Abstract
In this paper, we provide evidence that contrary to the current view, primates on the European continent did survive the dramatic extinction/origination event across the Eocene/Oligocene boundary 34 million years ago that severely affected the Eurasian mammal communities (the European “Grande Coupure” and the Asian “Mongolian Remodeling”). The survival of a mouse-sized omomyid for at least 2 million years, recorded in two localities of the Lower Stampian (Lower Oligocene) in a well dated stratigraphic series of fluviatile sediments in the north oriental sector of the Ebro Basin (Northeastern Spain), reflects the size-related survival pattern described recently for other coeval mammalian taxa.
At the beginning of this century, the Swiss paleontologist H. G. Stehlin (1) detected a dramatic faunal turnover at the Eocene/Oligocene boundary (34 million years ago; see Fig. 1 legend) that he denominated “Grande Coupure.” During this event, a large part of the Eocene mammals became extinct and new immigrants appeared. However, this faunal turnover was not as abrupt as supposed initially, but the changes occurred gradually from the Upper Eocene through the Lower Oligocene (4, 5). This faunal turnover was caused by a climatic crisis that affected not only the European continent but the entire northern hemisphere, i.e., North America (6) and Asia (7, 8).
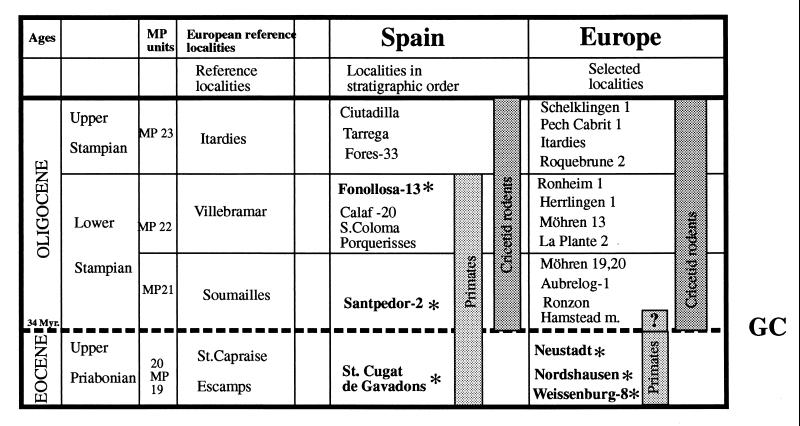
Figure 1.
The Eocene/Oligocene transition in Europe with the disappearance of primates and the appearance of cricetid rodents and diagnostic faunal components of post-“Grande Coupure” faunas. GC, “Grande Coupure;” ∗, localities with primates. Most paleontologists, apart from German paleontologists, agree to place the Eocene/Oligocene boundary for continental deposits at 34 million years ago (MP 20/21), thus coinciding with Stehlin’s “Grande Coupure” (1). The German definition of the Eocene/Oligocene boundary is guided by marine stratigraphy (2), where the beginning of the Oligocene is dated as MP 17/18 (Standard Levels Fons 4/La Débruge). Thus, German paleontologists situate the “Grande Coupure” at the Lower/Middle Oligocene boundary. In both conceptions, however, the “Grande Coupure” is placed between MP 20 and MP 21 (3), and in both conceptions, all European prosimians, apart from those of Santpedor and Fonollosa, are undoubtedly pre-“Grande Coupure.” In our paper, we use the most widely accepted definition with the Eocene/Oligocene boundary between MP 20 and MP 21.
Prosimian primates are excellent ecological indicators, because they are confined mostly to tropical or subtropical forests and highly susceptible to environmental changes. Therefore, their extinction on the European continent at the Eocene/Oligocene boundary is considered to be one of the paradigmatic events of the “Grande Coupure.” Although in North America there is one prosimian primate (9) [although this is debatable, because Ekgmoweshashala recently has been suggested to be a plagiomenid (10)] known to have survived this major climatic event, primates on the European continent are thought to fall under the large number of mammals that did not survive the “Grande Coupure.” Indeed, the lack of primate remains in the European Oligocene seemed to confirm this hypothesis. Only a single upper molar of an adapid found by an amateur on the Isle of White (United Kingdom) might suggest primate survival during the lowermost Oligocene of Europe (11 12). However, the inferred age [Mammal Paleogene European Chronostratigraphic Scale (MP) 21, Bouldnor Formation, lower part of Hamstead member] is not fully reliable (12), and in recent reviews, this specimen has not been considered (13).
During recent years, fieldwork in Northeastern Spain, in the province of Barcelona, not only allowed the reliable dating of an exceptional sequence of continental deposits from Upper Eocene through Middle Oligocene, but also provided evidence that European prosimian primates did survive the “Grande Coupure” for at least 2 million years, even longer than the possible date indicated by the Hamstead tooth.
