Abstract
In mammals, melanopsin is exclusively expressed in intrinsically photosensitive retinal ganglion cells (ipRGCs), which play an important role in circadian photoentrainment and other nonimage-forming functions. These ipRGCs reside in the inner retina, far removed from the pigment epithelium, which synthesizes the 11-cis retinal chromophore used by rod and cone photoreceptors to regenerate opsin for light detection. There has been considerable interest in the identification of the melanopsin chromophore and in understanding the process of photopigment regeneration in photoreceptors that are not in proximity to the classical visual cycle. We have devised an immuno-magnetic purification protocol that allows melanopsin-expressing retinal ganglion cells to be isolated and collected from multiple mouse retinas. Using this technique, we have demonstrated that native melanopsin in vivo exclusively binds 11-cis retinal in the dark and that illumination causes isomerization to the all-trans isoform. Furthermore, spectral analysis of the melanopsin photoproduct shows the formation of a protonated metarhodopsin with a maximum absorbance between 520 and 540 nm. These results indicate that even if melanopsin functions as a bistable photopigment with photo-regenerative activity native melanopsin must also use some other light-independent retinoid regeneration mechanism to return to the dark state, where all of the retinal is observed to be in the 11-cis form.
Keywords: 11-cis retinal, photoisomerization, retinal ganglion cells
Rod and cone photoreceptors and melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs) constitute the three classes of light-detecting cells in the mouse retina (1). Rods and cones are specialized ciliary cells that provide input for image-forming vision. Melanopsin-expressing ipRGCs, which comprise ≈1–2% of all RGCs in the mouse retina (2), provide input to regulate circadian activity and other nonimage-forming responses of the retina (1, 3–7). Melanopsin is a G protein-coupled receptor and belongs to the opsin class of this superfamily of proteins (8, 9). Melanopsin is unique among vertebrate opsins, however, because it shares greater sequence similarity with invertebrate rhabdomeric opsins than with any other vertebrate opsins (9). The amino acid sequence of the melanopsin suggests that the photopigment may have photo-activation and pigment regeneration properties similar to those of many invertebrate rhabdomeric photopigments (IRPs).
In the mammalian retina, rod and cone photoreceptors are juxtaposed to the retinal pigment epithelium (RPE), which contains the enzymatic machinery necessary to reconvert all-trans retinal released by rod and cone photopigments back to 11-cis retinal for photopigment regeneration. This chromophore regeneration cycle is essential for maintaining photosensitivity in rod and cone photoreceptors. In contrast, ipRGCs are found in the ganglion cell layer of the retina, which is the most distal retinal layer to the RPE. Therefore, it seems likely that melanopsin photopigment formation and regeneration uses a different mechanism than rod and cone opsins.
Previous studies have investigated the photochemical nature of melanopsin (10–15) and suggest that melanopsin can form a functional photopigment with a variety of retinaldehyde isomers. Measurements of ipRGCs' spectral sensitivity match the spectrum of an opsin bound to vitamin A-derived retinaldehyde chromophore (3). Heterologous expression studies have shown that melanopsin can be reconstituted with 11-cis, 9-cis, 13-cis, and all-trans retinal to form a functional photopigment that demonstrates light-dependent activation of a G protein (12–16). Furthermore, light responses in melanopsin-expressing cells persist in the absence of exogenously added chromophore, suggesting that melanopsin is capable of using the vitamin A-derived chromophore that is synthesized in mammalian cell lines (15, 17). These studies suggest that heterologously expressed melanopsin demonstrates a lack of specificity for a particular A1 chromophore isomer. In vivo experiments in mice deficient in the retinal ester isomerhydrolase, RPE65, have demonstrated that treatment with exogenous 9-cis and all-trans retinal can rescue defects in pupillary light responses (10). Although these studies collectively suggest that the melanopsin photopigment in the dark can bind an A1 retinaldehyde chromophore, they do not resolve the identity of the chromophore bound to native melanopsin in its inactive or active states.
