN3 (2 μg/ml, Fig. 1A or 10 μg/ml, Fig. 1B) was applied on G59 cells in the absence of EGTA. In the presence of 10 mM EGTA, GST-CCN3 (2 μg/ml) was added (Fig. 1C) and 12 mM CaCl2 was overloaded (Fig. 1D). The effects of fibulin-1C and GST-CCN2 (5 μg/ml) were compared to that of 5 μg/ml GST-CCN3 (Fig. 1E,1F)
The application of CCN3 resulted in a transient increase of the intracellular calcium concentration in G59 glioblastoma cells (fig. 1A). The control GST protein alone was without effect on the intracellular calcium level (data not shown). A similar transient increase in calcium was also seen with human neuroblastoma SK-N-SH cells upon CCN3 delivery [27]. In these experimental conditions, the calcium response reached a maximum and sustained effect (fig. 1B). Toxic effects (including the cells blowing up) were seen when higher concentrations of the GST fusion protein were used. To determine the origin of this transient calcium increase, experiments were carried out in the absence of extracellular calcium. Under these conditions, lower transient calcium increase in G59 cells persisted (fig. 1C). Furthermore, the addition of calcium to EGTA containing medium induced a sustained increase of intracellular calcium, suggesting a mixed interaction of CCN3 with the stimulation and release of intracellular calcium from the internal store and with the entry of extracellular calcium in G59 cells (fig. 1D). The addition of blockers of voltage-dependant calcium and/or sodium channels (verapamil and flunarizine) did not modify the transient calcium increase induced by GST-CCN3 in this cell line [27]. Finally, no increase was induced by the GST-fibulin-1C fusion protein, which contained the portion of fibulin-1C interacting with CCN3 [11] (fig. 1E). Conversely, a transient increase in intracellular calcium was also induced when another member of CCN family, GST-CCN2, was applied to G59 cells, but the effect of CCN2 was slightly lower than that of CCN3 at the same dose (fig. 1F). The presence of fibulin-1C or CCN2 did not modify the calcium response induced by CCN3, suggesting that these proteins did not compete with CCN3.
An interesting aspect of the relation between CCN3 function and calcium signaling was raised by the pronounced increase in intracellular calcium concentration, which was transiently induced in CCN3 treated G59. In these cells, both entry and the mobilization of the internal calcium store were induced by the addition of CCN3. Because the inhibitory effect of EGTA upon the calcium increase was reversed after the addition of CaCl2, CCN3 is unlikely to be an ionophore by itself.
The uptake of extracellular calcium induced by CCN3 proceeded even when voltage dependent channels were blocked. Therefore, it is tempting to speculate that it involves store operated calcium channels, a heterogeneous subset of plasma membrane calcium ion channels. The best characterized channel of this type was originally described in mast cells in which the depletion of calcium induced a sustained calcium inward current that was not voltage activated and therefore termed ICRAC (for calcium release activated calcium) [54-56]. A direct stimulation of these channels or of transient receptor potential (TRP) family of non-selective cation channels [57] could be postulated as a possible mode of action of CCN3.
The mobilization of intracellular calcium stores by CCN3 in G59 cells might involve specific receptors and IP3 activated channels. The transient calcium increase induced by CCN3 is sufficient to activate potassium current (BK/Ca) as recorded by whole-cell patch clamp on G59 cells (unpublished data). Along this line, the interaction of CCN1, CCN2, and CCN3 proteins [58-60] with integrins might be of relevance to induce a transient increase of intracellular calcium.
As discussed above, CCN3 can interact with fibulin-1C and Notch1. It will be interesting to determine whether the regulation of intracellular calcium concentrations by CCN3 affects in any way its association with these proteins and their biological activities.
The interaction of CCN3 with S100A4 and its ability to induce a transient increase of intracellular calcium by two different mechanisms add another degree of variety to the pleiotropic biological properties of the CCN proteins.
A growing body of evidence indicates that CCN3 is implicated in many fundamental aspects of cellular activity. Our observations suggest for the first time that CCN proteins functionally crosstalk with calcium related physiological and pathological processes and open new perspectives in understanding the functions of CCN proteins in normal and pathological conditions. The interaction of CCN3 protein with numerous potential partners could explain the multiplicity of functional implications of CCN3 in cellular calcium signaling.
