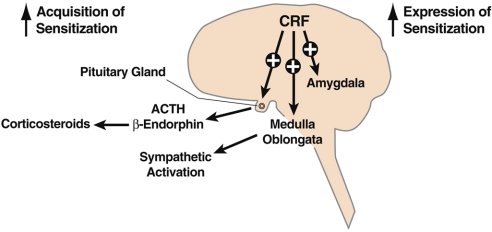
Sensitization is a ubiquitous biological phenomenon that has a role in the neuroadaptation of many different functions, from learning and memory to stress responsivity. Historically, a specific form of sensitization termed psychomotor sensitization (mistermed in my view as “behavioral sensitization”) is induced by repeated administration of drugs of abuse and has been linked to the neuroadaptive changes associated with increased drug-seeking behavior associated with addiction. Largely observed with psychostimulant drugs, psychomotor sensitization has been considered a model for the increased incentive salience contributing to the increased motivation to seek drugs in individuals with a previous history of drug use. Psychomotor sensitization also has been observed with repeated administration of ethanol in mice, but not in rats, and psychomotor sensitization to ethanol also has been linked to activation of both the hypothalamic-pituitary-adrenal (HPA) system and extrahypothalamic brain stress systems (Fig. 1). However, the exact mechanism for stress-induced sensitization has remained elusive.
Fig. 1.
CNS actions relevant to alcohol-induced psychomotor sensitization. CRF is a neuropeptide in the brain that controls autonomic, hormonal, and behavioral responses to stressors. New data show that CRF also has a role in the neuroplasticity associated with addiction. The present study extends the role of CRF to the psychomotor sensitization associated with repeated administration of alcohol. The hypothalamic-pituitary-adrenal responses produced by CRF appear to be more involved in the acquisition of sensitization, whereas extrahypothalamic CRF systems, likely to be in structures such as the amygdala, appear to be important for the expression of sensitization. These results, combined with previous studies on the role of CRF in the development of alcohol dependence, suggest a key role for CRF in the neuroplasticity associated with addiction.
Corticotropin-releasing factor (CRF) is a 41-amino-acid polypeptide that controls hormonal, sympathetic, and behavioral responses to stressors (1). CRF in the paraventricular nucleus of the hypothalamus controls the pituitary adrenal response to stress (2). Other peptides with structural homology to CRF, the urocortin family of peptides (urocortins 1, 2, and 3), also modulate behavioral and autonomic responses to stress and may have roles in other homeostatic functions, such as appetite. Substantial CRF- and urocortin 1-like immunoreactivity is present in the basal forebrain and autonomic midbrain and hindbrain nuclei. The endogenous selective CRF2 agonists—the type 2 urocortins—include urocortin 2 and urocortin 3 and have neuroanatomical distributions that are distinct from those of CRF and urocortin 1, notably in hypothalamic nuclei. Two CRF receptors have been identified, CRF1 and CRF2. The CRF1 receptor has abundant, widespread expression in the brain that overlaps significantly with the distribution of CRF and urocortin 1. The CRF2(a) receptor is localized neuronally in brain areas that are distinct from those of the CRF/urocortin 1/CRF1 receptor system and overlaps significantly with the distribution of the type 2 urocortins. CRF and urocortin 1 bind to both the CRF1 and the CRF2 receptors. Type 2 urocortins also differ from urocortin 1 and CRF with respect to their high functional selectivity for the CRF2 receptor.
In this issue of PNAS, Pastor et al. (3) show that mice lacking CRF1 and CRF2 receptors (double-constitutive knockouts) do not show psychomotor sensitization to ethanol. Further analysis revealed that knockout of the CRF1 receptor also blocked the psychomotor sensitization to ethanol. Administration of a CRF1 receptor antagonist attenuated the acquisition and blocked the expression of ethanol-induced psychomotor sensitization. Knockout of the CRF2 receptor, knockout of urocortin-1, and blockade of glucocorticoid receptors failed to block expression of ethanol-induced psychomotor sensitization. The authors hypothesize that CRF may have a key role in ethanol-induced psychomotor sensitization in acquisition (via activation of the HPA axis) and in expression (via the extrahypothalamic CRF stress system).
These results provide new insights into the role of the brain and pituitary stress systems in the neuroadaptations associated with chronic administration of drugs of abuse and alcohol. In particular, the study provides an elegant demonstration of the power of the molecular genetic approach in solving a problem and provides novel directions for therapeutic intervention in a chronic relapsing disorder. Previous studies using pharmacological approaches showed that CRF could facilitate psychomotor sensitization either via the HPA axis (4) or via extrahypothalamic sites (5–7). However, the specific CRF-like peptide, the specific CRF receptor, and the specific functional role had not been identified. Here, Pastor et al. (3) demonstrates unequivocally that the neuropeptide must be CRF and the receptor must be CRF1. Positive results with knockout mice provide a powerful means of testing a hypothesis, and one must employ a tortured compensatory logic to argue the contrary.