Morphologically, ipRGCs are unlike rods and cones in that they lack specialized photoreceptive structures such as an outer segment or a rhabdomere, which contain a readily isolated source of concentrated photopigment. IpRGCs express melanopsin throughout their dendrites and soma, forming a photoreceptive net across the retina (3, 18). We have calculated that there are ≈7 pmols of melanopsin in a single mouse retina compared with 400 pmols of rhodopsin (19) [supporting information (SI) Fig. S1]. For these reasons it has been difficult to directly measure the properties and spectral characteristics of the native melanopsin photopigment. By selectively isolating melanopsin-expressing cells from other photoreceptors in the mouse retina, we have been able to measure directly the spectral properties of endogenous mouse melanopsin and to identify its native chromophore. We found that native mouse melanopsin was exclusively bound to 11-cis retinal exclusively in the dark; subsequent exposure to 480-nm light induced isomerization to all-trans retinal, resulting in the formation of two spectrally distinct isoforms of the photopigment.
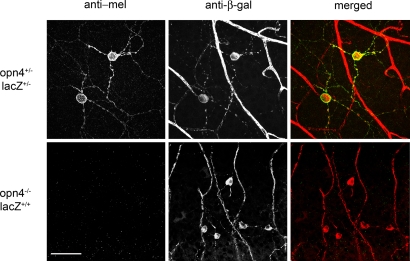
Results
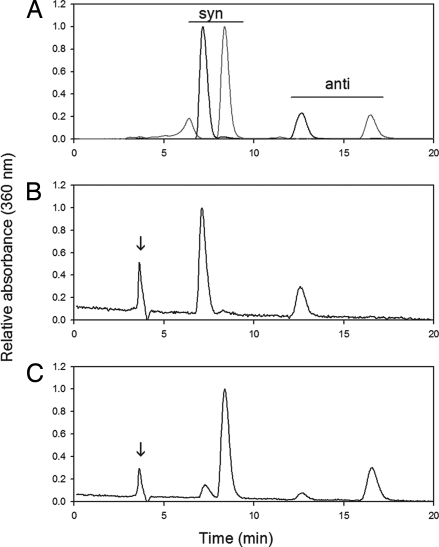
The chromophore of an opsin photopigment is the only light-sensitive portion of the molecule. The absorption of a photon of light causes the chromophore to isomerize, and as a result the visual pigment switches from an inactive confirmation to an active state. To understand the mechanism of light activation in melanopsin, it is necessary to understand the effect of light on the bound chromophore. To obtain endogenous melanopsin for this determination, we have designed a protocol using immuno-labeled magnetic beads (Miltenyi Biotec) that allows us to selectively separate melanopsin-containing ipRGCs from other photoreceptors in the mouse retina by using a melanopsin-specific antibody (Fig. 1). As shown in Fig. 2, this antibody is highly specific for melanopsin (opn4). In heterozygous animals expressing the tau-β-gal fusion protein under control of the melanopsin promoter (opn4+/− lacZ+/−), melanopsin was detected only in coincidence with β-gal (Fig. 2 Upper); furthermore, melanopsin staining was absent in retinas from melanopsin knockout mice (opn4−/− lacZ+/+), leaving only the β-gal staining (Fig. 2 Lower). To isolate highly purified melanopsin, we designed a procedure that emphasized purity at the expense of isolating total endogenous melanopsin. The purity of the preparation was probed by RT-PCR (Fig. 1A) and Western blot analysis (Fig. 1 B and C). The extracted ipRGCs were then solubilized with 1% digitonin in PBS, and the melanopsin-bound chromophore was extracted with hydroxylamine, which reacts with the Schiff base to produce free retinal oxime and opsin. The extracted chromophore was identified by using HPLC separation (Fig. 3). Chromophore extraction from dark-adapted retinas demonstrated that native mouse melanopsin binds 11-cis retinal (Fig. 3B). Irradiation with 480-nm monochromatic light caused the 11-cis retinal to isomerize to all-trans retinal (Fig. 3C). Despite its ability to bind various isoforms of the A1 chromophore in heterologous systems, in vivo, melanopsin binds only the 11-cis isoform in the dark, and light exposure isomerizes the 11-cis to all-trans retinal.