The fossils described in this paper come from the northeastern sector of the Ebro Basin. In this region, an exceptional sequence of continental deposits from the Upper Eocene through the Middle Oligocene crops out over a wide area (14). It contains a series of recently discovered fossiliferous horizons that permit detailed dating (15, 16). Three sites yielded fossil primates. The relative stratigraphic position of these localities is well established, and a detailed geological study of the area allowed the reliable correlation of the three sites with each other (14). The oldest locality is Sant Cugat de Gavadons from the Upper Eocene, the middle one is the Lower Oligocene locality of Santpedor (Stampian), and the youngest locality is Fonollosa-13 (Stampian). An exhaustive biostratigraphic study, essentially based on the record of rodent faunas, allowed the precise situation of these localities in the MP scale (15). The presence of cricetid rodents, in particular, of Eucricetodon atavus and the theridomyid Theridomys aff. aquatilis, permits the correlation of Santpedor with the Belgian locality Hoogbutsel (15, 16), which situates Santpedor unambiguously as “post-Grande Coupure” in MP 21 (Lower Oligocene). The rich micromammal assemblage of Fonollosa-13 yielded Theridomys calafensis, a descendent of Theridomys aff. aquatilis. This allows the correlation of this locality with the French locality Villebramar (15, 16) and situates Fonollosa-13 in MP 22 (highest Lower Stampian).
Systematics
Primates; Omomyiformes; Microchoeridae; Pseudoloris; Pseudoloris godinoti n. sp.
Etymology.
This species is dedicated to Marc Godinot (Institut de Paléontologie, Paris) for his important contribution to the knowledge of European Paleogene primates.
Holotype.
The upper tooth row with M1/, P4/, P3/, and P2/is stored in the Institute of Paleontology M. Crusafont (Catalog no. IPMC 14041) (Fig. 2).
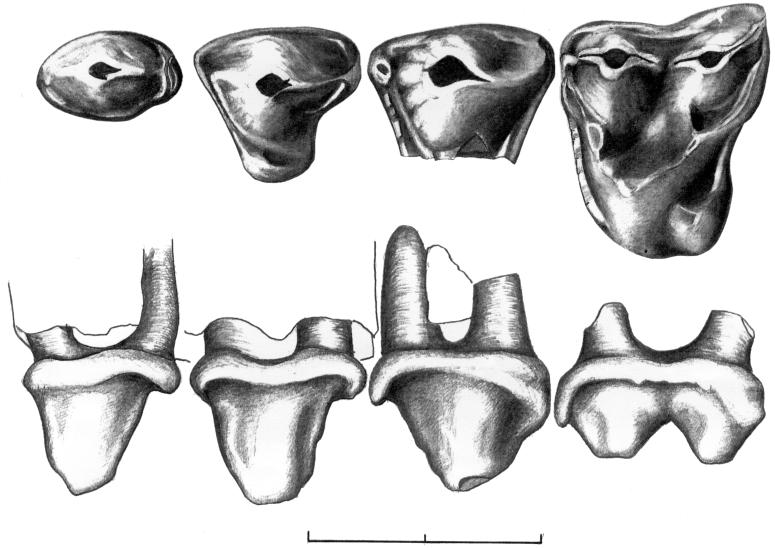
Figure 2.
Upper tooth row of Pseudoloris godinoti n. sp. with M1/through P2/, from Fonollosa-13, Barcelona. Holotype: (Upper) occlusal view; (Lower) labial view. (Scale = 2 mm.)
Type locality, age, and other localities.
The type locality is Fonollosa-13 (Fonollosa, Barcelona), Ebro Basin, Spain. The age of the species is MP 22, Stampian, Lower Oligocene. Other localities include Santpedor (Ebro Basin), MP 21, Stampian.
Material.
For Fonollosa-13 (VF-13): upper tooth row with P2/through M1/, lacking the lingual cusp of the P4/(holotype), a lower M/1, and a lower P/3. Measurements (length × width): M/1, 1.77 × 1.44; M1/, 1.71 × 2.02; P4/, 1.53 × P4/, 1.53 × – (tooth incomplete); P3/, 1.35 × 1.28; P2/, 1.19 × 0.76; P/3, 1.37 × 0.82. For Santpedor-2 (SP-2): two isolated trigones of lower molars, probably M/1 and M/2.
Diagnosis.
A large species of Pseudoloris was found that is similar in size to P. crusafonti from Grissolles (France) and Pseudoloris n. sp. from Weissenburg-8 (Germany) and that differs from all Pseudoloris species by having noticeably longer and narrower upper molars and premolars with mediolaterally considerably compressed labial cusps. The upper first molar shows a very reduced trigone, with reduced metaconule and paraconule situated close to paracone and metacone, with small hypocone, reduced premetacrista, and reduced cingula. P3/shows a very reduced lingual cusp; P2/is very long and narrow.