The multimodular organization of the CCN proteins provides a structural base that may account for its interaction with different target proteins. This type of multimodular organization is not unique to the CCN proteins. For example, the Caspase proteins family also show a modular organization [61]. They contain different domains (DD, CARD, etc.) that can be bound by adaptative proteins such as FADD and Apaf-1 [62] or BAR [63], which are involved in controlling apoptosis.
The regulation of intracellular calcium levels can be achieved by several ways. Recently, a PKC binding protein – enigma homolog (ENH)- has been reported to specifically interact with both PKCε and N-type Ca2+ channels, thereby forming a PKCε-ENH-Ca2+ channel macromolecular complex [64] that facilitated modulation of N-type Ca2+ channel activity by PKC.
The increased intracellular calcium level induced by CCN3 might also involve different voltage-independent calcium channels, among which receptor operated calcium channel and capacitative calcium entry channel may be of considerable importance in regulating this calcium influx.
Conclusions
We have hypothesized [7] that CCN3 might function as an adaptative protein by interacting with different regulatory proteins. Thus, CCN3 was proposed to coordinate signaling pathways by bringing together regulators, which require calcium for their biological effects.
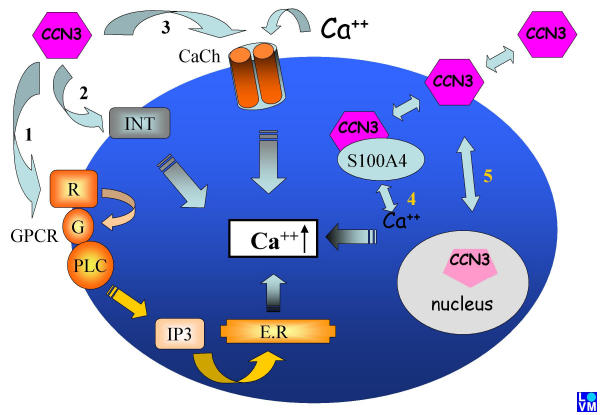
In figure 2 we have summarized non-exclusive possible pathways that may account for the calcium-related functions of CCN3.
Figure 2.
Schematic representation of different signaling pathways that may account for the calcium-related functions of CCN3. 1. Activation of GPCRs 2. Binding to integrins 3. Opening of voltage-independent calcium channels 4. Interaction with calcium binding proteins inside the cell 5. Modification of the calcium status inside the nucleus and modulation of gene expression
In the first case, CCN3 is shown to interact with members of the G Protein-Coupled Receptors (GPCR) family whose activation is known to result in Inositol 1,4,5-triPhosphate (IP3) production and calcium release from endoplasmic reticulum. IGFBPs were recently reported to specifically recognize these receptors and induce a transient increase of intracellular calcium [65]. The possibility that the IGFBP module is responsible for its effects on cellular calcium is under current investigation.
The second pathway by which CCN3 could induce the calcium increase involves binding to integrins. Effects of integrins on calcium have been extensively documented. The physical interaction of CCN3 with integrins makes it a major possibility.
In the third case, interaction of CCN3 with voltage-independent channels would lead to an uptake of calcium from external sources. As discussed above, store operated channels are among the likely candidates.
The fourth level of action would imply S100A4 whose physical interaction with CCN3 might interfere with its ability to bind calcium ions and play its buffer functions. We are presently in process of determining whether other proteins of the S100 family are also interacting with CCN3.
In the fifth case, translocation of CCN3 to the nucleus [66], is thought to account for the detection of the truncated CCN3 nuclear isoforms [12]. The importance of calcium in the regulation of gene expression is well established. It has been proposed that CCN3 play a direct role in the regulation of transcription by interacting with the rpb7 subunit of RNA polymerase II [12]. The levels of intracellular calcium ions might therefore be a key factor in controlling the interactions of CCN3 with its nuclear partners.
In any case, the links that we have established between CCN proteins and calcium signaling opens new avenues that should help to decipher the role of these regulatory proteins in processes governing cell growth control, development, and normal and pathological physiology.
Competing interests
None declared.
Acknowledgments
Acknowledgements
The work has been supported by grants from ARC, LNCC, FRM, and AFM. We are grateful to R. Mochino for financial support.
Contributor Information
Alain Lombet, Email: alain.lombet@ccml.u-psud.fr.
Nathalie Planque, Email: planque@ext.jussieu.fr.
Anne-Marie Bleau, Email: bleauam@hotmail.com.