Fig. 1.
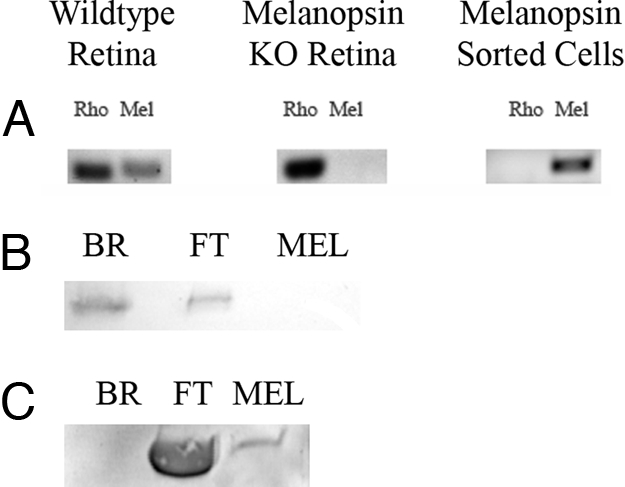
RNA and protein screening of immuno-magnetically isolated melanopsin-containing RGCs. (A) Agarose gel analysis of RT-PCR products visualized with ethidium bromide. Total RNA extracted from mouse WT (C57BL/6) retina, opn4−/− melanopsin KO retina, and immunomagentically isolated melanopsin cells was screened by using RT-PCR with DNA primers specific for mouse rhodopsin and melanopsin (see Experimental Procedures for primer sequences). (B) Rhodopsin protein detection using Western analysis. Protein from bovine rod outer segments (BR), melanopsin-sorting column flow through (FT), and melanopsin-sorted cells (MEL) were separated by SDS/PAGE and transferred to nitrocellulose. The immobilized protein was probed with anti-1D4 antibody (raised against the last 18 aa of bovine rhodopsin) that recognizes mouse rhodopsin. (C) Melanopsin protein detection using Western analysis. The immobilized protein was probed with an anti-melanopsin antibody that was raised against the carboxyl terminus of rat melanopsin and also recognizes mouse melanopsin. For B and C, protein expression was visualized on the Storm 860 phosphorimaging system (as described in Experimental Procedures). The prominent band at -50,000 KD in the FT in C is the heavy chain of the rabbit IgG.
Fig. 2.
Immunohistochemical detection of melanopsin-expressing cells in a mouse retina. In melanopsin KO mice, the melanopsin gene (opn4) was replaced with DNA encoding a tau-β-gal fusion protein. Retinal whole-mounts from heterozygous mice (Upper) and homozygous KO mice (Lower) were immunostained for melanopsin (Left) and β-gal (Center). Staining in the merged images (Right) have been pseudocolored (melanopsin, red; and β-gal, green) with yellow indicating colocalization. The large, branched structures visible in the anti-β-gal images (Center) are retinal blood vessels caused by the reaction of the anti-mouse secondary antibodies with endogenous mouse IgG. The total lack of anti-melanopsin staining in the KO retina indicates that the anti-melanopsin antibody used in this study is highly specific. (Scale bar: 20 μM)
Fig. 3.
Native melanopsin chromophore chromatogram. All procedures were performed under dim red light (Kodak filter 1A). Melanopsin-containing RGCs from 14 mouse retinas were isolated by using the immuno-magnetic sorting procedure described in SI Text and solubilized with 1% digitonin in PBS, pH 7.2. Concentrated HCl was added to each sample to a final concentration of 1 M. To extract the chromophore, hydroxylamine was used to remove the chromophore from the photopigment and to form retinal oximes. Chromophore was extracted from solution (procedure in Experimental Procedures), and the retinoids were dried under argon. For HPLC analysis the samples were resuspended in HPLC mobile phase (11.2% ethyl acetate/2.0% dioxane/1.4% octanol in hexane). The retinoids were separated by using a Lichrosphere-60 5-μm column (Alltech Associates). (A) Chromatogram of free 11-cis retinal (black line) and free all-trans retinal (gray line) treated with 50 mM hydroxylamine (chromophore standards). (B) Chromatogram of chromophore extracted from dark melanopsin photopigment (n = 3). (C) Chromatogram of chromophore extracted from light-irradiated melanopsin (n = 3). The peaks at 4 min in chromatograms B and C are predicted to be retinyl esters.
