Abstract
Kinesin-1 is a molecular motor protein that transports cargo along microtubules. Inside cells, the vast majority of kinesin-1 is regulated to conserve ATP and to ensure its proper intracellular distribution and coordination with other molecular motors. Regulated kinesin-1 folds in half at a hinge in its coiled-coil stalk. Interactions between coiled-coil regions near the enzymatically active heads at the N terminus and the regulatory tails at the C terminus bring these globular elements in proximity and stabilize the folded conformation. However, it has remained a mystery how kinesin-1's microtubule-stimulated ATPase activity is regulated in this folded conformation. Here, we present evidence for a direct interaction between the kinesin-1 head and tail. We photochemically cross-linked heads and tails and produced an 8-Å cryoEM reconstruction of the cross-linked head–tail complex on microtubules. These data demonstrate that a conserved essential regulatory element in the kinesin-1 tail interacts directly and specifically with the enzymatically critical Switch I region of the head. This interaction suggests a mechanism for tail-mediated regulation of the ATPase activity of kinesin-1. In our structure, the tail makes simultaneous contacts with the kinesin-1 head and the microtubule, suggesting the tail may both regulate kinesin-1 in solution and hold it in a paused state with high ADP affinity on microtubules. The interaction of the Switch I region of the kinesin-1 head with the tail is strikingly similar to the interactions of small GTPases with their regulators, indicating that other kinesin motors may share similar regulatory mechanisms.
Keywords: cross-linking, electron microscopy, regulation, switch
The motor protein kinesin-1 uses energy from ATP hydrolysis to move intracellular cargo to the microtubule plus end. Kinesin-1 is either regulated or activated for cargo movement in response to various cues, ensuring its proper localization and facilitating cargo transport to the right destination. The mechanism by which active kinesin-1 converts ATP hydrolysis into movement is fairly well established, but little is known about how kinesin-1 is regulated when it is not needed for cargo transport.
Regulated kinesin-1 adopts a folded conformation in which it remains very tightly bound to ADP and does not bind strongly to microtubules (1, 2). This folding can occur in the absence of kinesin light chains, although the light chains confer additional regulatory function (1, 3). In the folded conformation, the hinge II region of kinesin-1's coiled-coil stalk bends, and an interaction occurs between the neck coiled-coil and the tail coiled-coil (4–6). This interaction stabilizes the folded conformation, positioning the C-terminal globular tail domain near the enzymatically active heads [supporting information (SI) Fig. S1] (7, 8).
Kinetic data on tail-mediated regulation have shown that the folded conformation of kinesin-1 is not strictly necessary, nor is it sufficient for inhibition of ADP release by the heads. The conserved QIAKPIRP sequence in the C-terminal globular portion of the tail (residues 919–926 in human kinesin-1) is not required for kinesin-1 to fold, but deletion or mutation of this sequence abolishes regulation (1, 2, 9). The QIAKPIRP sequence specifically inhibits kinesin-1's initial microtubule-stimulated ADP release step, when it first engages on the microtubule (2). Short peptides containing the QIAKPIRP sequence but lacking any tail coiled-coil elements have also been shown to inhibit ADP release by truncated kinesin-1 heads (10). Oddly enough, a direct head–tail interaction has never been identified for kinesin-1, even in experiments using extensive mutagenesis and screening approaches, despite the fact that the kinetic data appear to demand it (6, 9). The interaction between the neck coiled-coil and tail coiled-coil elements could provide structural support for a relatively weak but direct head–tail interaction involving the critical QIAKPIRP sequence. The proposed direct head–tail interaction would then perform the critical regulatory function of preventing microtubule-stimulated ADP release and subsequent movement by kinesin-1.