Chang Long Li, Email: clli@ccr.jussieu.fr.
Bernard Perbal, Email: perbal@ccr.jussieu.fr.
References
- Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993;327:125–30. doi: 10.1016/0014-5793(93)80155-N. [DOI] [PubMed] [Google Scholar]
- Brigstock DR, Goldschmeding R, Katsube KI, Lam SC, Lau LF, Lyons K, Naus C, Perbal B, Riser B, Takigawa M, Yeqer H. Proposal for a unified CCN nomenclature. Mol Pathol. 2003;56:127–128. doi: 10.1136/mp.56.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot V, Martinerie C, Dambrine G, Plassiart G, Brisac M, Crochet J, Perbal B. Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced nephroblastomas. Mol Cell Biol. 1992;12:10–21. doi: 10.1128/mcb.12.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. Contribution of MAV-1-induced nephroblastoma to the study of genes involved in human Wilms' tumor development. Crit Rev Oncog. 1994;5:589–613. [PubMed] [Google Scholar]
- Hashimoto Y, Shindo-Okada N, Tani M, Nagamachi Y, Takeuchi K, Shiroishi T, Toma H, Yokota J. Expression of the Elm1 gene, a novel gene of the CCN (connective tissue growth factor, Cyr61/Cef10, and neuroblastoma overexpressed gene) family, suppresses In vivo tumor growth and metastasis of K-1735 murine melanoma cells. J Exp Med. 1998;187:289–296. doi: 10.1084/jem.187.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Averboukh L, Zhu W, Zhang H, Jo H, Dempsey PJ, Coffey RJ, Pardee AB, Liang P. Identification of rCop-1, a new member of the CCN protein family, as a negative regulator for cell transformation. Mol Cell Biol. 1998;18:6131–6141. doi: 10.1128/mcb.18.10.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol Pathol. 2001;54:57–79. doi: 10.1136/mp.54.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. doi: 10.1210/er.20.2.189. [DOI] [PubMed] [Google Scholar]
- Planque N, Perbal B. A structural approach to the role of CCN proteins in tumorigenesis. Cancer Cell Int. 2003. [DOI] [PMC free article] [PubMed]
- Perbal B, Martinerie C, Sainson R, Werner M, He B, Roizman B. The C-terminal domain of the regulatory protein NOVH is sufficient to promote interaction with fibulin 1C: a clue for a role of NOVH in cell-adhesion signaling. Proc Natl Acad Sci U S A. 1999;96:869–874. doi: 10.1073/pnas.96.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. Nuclear localisation of NOVH protein: a potential role for NOV in the regulation of gene expression. Mol Pathol. 1999;52:84–91. doi: 10.1136/mp.52.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argraves WS, Tran H, Burgess WH, Dickerson K. Fibulin is an extracellular matrix and plasma glycoprotein with repeated domain structure. J Cell Biol. 1990;111:3155–3164. doi: 10.1083/jcb.111.6.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran H, Tanaka A, Litvinovich SV, Medved LV, Haudenschild CC, Argraves WS. The interaction of fibulin-1 with fibrinogen. A potential role in hemostasis and thrombosis. J Biol Chem. 1995;270:19458–19464. doi: 10.1074/jbc.270.33.19458. [DOI] [PubMed] [Google Scholar]
- Brooke JS, Cha JH, Eidels L. Latent transforming growth factor beta-binding protein-3 and fibulin-1C interact with the extracellular domain of the heparin-binding EGF-like growth factor precursor. BMC Cell Biol. 2002;3:2. doi: 10.1186/1471-2121-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran H, VanDusen WJ, Argraves WS. The self-association and fibronectin-binding sites of fibulin-1 map to calcium-binding epidermal growth factor-like domains. J Biol Chem. 1997;272:22600–22606. doi: 10.1074/jbc.272.36.22600. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hu DD, Hoyer JR, Smith JW. Ca2+ suppresses cell adhesion to osteopontin by attenuating binding affinity for integrin alpha v beta 3. J Biol Chem. 1995;270:9917–9925. doi: 10.1074/jbc.270.17.9917. [DOI] [PubMed] [Google Scholar]