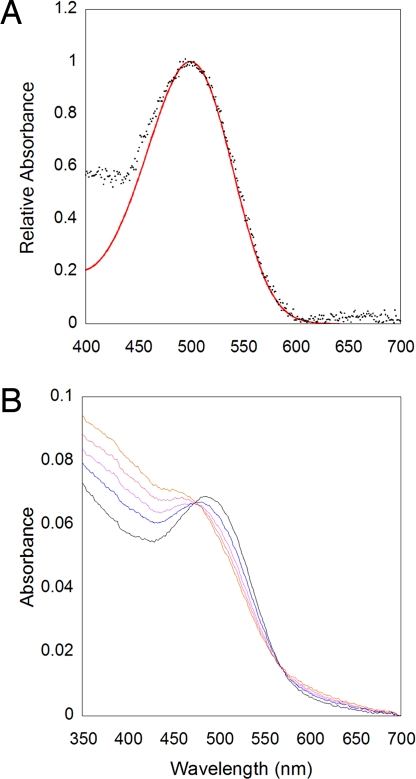
For spectral analysis of native melanopsin photopigment, melanopsin-containing cells were immuno-magnetically purified from dark-adapted retinas and solubilized in PBS containing 1% digitonin. In the dark, detergent-solubilized melanopsin had an absorbance maximum of 500 nm (Fig. 4A), which is slightly red-shifted from the spectral sensitivity of 480 nm determined from the action spectrum of ipRGCs (3). Furthermore, melanopsin remained functional after detergent solubilization, and, upon illumination, was capable of activating bovine rod transducin (Gt) in a light-dependent manner (Fig. S2). Thus, the unilluminated melanopsin represents the inactive state of the photopigment. In the dark, the bound 11-cis retinal keeps the activity of the photopigment low, and light isomerizes the chromophore to all-trans, driving the photopigment into an active state (i.e., a metarhodopsin). Light activation of the melanopsin photopigment also caused a spectral shift in its absorbance maximum. Irradiation with 480-nm light produced a decrease in the absorbance at 500 nm and a rise in absorbance in both the UV spectral range and the red (560–700 nm) portions of the spectrum (Fig. 4B). The melanopsin photoproduct was uniformly produced with each subsequent light pulse. In contrast to rhodopsin, an overlay of the series of light-irradiated spectra shows two isobestic points in the plots (Fig. 4B).
Fig. 4.
Absorbance spectra of native melanopsin photopigment from dark-adapted mouse retinas. Melanopsin-containing RGCs were isolated and then solubilized with 1% digitonin in PBS. (A) Normalized absorbance spectrum of the dark photopigment. Nomogram (red line) of an opsin photopigment with an absorbance maximum of 500 nm. (B) Absorbance spectra melanopsin light bleaching. Sample was treated with five consecutive 15-s pulses of 480-nm light (2.66 × 1014 photons/cm2 per s), and the absorbance spectrum was taken after each light pulse (black line, 15 s; blue line, 30 s; purple line, 45 s; magenta line, 60 s, and red line, 75 s).