Description.
The most outstanding feature of the upper first molar is the large size, the lengthening of paracone and metacone, and the reduction of the trigone. Thus, the tooth appears to be long and narrow. This morphology is accompanied by a typical wasting of the posterior part of the tooth. Metaconule and, especially, paraconule are reduced and situated close to paracone and metacone, hypocone is small, and the premetacrista is reduced considerably (Fig. 2).
Upper premolars.
Unfortunately, the P4/lacks the lingual cusp. Nevertheless, the preserved part suggests that the premolar was long and narrow, with an anteriorly placed, asymmetric and mediolaterally compressed labial cusp. From the top of this cusp, a sharp crest runs down posteriorly. There is a strong style on the anterior half of the tooth. The P3/is well preserved. It is long and triangular, similar to the pattern of the P4/, and its lingual cusp is reduced considerably. The P2/is a long and narrow tooth with a prominent posterior cingulum (Fig. 2).
Lower molars.
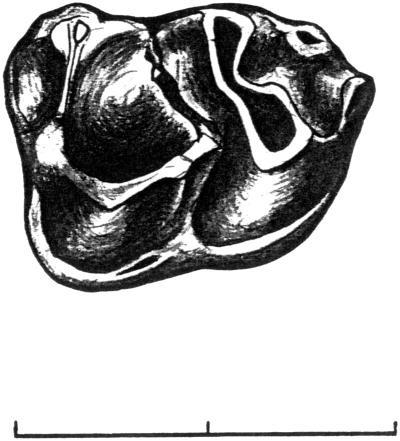
The isolated lower first molar from VF-13 shows the simple pattern of Pseudoloris, the same as the isolated trigones from Santpedor. Protoconid is considerably larger than metaconid. A cingulum runs close to the anterolingual fovea of the trigonid. The anterior cingula of the two isolated trigonids of SP-2 from Santpedor are better developed (Fig. 3).
Figure 3.
Lower first molar of Pseudoloris godinoti n. sp. from Fonollosa-13, Barcelona. Occlusal view. (Scale = 2 mm.)
Comparisons
Up to now, three species of Pseudoloris have been described: P. parvulus, P. crusafonti, and P. reguanti. The most common species is P. parvulus (17). It is well known and cited from many Upper Eocene sites (17). P. crusafonti is a Bartonian species and comes from the French locality Grissolles (18). A third species, P. reguanti (19), has been described from Upper Eocene levels (MP 19) of Sant Cugat de Gavadons, situated in the geographic area of our study. This latter species is considered nomen nudum (20), because it is described but not figured. It is based on a single tooth, the holotype, which was lost in the 1970s in the collections of Sabadell. Three Pseudoloris teeth recovered from Weissenburg-8 provide too little information for establishing a new species (20).
Pseudoloris godinoti n. sp. is larger than P. parvulus (17, 21), but similar in size to P. crusafonti (18) and Pseudoloris sp. from Weissenburg (Germany) (20). Morphologically, P. godinoti n. sp. differs from the other known Pseudoloris species in a series of features. The upper molars of P. godinoti n. sp. are more triangular, more elongated, and narrower. Trigone, metaconule, premetacrista, and the trigone basin are reduced. Also, the cingula are reduced considerably. The upper premolars are longer and narrower, and their cusps are mediolaterally compressed. The P3/has a reduced lingual cusp. Also, the lower P/3 is considerably more elongated and narrower. In some characters, however, P. godinoti n. sp. resembles P. crusafonti (18). This species reduced the posterior cingulum, paraconule, and hypoconule, and the P4/shows an asymmetric labial cusp with a sharp posterior crest. The material from Weissenburg, however, resembles that of P. parvulus rather than that of P. godinoti, but has a larger size (20). All suggest that P. godinoti n. sp. is more closely related to P. crusafonti than to P. parvulus and Pseudoloris n. sp. from Weissenburg-8.
Results and Discussion
In recent years, it has become increasingly clear that the drastic change in faunal structure during the “Grande Coupure” was accompanied by an important decrease in temperature and humidity, with a steep latitudinal gradient that caused the vast tropical/subtropical forests of the Eurasian continent to diminish (5, 6, 22). From most recent studies of the Mongolian faunal sequences, a turnover pattern emerges in which large and especially medium-sized forms are replaced by much smaller immigrants (“Mongolian Remodeling”) (8). This decrease in body size from Eocene to Oligocene faunas (see legend to Fig. 1) is accompanied by a shift from low-crowned to higher-crowned molars within primary consumers, reflecting adaptation to tougher diets, undoubtedly related to the expansion of more open and dryer habitats.