- Mould AP, Akiyama SK, Humphries MJ. Regulation of integrin alpha 5 beta 1-fibronectin interactions by divalent cations. Evidence for distinct classes of binding sites for Mn2+, Mg2+, and Ca2+ J Biol Chem. 1995;270:26270–26277. doi: 10.1074/jbc.270.44.26270. [DOI] [PubMed] [Google Scholar]
- Hu DD, Barbas CF, Smith JW. An allosteric Ca2+ binding site on the beta3-integrins that regulates the dissociation rate for RGD ligands. J Biol Chem. 1996;271:21745–21751. doi: 10.1074/jbc.271.36.21745. [DOI] [PubMed] [Google Scholar]
- Lin C, Leu SJ, Chen N, Tebeau CM, Lin SX, Yeung CY, Lau LF. CCN3 (NOV) is a novel angiogenic regulator of the CCN protein family. J Biol Chem. 2003;278:24200–24208. doi: 10.1074/jbc.M302028200. [DOI] [PubMed] [Google Scholar]
- Leitinger B, McDowall A, Stanley P, Hogg N. The regulation of integrin function by Ca(2+) Biochim Biophys Acta. 2000;1498:91–98. doi: 10.1016/S0167-4889(00)00086-0. [DOI] [PubMed] [Google Scholar]
- Sjaastad MD, Nelson WJ. Integrin-mediated calcium signaling and regulation of cell adhesion by intracellular calcium. Bioessays. 1997;19:47–55. doi: 10.1002/bies.950190109. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Yamaguchi S, Ando R, Miyawaki A, Kabasawa Y, Takagi M, Li CL, Perbal B, Katsube K. The nephroblastoma overexpressed gene (NOV/ccn3) protein associates with Notch1 extracellular domain and inhibits myoblast differentiation via Notch signaling pathway. J Biol Chem. 2002;277:29399–29405. doi: 10.1074/jbc.M203727200. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Rand MD, Grimm LM, Artavanis-Tsakonas S, Patriub V, Blacklow SC, Sklar J, Aster JC. Calcium depletion dissociates and activates heterodimeric notch receptors. Mol Cell Biol. 2000;20:1825–1835. doi: 10.1128/MCB.20.5.1825-1835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CL, Martinez V, He B, Lombet A, Perbal B. A role for CCN3 (NOV) in calcium signalling. Mol Pathol. 2002;55:250–261. doi: 10.1136/mp.55.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizmann CW, Hunziker W. Intracellular calcium-binding proteins: more sites than insights. Trends Biochem Sci. 1991;16:98–103. doi: 10.1016/0968-0004(91)90041-S. [DOI] [PubMed] [Google Scholar]
- Linse S, Forsen S. Determinants that govern high-affinity calcium binding. Adv Second Messenger Phosphoprotein Res. 1995;30:89–151. doi: 10.1016/s1040-7952(05)80005-9. [DOI] [PubMed] [Google Scholar]
- Schafer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996;21:134–140. doi: 10.1016/0968-0004(96)10020-7. [DOI] [PubMed] [Google Scholar]
- Barraclough R. Calcium-binding protein S100A4 in health and disease. Biochim Biophys Acta. 1998;1448:190–199. doi: 10.1016/S0167-4889(98)00143-8. [DOI] [PubMed] [Google Scholar]
- Heizmann CW, Cox JA. New perspectives on S100 proteins: a multi-functional Ca(2+)-, Zn(2+)- and Cu(2+)-binding protein family. Biometals. 1998;11:383–397. doi: 10.1023/A:1009212521172. [DOI] [PubMed] [Google Scholar]
- Gibbs FE, Barraclough R, Platt-Higgins A, Rudland PS, Wilkinson MC, Parry EW. Immunocytochemical distribution of the calcium-binding protein p9Ka in normal rat tissues: variation in the cellular location in different tissues. J Histochem Cytochem. 1995;43:169–180. doi: 10.1177/43.2.7822773. [DOI] [PubMed] [Google Scholar]
- Ilg EC, Schafer BW, Heizmann CW. Expression pattern of S100 calcium-binding proteins in human tumors. Int J Cancer. 1996;68:325–332. doi: 10.1002/(SICI)1097-0215(19961104)68:3<325::AID-IJC10>3.3.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Takenaga K, Nakanishi H, Wada K, Suzuki M, Matsuzaki O, Matsuura A, Endo H. Increased expression of S100A4, a metastasis-associated gene, in human colorectal adenocarcinomas. Clin Cancer Res. 1997;3:2309–2316. [PubMed] [Google Scholar]