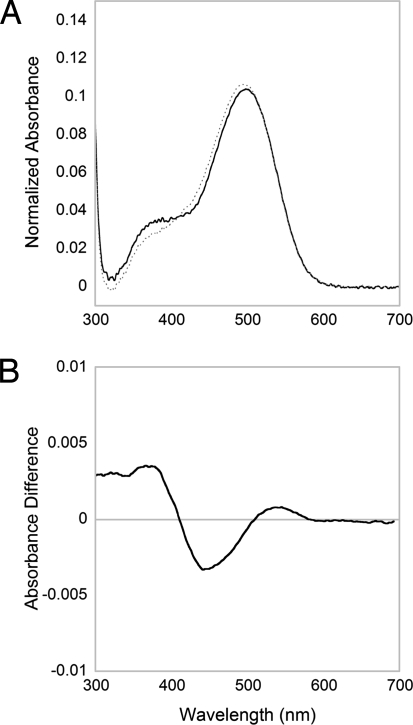
The experiment in Fig. 4B suggests that at least two photoproducts are formed by illumination of melanopsin. The spectral identity of the photoproduct was further explored in the experiment depicted in Fig. 5. In addition to these spectral changes, light activation of melanopsin causes a decrease in the stability of the photopigment. Unilluminated melanopsin photopigment remains stable from pH 7.4 down to at least pH 5.8. After illumination, the photoproducts were readily denatured by decreasing the pH of the solution from 6.8 to 5.8, as illustrated by the shift in the absorbance maximum of the photoproducts to 440 nm as the Schiff base is protonated (Fig. 5A, dashed lines). The difference spectrum of the acid-trapped metarhodopsin-like state shows that there were at least two photoproducts: one photoproduct had an absorbance in the UV (≈380 nm), and a second photoproduct had an absorbance maximum near 520–540 nm (Fig. 5B). Results from experiments described earlier (Fig. 3) indicate that both photoproducts are bound to all-trans retinal. The difference in the spectral absorbance of the photoproducts depends on the protonation of the metarhodopsin's Schiff base. The Schiff base of the UV-absorbing photoproduct is deprotonated, whereas the longer wavelength absorbing photoproduct is protonated.
Fig. 5.
Acid trapping of the melanopsin photoproducts. Melanopsin from solubilized ipRGCs was given a 15-s treatment with 480-nm light (2.66 × 1014 photons/cm2 per s). (A) Spectrum of melanopsin photopigment after light irradiation at pH 6.8 (solid line); 1 M HCl was added to reduce the pH of the photopigment to 5.8. Spectrum of melanopsin photopigment after HCl was added to lower the pH to 5.8 (dotted line). (B) Difference spectrum of the light-irradiated melanopsin at pH 6.8 minus pH 5.8.
Discussion
The biochemical study of the endogenous melanopsin photopigment has been limited by its expression in a very small subset of retinal ganglion cells. We have overcome this limitation by developing a protocol that selectively isolates ipRGCs from multiple retinas into a single sample. Using native photopigment, we have shown that melanopsin in dark-adapted retinas exclusively binds 11-cis retinal. The 11-cis retinal functions as an inverse agonist, keeping the photopigment in the inactive state. Upon exposure to light, the 11-cis form isomerizes to the all-trans isoform. It has been suggested that melanopsin acts as a bistable photopigment and that dark melanopsin can be regenerated by light. After light exposure, this type of regeneration mechanism would be expected to result in a photoequilibrium situation where there would be a mixed population of melanopsin containing both 11-cis retinal and all-trans retinal. However, the observation that only 11-cis retinal was found in melanopsin extracted from dark-adapted retinas suggests that, even with putative photo-regenerative properties, melanopsin must use some other light-independent mechanism to regenerate the photopigment. In Drosophila photoreceptors, Rh1 photopigment has the capacity to be photo-regenerated (20), yet Rh1 expression and pigment formation depend on the vitamin A retinoid synthesis pathway (21). Considering the distance and the presence of several layers of cells separating ipRGCs from the RPE, the regeneration of melanopsin with 11-cis retinal in ipRGCs remains an important unresolved question.