A parallel “sensitization” phenomenon that is important for alcohol dependence is hypothesized to involve neuroplasticity in the activation of both HPA and extrahypothalamic CRF systems. Progressive changes in the HPA axis are observed during the transition from acute administration to chronic administration of drugs of abuse. Acute administration in animals of most drugs of abuse, including cocaine, opiates, nicotine, and alcohol, activates the HPA axis and has been hypothesized to contribute to the acute reinforcing effects of these drugs (e.g., refs. 8–11). However, as the cycle of drug taking and withdrawal continues, the HPA axis response shows tolerance, but repeated exposure of the brain to high levels of glucocorticoids can continue to have profound effects on the extrahypothalamic brain stress systems. In fact, strong evidence suggests that glucocorticoids “sensitize” the CRF system in the extended amygdala (12). Increases in extrahypothalamic CRF drives anxiety-like responses associated with acute withdrawal from ethanol (13), excessive drinking during acute and protracted withdrawal from ethanol (14, 15), and stress-induced reinstatement of ethanol-seeking (16). Engagement of the brain stress systems, in contrast to the HPA axis, may contribute to the negative emotional state that dissipates with time after a single injection of a drug, but with repeated administration of drug grows larger with time (or fails to return to normal homeostatic baseline), setting up a negative reinforcement mechanism and representing another form of sensitization (17). The results reported by Pastor et al. (3), combined with the established role of CRF in drug dependence, provide an even more integrative role for CRF in the neuroplasticity of addiction. The integrative role for CRF in the neuroplasticity of reward, psychomotor sensitization, and dependence makes even more compelling the argument that the CRF system is an excellent target for future therapeutic strategies (18).
Footnotes
The author declares no conflict of interest.
See companion article on page 9070.
References
- 1.Bale TL, Vale WW. CRF and CRF receptors: Role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 2.Turnbull AV, Rivier C. Corticotropin-releasing factor (CRF) and endocrine responses to stress: CRF receptors, binding protein, and related peptides. Proc Soc Exp Biol Med. 1997;215:1–10. doi: 10.3181/00379727-215-44108. [DOI] [PubMed] [Google Scholar]
- 3.Pastor R, et al. Corticotropin-releasing factor-1 receptor involvement in behavioral neuroadaptation to ethanol: A urocortin-1-independent mechanism. Proc Natl Acad Sci USA. 2008;105:9070–9075. doi: 10.1073/pnas.0710181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deroche V, et al. Stress-induced sensitization to amphetamine and morphine psychomotor effects depend on stress-induced corticosterone secretion. Brain Res. 1992;598:343–348. doi: 10.1016/0006-8993(92)90205-n. [DOI] [PubMed] [Google Scholar]
- 5.Cador M, Cole BJ, Koob GF, Stinus L, Le Moal M. Central administration of corticotropin releasing factor induces long-term sensitization to D-amphetamine. Brain Res. 1993;606:181–186. doi: 10.1016/0006-8993(93)90982-s. [DOI] [PubMed] [Google Scholar]
- 6.Cole BJ, et al. Central administration of a CRF antagonist blocks the development of stress-induced behavioral sensitization. Brain Res. 1990;512:343–346. doi: 10.1016/0006-8993(90)90646-S. [DOI] [PubMed] [Google Scholar]
- 7.Koob GF, Cador M. Psychomotor stimulant sensitization: The corticotropin-releasing factor-steroid connection. Behav Pharmacol. 1993;4:351–354. [PubMed] [Google Scholar]
- 8.Goeders NE. A neuroendocrine role in cocaine reinforcement. Psychoneuroendocrinology. 1997;22:237–259. doi: 10.1016/s0306-4530(97)00027-9. [DOI] [PubMed] [Google Scholar]
- 9.Goeders NE, Guerin GF. Effects of surgical and pharmacological adrenalectomy on the initiation and maintenance of intravenous cocaine self-administration in rats. Brain Res. 1996;722:145–152. doi: 10.1016/0006-8993(96)00206-5. [DOI] [PubMed] [Google Scholar]
- 10.Piazza PV, et al. Corticosterone in the range of stress-induced levels possesses reinforcing properties: Implications for sensation-seeking behaviors. Proc Natl Acad Sci USA. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piazza PV, Le Moal M. Glucocorticoids as a biological substrate of reward: Physiological and pathophysiological implications. Brain Res Rev. 1997;25:359–372. doi: 10.1016/s0165-0173(97)00025-8. [DOI] [PubMed] [Google Scholar]
- 12.Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: A hybridization histochemical study in the rat. J Comp Neurol. 1989;285:413–435. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- 13.Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valdez GR, et al. Increased ethanol self-administration and anxiety-like behavior during acute withdrawal and protracted abstinence: Regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: Exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 18.Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]