- Burren CP, Wilson EM, Hwa V, Oh Y, Rosenfeld RG. Binding properties and distribution of insulin-like growth factor binding protein-related protein 3 (IGFBP-rP3/NovH), an additional member of the IGFBP Superfamily. J Clin Endocrinol Metab. 1999;84:1096–1103. doi: 10.1210/jc.84.3.1096. [DOI] [PubMed] [Google Scholar]
- Sherbet GV, Lakshmi MS. S100A4 (MTS1) calcium binding protein in cancer growth, invasion and metastasis. Anticancer Res. 1998;18:2415–2421. [PubMed] [Google Scholar]
- Davies M, Harris S, Rudland P, Barraclough R. Expression of the rat, S-100-related, calcium-binding protein gene, p9Ka, in transgenic mice demonstrates different patterns of expression between these two species. DNA Cell Biol. 1995;14:825–832. doi: 10.1089/dna.1995.14.825. [DOI] [PubMed] [Google Scholar]
- Duarte WR, Iimura T, Takenaga K, Ohya K, Ishikawa I, Kasugai S. Extracellular role of S100A4 calcium-binding protein in the periodontal ligament. Biochem Biophys Res Commun. 1999;255:416–420. doi: 10.1006/bbrc.1999.0214. [DOI] [PubMed] [Google Scholar]
- Perbal B, Brigstock DR, Lau LF. Report on the second international workshop on the CCN family of genes. Mol Pathol. 2003;56:80–85. doi: 10.1136/mp.56.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford HL, Silver DL, Kachar B, Sellers JR, Zain SB. Effect of Mts1 on the structure and activity of nonmuscle myosin II. Biochemistry. 1997;36:16321–16327. doi: 10.1021/bi971182l. [DOI] [PubMed] [Google Scholar]
- Kriajevska M, Bronstein IB, Scott DJ, Tarabykina S, Fischer-Larsen M, Issinger O, Lukanidin E. Metastasis-associated protein Mts1 (S100A4) inhibits CK2-mediated phosphorylation and self-assembly of the heavy chain of nonmuscle myosin. Biochim Biophys Acta. 2000;1498:252–263. doi: 10.1016/S0167-4889(00)00100-2. [DOI] [PubMed] [Google Scholar]
- Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- Keirsebilck A, Bonne S, Bruyneel E, Vermassen P, Lukanidin E, Mareel M, van Roy F. E-cadherin and metastasin (mts-1/S100A4) expression levels are inversely regulated in two tumor cell families. Cancer Res. 1998;58:4587–4591. [PubMed] [Google Scholar]
- Merzak A, Parker C, Koochekpour S, Sherbet GV, Pilkington GJ. Overexpression of the 18A2/mts1 gene and down-regulation of the TIMP-2 gene in invasive human glioma cell lines in vitro. Neuropathol Appl Neurobiol. 1994;20:614–619. doi: 10.1111/j.1365-2990.1994.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Bjornland K, Winberg JO, Odegaard OT, Hovig E, Loennechen T, Aasen AO, Fodstad O, Maelandsmo GM. S100A4 involvement in metastasis: deregulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in osteosarcoma cells transfected with an anti-S100A4 ribozyme. Cancer Res. 1999;59:4702–4708. [PubMed] [Google Scholar]
- Parker C, Whittaker PA, Usmani BA, Lakshmi MS, Sherbet GV. Induction of 18A2/mts1 gene expression and its effects on metastasis and cell cycle control. DNA Cell Biol. 1994;13:1021–1028. doi: 10.1089/dna.1994.13.1021. [DOI] [PubMed] [Google Scholar]
- Grigorian M, Andresen S, Tulchinsky E, Kriajevska M, Carlberg C, Kruse C, Cohn M, Ambartsumian N, Christensen A, Selivanova G, Lukonidin E. Tumor suppressor p53 protein is a new target for the metastasis-associated Mts1/S100A4 protein: functional consequences of their interaction. J Biol Chem. 2001;276:22699–22708. doi: 10.1074/jbc.M010231200. [DOI] [PubMed] [Google Scholar]