Based primarily on its amino acid sequence, melanopsin has been hypothesized to function like an IRP. Spectral analysis of melanopsin demonstrates that light irradiation produces two spectrally distinct photoproducts. These can be resolved by trapping the illuminated form of melanopsin with acid, revealing one product that has a maximum absorbance at ≈380 nm and a second product that absorbs maximally at ≈520–540 nm. In vertebrate ciliary photopigments like bovine rhodopsin, the formation of metarhodopsin causes a spectral shift in absorbance from 500 to 380 nm (22, 23). In the metarhodopsin state, the Schiff base of bovine rhodopsin becomes deprotonated, causing a blue-shift in the absorbance of the photopigment (24). For many IRPs like Drosophila or squid rhodopsin, the Schiff base of the metarhodopsin state in vitro can be either protonated or deprotonated (25, 26). The protonated form is called the acidic metarhodopsin, and its spectral absorbance maximum is red-shifted from that of the inactive state, whereas the deprotonated metarhodopsin is shifted to shorter wavelengths (25–27). Experiments with Drosophila and squid rhodopsin have shown that the equilibrium between the protonated and deprotonated forms in vitro is affected by both the photopigment's interaction with visual arrestin and temperature (25, 26, 28, 29). For native melanopsin, light irradiation results in the formation of both acidic and alkaline metarhodopsin. Under our current experimental conditions there appears to be a rapid decay of the acidic metarhodopsin to the deprotonated alkaline metarhodopsin. Further analysis of the formation of the melanopsin photoproducts will require the use of low-temperature spectroscopy, which has been a successful method for stabilizing and identifying photo-intermediates of IRPs (25, 28). For melanopsin, it will also be necessary to further optimize our cell sorting procedure to reach the pigment concentrations needed for low-temperature spectroscopy. Our results suggest that light activation of melanopsin forms photo-intermediates that are similar to those of IRPs. Based on the photochemistry of some invertebrate visual pigments, the formation of the red-shifted acidic metarhodopsin suggests that activated melanopsin may be photostable and therefore contains the potential to be photoconverted back to the inactive state.
Experimental Procedures
Immuno-Magnetic Cell Sorting of ipRGCs.
Mice were dark-adapted overnight, and the retinas were dissected from the eyecup under infrared light by using image converters. Retinal cells from 12–14 retinas were dispersed by using 1 mg/ml of Collagenase type D (Roche; 15 min at room temperature) in DMEM (Gibco). The cell sorting procedures were performed under dim red light (Kodak filter 1A) at 6°C. After trituration, the suspension of retinal cells was centrifuged for 5 min (1,000 × g) and resuspended in 2 ml of MACS buffer (10 mM PBS, pH 7.2, 0.5% BSA, and 2 mM EDTA). After one wash with MACS buffer, the cells were passed through a 100-μm cell strainer, centrifuged for 5 min (1,000 × g), and resuspended in 5 ml of anti-melanopsin antibody raised in rabbit against the extracellular amino terminal of mouse melanopsin (1:10,000 dilution in MACS buffer). The cell sample was incubated for 15 min, centrifuged for 5 min (1,000 × g), and resuspended in 2 ml of MACS buffer. The cells were then washed one additional time, centrifuged for 5 min (1,000 × g), resuspended in 320 μl of MACS buffer containing 20 μl of magnetic microbeads coated with a goat anti-rabbit IgG antibody (Militenyi Biotec), and incubated for 15 min. After the incubation, 1.66 ml of MACs buffer was added to the cell suspension, which was centrifuged for 5 min (1,000 × g), and resuspended in 0.5 ml of MACS buffer. Cells were once again passed through a 100-μm cell strainer and diluted with 2 ml of MACS buffer. Bead-bound cells were separated from other retinal cells by applying a magnetic field to a separation column (MS column; Militenyi) and passing the cell suspension through the column. After collecting the bead-bound ipRGCs, the column was washed three times with 500 μl of MACS buffer. After washing, the column was removed from the magnet, and the bead-bound cells were eluted with 1 ml of MACS buffer. The eluant was centrifuged at (14,000 × g) for 5 min, and the supernatant was removed.
RT-PCR Analysis.
Total cellular RNA was extracted from immuno-magnetic bead-sorted ipRGC cell pellets with an Absolutely RNA nanoprep kit (Stratagene). The extracted RNA was used to synthesize cDNA template with SuperScript III reverse transcriptase. The cDNA template was used in PCRs with gene-specific oligonucleotide primers to detect the expression of rhodopsin (forward, CCACAGGCTGTAATCTCGAGG GCTT; reverse, CGGAAGTTGCTCATCGGCTTGCAG) or melanopsin (forward, GCGGACACCAGCAAACATGTTC; reverse, CCGCAGAAGGCATAGAACTCGCAAC).