The Eocene–Oligocene primate record reflects a similar extinction/survival pattern. Eocene primates show a considerable diversity in their body size, with a noticeable size increase in some specialized folivorous genera (up to 8-kg body weight) at the end of the Eocene (23). Pseudoloris is the only primate genus that survived the major climatic changes across the Eocene/Oligocene boundary (see legend to Fig. 1). Pseudoloris species fall under the smallest Eocene prosimian primates and are comparable in size, skeletal structure (as far as is known), and dietary habits to the smallest living prosimians, the mouse lemur (Microcebus), and the smallest bushbaby (Galago demidovii, 45–120 g) (23). Microcebus and Galago demidovii are quite unspecialized and ubiquitous faunivores, which are abundant in the undergrowth of secondary forests and virtually in all forest types (23). The overall resemblances to these small, extant prosimians suggest a similar ecological niche for Pseudoloris species. Most likely, they were relatively independent from humid and densely forested habitats and, as secondary consumers, less susceptible to drastic changes in floral composition than the large folivorous adapids (23). Possibly, their lifestyle was a preadaptation to endure the disappearance of tropical forests under the harder environmental conditions during the Lower Oligocene, which were less suitable for the much larger and more specialized contemporaneous primates.
Acknowledgments
We thank S. Arbiol and A. Saez, who discovered these interesting sites.
Abbreviation
- MP
Mammal Paleogene European Chronostratigraphic Scale
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Stehlin H G. Bull Soc Geol Fr. 1909;4:488–520. [Google Scholar]
- 2.Tobien H. Münch Geowiss Abh. 1987;10:197–202. [Google Scholar]
- 3.Schmidt-Kittler N. Münch Geowiss Abh. 1987;10:1–311. [Google Scholar]
- 4.Brunet M. Les grands Mammifères, Chefs de File de l’immigration oligocène et le Problème de la Limite Eocène-Oligocène en Europe. Paris, France: Singer-Polignac; 1979. p. 325. [Google Scholar]
- 5.Legendre S. Münch Geowiss Abh. 1989;16:1–110. [Google Scholar]
- 6.Prothero D R. In: Mass Extinctions: Processes and Evidence. Donovan S K, editor. New York: Columbia Univ. Press; 1989. pp. 217–234. [Google Scholar]
- 7.Russell D E, Tobien H. In: Terminal Eocene Events. Pomerol C, Premoli-Silva I R, editors. Amsterdam: Elsevier; 1987. pp. 299–307. [Google Scholar]
- 8.Meng J, McKenna M C. Nature (London) 1998;394:364–367. [Google Scholar]
- 9.Macdonald J R. Bull Amer Mus Nat Hist. 1963;125:139–238. [Google Scholar]
- 10.McKenna M D. Geol Soc Amer Spec Pap. 1990;243:211–234. [Google Scholar]
- 11.Hooker J. Bull Br Mus Nat Hist. 1986;39(4):191–478. [Google Scholar]
- 12.Hooker J. Münch Geowiss Abh. 1987;10:109–116. [Google Scholar]
- 13.Brunet M, Vianey-Liaud M. Münch Geowiss Abh. 1987;10:30–31. [Google Scholar]
- 14.Saez A. Ph.D. thesis. Universidad de Barcelona; 1987. [Google Scholar]
- 15.Agusti J, Anadon P, Arbiol S, Cabreara L, Colombo F, Saez A. Münch Geowiss Abh. 1987;10:35–42. [Google Scholar]
- 16.Arbiol S, Saez A. Act Geol Hisp. 1988;1:47–50. [Google Scholar]
- 17.Godinot M. Palaeontographica. 1988;205:113–127. [Google Scholar]
- 18.Sudre J. Problèmes actuels de Paléontologie-Evolution des Vertébrés. Paris: Centre National de la Recherche Scientifique; 1975. pp. 805–828. [Google Scholar]
- 19.Crusafont M. Problèmes actuels de Paléontologie-Evolution des Vertébrés. Vol. 163. Paris: Centre National de la Recherche Scientifique; 1967. pp. 611–632. [Google Scholar]
- 20.Schmidt-Kittler N. Mitt Bayer Staatsslg Paläont Hist Geol. 1977;17:177–195. [Google Scholar]
- 21.Tobien H. Notizbl Hess L-Amt Bodenf. 1971;99:9–29. [Google Scholar]
- 22.Legendre D, Hartenberger J L. In: Eocene-Oligocene Climatic and Biotic Evolution. Prothero D R, Berggren W A, editors. Princeton, NJ: Princeton Univ. Press; 1992. pp. 516–528. [Google Scholar]
- 23.Fleagle J. Primate Adaptation and Evolution. San Diego: Academic; 1998. pp. 1–596. [Google Scholar]