- Chen H, Fernig DG, Rudland PS, Sparks A, Wilkinson MC, Barraclough R. Binding to intracellular targets of the metastasis-inducing protein, S100A4 (p9Ka) Biochem Biophys Res Commun. 2001;286:1212–1217. doi: 10.1006/bbrc.2001.5517. [DOI] [PubMed] [Google Scholar]
- Roberts DD. Regulation of tumor growth and metastasis by thrombospondin-1. Faseb J. 1996;10:1183–1191. [PubMed] [Google Scholar]
- Ambartsumian N, Klingelhofer J, Grigorian M, Christensen C, Kriajevska M, Tulchinsky E, Georgiev G, Berezin V, Bock E, Rygaard J, Cao R, Cao Y, Lukanidin E. The metastasis-associated Mts1(S100A4) protein could act as an angiogenic factor. Oncogene. 2001;20:4685–4695. doi: 10.1038/sj.onc.1204636. [DOI] [PubMed] [Google Scholar]
- Nelson MR, Chazin WJ. Structures of EF-hand Ca(2+)-binding proteins: diversity in the organization, packing and response to Ca2+ binding. Biometals. 1998;11:297–318. doi: 10.1023/A:1009253808876. [DOI] [PubMed] [Google Scholar]
- Bourcier T, Berbar T, Paquet S, Rondeau N, Thomas F, Borderie V, Laroche L, Rostene W, Haour F, Lombet A. Characterization and functionality of CXCR4 chemokine receptor and SDF-1 in human corneal fibroblasts. Mol Vis. 2003;9:96–102. [PubMed] [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Shin DM, Shcheynikov N, Kurosaki T, Muallem S. Regulation of Ca2+-release-activated Ca2+ current (Icrac) by ryanodine receptors in inositol 1,4,5-trisphosphate-receptor-deficient DT40 cells. Biochem J. 2001;360:17–22. doi: 10.1042/0264-6021:3600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- Chen N, Chen CC, Lau LF. Adhesion of human skin fibroblasts to Cyr61 is mediated through integrin alpha 6beta 1 and cell surface heparan sulfate proteoglycans. J Biol Chem. 2000;275:24953–24961. doi: 10.1074/jbc.M003040200. [DOI] [PubMed] [Google Scholar]
- Grzeszkiewicz TM, Kirschling DJ, Chen N, Lau LF. CYR61 stimulates human skin fibroblast migration through Integrin alpha vbeta 5 and enhances mitogenesis through integrin alpha vbeta 3, independent of its carboxyl-terminal domain. J Biol Chem. 2001;276:21943–21950. doi: 10.1074/jbc.M100978200. [DOI] [PubMed] [Google Scholar]
- Chen CC, Chen N, Lau LF. The angiogenic factors Cyr61 and connective tissue growth factor induce adhesive signaling in primary human skin fibroblasts. J Biol Chem. 2001;276:10443–10452. doi: 10.1074/jbc.M008087200. [DOI] [PubMed] [Google Scholar]
- Hofmann K. The modular nature of apoptotic signaling proteins. Cell Mol Life Sci. 1999;55:1113–1128. doi: 10.1007/s000180050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzinet A, Herincs Z, Hueber AO. Régulation de la mort cellulaire programmée: vers une conception plus dynamique. Médecine/Sciences. 2002;18:841–852. [Google Scholar]
- Zhang H, Xu Q, Krajewski S, Krajewska M, Xie Z, Fuess S, Kitada S, Pawlowski K, Godzik A, Reed JC. BAR: An apoptosis regulator at the intersection of caspases and Bcl-2 family proteins. Proc Natl Acad Sci U S A. 2000;97:2597–2602. doi: 10.1073/pnas.97.6.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno-Hikichi Y, Chang S, Matsumura K, Lai M, Lin H, Nakagawa N, Kuroda S, Zhang JF. A PKCepsilon-ENH-channel complex specifically modulates N-type Ca2+ channels. Nat Neurosci. 2003;6:468–475. doi: 10.1038/nn1041. [DOI] [PubMed] [Google Scholar]
- Ricort JM, Lombet A, Lassarre C, Binoux M. Insulin-like growth factor binding protein-3 increases intracellular calcium concentrations in MCF-7 breast carcinoma cells. FEBS Lett. 2002;527:293–297. doi: 10.1016/S0014-5793(02)03250-7. [DOI] [PubMed] [Google Scholar]
- Thomopoulos GN, Kyurkchiev S, Perbal B. Immunocytochemical localization of NOVH protein and ultrastructural characteristics of NCI-H295R cells. J Submicrosc Cytol Pathol. 2001;33:251–260. [PubMed] [Google Scholar]