Immunoblotting and Immunohistochemical Analysis.
Immunological detection of immobilized proteins was performed by using a protocol adapted from the Western blotting protocol of Burnette (30). Rhodopsin expression was detected by using an anti-1D4 antibody (31) (1:10,000 dilution) and an anti-mouse IgG secondary antibody conjugated to alkaline phosphatase (Promega). Melanopsin expression was detected by using the anti-melanopsin antibody that targets the carboxyl terminus (2) (1:5,000 dilution) and an anti-rabbit IgG secondary antibody conjugated to alkaline phosphatase (Promega). Fluorescent alkaline phosphatase substrate (Attophos; Promega) was used to detect antibody binding, and blots were visualized on the Storm 860 phosphorimaging system (Molecular Dynamics).
Immunohistochemistry was performed on mouse retinal whole mounts as described (32). The anti-melanopsin antibody was generated against a peptide containing the first 15 amino-terminal residues of melanopsin conjugated to keyhole limpet hemocyanin and was affinity-purified by using the peptide before use (1:1,000). The anti-β-gal antibody was a mouse monoclonal (clone 40-1a) obtained from the Developmental Studies Hybridoma Bank at the University of Iowa, Iowa City.
Melanopsin Absorbance Spectra.
All procedures were performed under dim red light (Kodak filter 1A). Immuno-magnetic bead-sorted, melanopsin-containing RGC cell pellets were resuspended in 1% digitonin in PBS. Samples were rotated for 2 h at 4°C and then centrifuged for 20 min (14,000 × g) at 4°C. The supernatant was removed and analyzed with a Hitachi model U-3300 dual-path spectrophotometer.
Retinoid Extraction and Analysis.
All procedures were performed under dim red light (Kodak filter 1A). Melanopsin-containing RGCs from 12–14 mouse retinas were isolated by using the immuno-magnetic sorting procedure and solubilized with 1% digitonin in PBS. Concentrated HCl was added to each 120-μl sample to a final concentration of 1 M. To extract the chromophore, methanol (300 μl) and 1 M hydroxylamine (60 μl) were added, and the samples were vortexed for 30 s. Samples were then incubated at room temperature for 5 min. Methylene chloride (300 μl) was added to each of the samples, which were then vortexed for 30 s and centrifuged (14,000 × g, 1 min). The lower organic phase of the sample was removed, and the extraction procedure was repeated on the upper aqueous phase. After the second extraction, the organic phase from both extractions were combined and dried under argon. For HPLC analysis the samples were resuspended in HPLC mobile phase (11.2% ethyl acetate/2.0% dioxane/1.4% octanol in hexane). The retinoids were separated by HPLC using a Lichrosphere-60 5-μm column (Alltech Associates).
Supplementary Material
Acknowledgments.
We thank R. K. Crouch and J. G. Beall (Medical University of South Carolina, Charleston) for the HPLC analysis of all retinoid samples. This research was supported by National Institutes of Health National Research Service Award 1F31EY015927-01 (to M.T.W.), National Institutes of Health Initiative for Minority Student Development Grant R25-GM55036 (to M.T.W.), National Science Foundation Grants IOB 0615569 and IBN 0119102 (to P.R.R.) and IOS 0721608 (to T.W.C. and P.R.R.), and National Institute of Mental Health Grant MH67,094 (to R.L.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711397105/DCSupplemental.