The QIAKPIRP sequence of the tail inhibits kinesin-1's initial microtubule-stimulated ADP release step, while a region of the tail roughly 15 residues N-terminal to this sequence binds to microtubules (2, 5). If these two activities occurred simultaneously, kinesin-1 could pause in a state that is tightly bound to microtubules but inhibited in its ATPase activity and thus movement. Consistent with this idea, pauses have been observed in processive runs by single molecules of full-length but not truncated kinesin-1 (4). While regulation primarily prevents the heads from productively engaging with microtubules, it is intriguing to suggest that a second regulatory mechanism of the kinesin-1 tail may affect its movement on microtubules by enabling it to pause its enzymatic activity but remain microtubule-bound.
In this work, we show that a direct interaction occurs between the inhibitory QIAKPIRP sequence of the tail domain and the Switch I region in the head of kinesin-1 (residues 190–205). For kinesin motors and for small GTPases, Switch I plays a critical role in nucleotide binding and/or release (11–14). Thus, a Switch I–tail interaction is consistent with the known kinetic mechanism of tail-mediated regulation (2) and is analogous to the mechanisms by which GDP dissociation inhibitor proteins (GDIs) inhibit small GTPases¶ (10, 15). Our results also show that the tail can, at least in principle, bind to the heads and microtubule at the same time. This may create a paused state for kinesin-1 that can be modulated by other factors for additional regulation of motor activity.
Results and Discussion
Specific Association and Photocross-Linking of Kinesin-1 Heads and Tails.
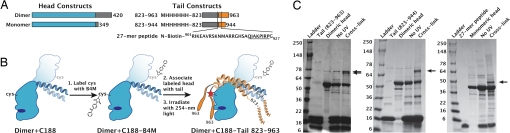
We hypothesized that kinesin-1 regulation occurs through a direct interaction of the enzymatically active head with the C-terminal tail. To identify head–tail interactions, we combined separate truncated head and tail constructs (Fig. 1A) and allowed them to associate in trans. We used either monomeric or dimeric untagged human kinesin-1 heads having all reactive cysteines removed (referred to as cysteine-light). Truncated dimeric tail constructs consisted of either residues 823–963 or 823–944 with an N-terminal 6x-histidine tag. A monomeric N-terminally biotinylated 27-mer peptide containing the critical QIAKPIRP sequence of the tail was also used.
Fig. 1.
Photocross-linking of kinesin-1 head and tail domains. (A) Head and tail constructs. Head residues are cyan, coiled-coil residues gray, and predicted globular tail residues are orange (17). Cysteine-light monomeric and dimeric head constructs have been described (19, 24). Dimeric tail constructs contain N-terminal 6x-histidine tags and residues 823–963 or 823–944. The 27-mer tail peptide (residues 901–927 with an N-terminal biotin tag) corresponds to the bracketed region of the dimeric tail constructs and is shown with the conserved QIAKPIRP underlined. (B) Photocross-linking scheme. Heads and tails are colored as in A with blue outlining on the neck coiled-coil and orange outlining on the predicted tail coiled-coil. The head in the foreground is positioned as if it were docked on a microtubule with the plus end up, the same orientation as the head in Fig. 3A. Cys-188 and bound ADP (blue ellipse) are shown. After conjugation to the maleimide moiety of the B4M (Center), heads were associated with tails, then irradiated to initiate photocross-linking (see Methods). A red star marks the cross-linked site (Right). Dimeric heads and tails are shown, but cross-linking was observed at position 188 using any combination of the head and tail constructs shown in A. We cannot determine whether one or both heads of a kinesin-1 dimer interact with the dimeric tail. This diagram model is consistent with our observed <50% cross-linking efficiencies (see Methods). An interaction between the neck coiled-coil and tail coiled-coil is shown, based on previous studies (4, 9). (C) SDS/PAGE gels (4–20%) showing photocross-linking. For the photo cross-linking experiment shown in the left gel, the heads contained Cys 188 and the G234A mutation, whereas in the others, the heads contain only the Cys 188 mutation. The tail constructs used for these experiments are indicated on each gel. Lanes for all gels are as follows: SeeBlue Plus2 Prestained Standard (Invitrogen), tail, head + Cys 188, head + tail before UV exposure, head + tail after 5-min UV exposure. Bands marked with arrows contained both head and tail sequences, as verified by MALDI-MS.