References
- 1.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:75–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hattar S, et al. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 4.Lucas RJ, et al. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 5.Panda S, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 6.Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int. 2003;20:989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- 7.Warren EJ, Allen CN, Brown RL, Robinson DW. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur J Neurosci. 2003;17:1727–1735. doi: 10.1046/j.1460-9568.2003.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Provencio I, et al. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Provencio I, et al. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu Y, et al. Intrinsically photosensitive retinal ganglion cells detect light with a vitamin A-based photopigment, melanopsin. Proc Natl Acad Sci USA. 2005;102:10339–10344. doi: 10.1073/pnas.0501866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koyanagi M, et al. Cephalochordate melanopsin: Evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr Biol. 2005;15:1065–1069. doi: 10.1016/j.cub.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 12.Melyan Z, et al. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- 13.Newman LA, et al. Melanopsin forms a functional short-wavelength photopigment. Biochemistry. 2003;42:12734–12738. doi: 10.1021/bi035418z. [DOI] [PubMed] [Google Scholar]
- 14.Panda S, et al. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- 15.Qiu X, et al. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 16.Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflügers Arch. 2007;454:849–855. doi: 10.1007/s00424-007-0242-2. [DOI] [PubMed] [Google Scholar]
- 17.Ukai H, et al. Melanopsin-dependent photo-perturbation reveals desynchronization underlying the singularity of mammalian circadian clocks. Nat Cell Biol. 2007;9:1327–1334. doi: 10.1038/ncb1653. [DOI] [PubMed] [Google Scholar]
- 18.Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina: This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- 19.Hanson SM, et al. Each rhodopsin molecule binds its own arrestin. Proc Natl Acad Sci USA. 2007;104:3125–3128. doi: 10.1073/pnas.0610886104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirschfeld K, Franceschini N, Minke B. Evidence for a sensitizing pigment in fly photoreceptors. Nature. 1977;269:386–390. doi: 10.1038/269386a0. [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Jiao Y, Montell C. Dissection of the pathway required for generation of vitamin A and for Drosophila phototransduction. J Cell Biol. 2007;177:305–316. doi: 10.1083/jcb.200610081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews RG, Hubbard R, Brown PK, Wald G. Tautomeric forms of metarhodopsin. J Gen Physiol. 1963;47:215–240. doi: 10.1085/jgp.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wald G. Molecular basis of visual excitation. Science. 1968;162:230–239. doi: 10.1126/science.162.3850.230. [DOI] [PubMed] [Google Scholar]
- 24.Zhukovsky EA, Oprian DD. Effect of carboxylic acid side chains on the absorption maximum of visual pigments. Science. 1989;246:928–930. doi: 10.1126/science.2573154. [DOI] [PubMed] [Google Scholar]
- 25.Yoshizawa T, Shichida Y. Low-temperature spectrophotometry of intermediates of rhodopsin. Methods Enzymol. 1982;81:333–354. doi: 10.1016/s0076-6879(82)81051-3. [DOI] [PubMed] [Google Scholar]
- 26.Schwemer J, Langer H. Insect visual pigments. Methods Enzymol. 1982;81:182–190. doi: 10.1016/s0076-6879(82)81030-6. [DOI] [PubMed] [Google Scholar]
- 27.Stavenga D, Schwemer J. Visual Pigments of Invertebrates. In: Ali MA, editor. Photoreception and Vision in Invertebrates. Vol 74. New York: Plenum; 1984. pp. 11–60. [Google Scholar]
- 28.Vought BW, et al. Characterization of the primary photointermediates of Drosophila rhodopsin. Biochemistry. 2000;39:14128–14137. doi: 10.1021/bi001135k. [DOI] [PubMed] [Google Scholar]
- 29.Kiselev A, Subramaniam S. Activation and regeneration of rhodopsin in the insect visual cycle. Science. 1994;266:1369–1373. doi: 10.1126/science.7973725. [DOI] [PubMed] [Google Scholar]
- 30.Burnette WN. Western blotting: Electrophoretic transfer of proteins from sodium dodecyl sulfate–polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 31.Molday RS, MacKenzie D. Monoclonal antibodies to rhodopsin: Characterization, cross-reactivity, and application as structural probes. Biochemistry. 1983;22:653–660. doi: 10.1021/bi00272a020. [DOI] [PubMed] [Google Scholar]
- 32.Warren EJ, Allen CN, Brown RL, Robinson DW. The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells. Eur J Neurosci. 2006;23:2477–2487. doi: 10.1111/j.1460-9568.2006.04777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.