To demonstrate that kinesin-1 heads and tails associate, we performed a co-elution experiment (Fig. S2). We combined dimeric heads with dimeric tails containing residues 823–944. We flowed the combined heads and tails over TALON resin, which binds the 6x-histidine tag on the tails. After extensive washing, we performed sequential elutions using 10 and 100 μM 27-mer tail peptide, followed by an imidazole elution. Kinesin-1 heads did not bind to TALON resin (Fig. S2A), but heads that were associated with tails were retained on the column. Because binding of heads to tails is relatively weak, some heads are visible in washes. Additional heads eluted after the addition of the 27-mer peptide, and the remaining heads and tails coeluted from the TALON resin after addition of imidazole (Fig. S2B). This result suggests that dimeric heads and tails interact, and the 27-mer tail peptide competes at least partially for the same binding site on the heads as the dimeric tail construct.
To test whether the head–tail interaction is specific, we added single reactive cysteines back to known locations on the monomeric or dimeric heads and photocross-linked them using benzophenone-4-maleimide (B4M), as described in Fig. 1B. We labeled the added cysteine with the maleimide moiety of the photocross-linker B4M, then combined heads and tails under conditions that allow them to associate (see Methods). We next irradiated the combined B4M heads and tails with 254-nm UV light, which initiates cross-linking of the benzophenone moiety to carbon-hydrogen bonds within 9 Å (16) (Fig. 1B). Formation of a head–tail cross-link indicates the tail may interact with the head near the position of the added cysteine. The collective results of several experiments suggest that cross-linking is specific. Head–head cross-links were not observed in any of our experiments, and efficient head–tail cross-linking was observed only for cysteines at certain locations in the head. In particular, cross-links were formed between tail proteins and cysteines added back to heads at positions 188 in the α3 helix (Fig. 1C) and positions 193 and 197 in Switch I (data not shown). In contrast, head–tail cross-links were not detected by using heads containing single cysteines in the neck linker region at positions 328, 330, or 333 (data not shown; see Fig. 3 and Movie S1 for cysteine locations).
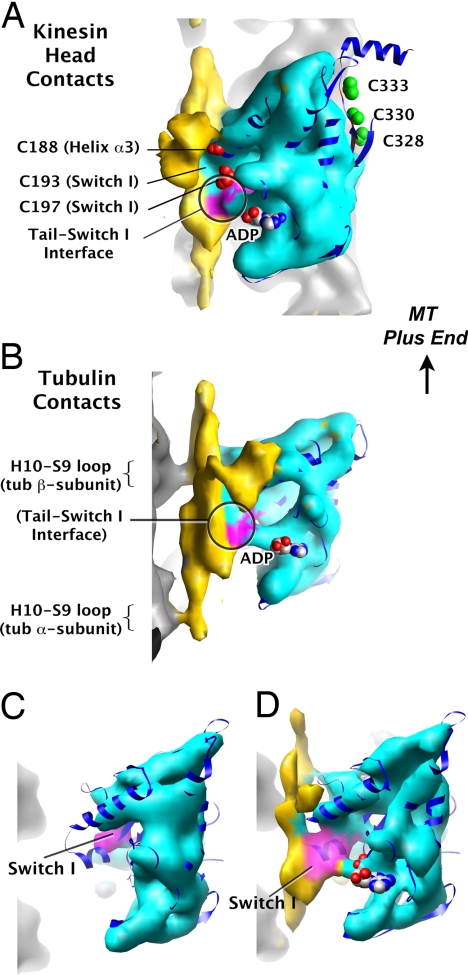
Fig. 3.
CryoEM map of cross-linked head and tail bound to microtubules at 8-Å resolution. (A) View from outside the microtubule, with the plus end pointing up. Cyan head density, magenta Switch I density, white microtubule density, and yellow tail density were rendered by using the “Color Zone” function of University of California, San Francisco (UCSF) Chimera (27), by coloring the isosurface based on proximity to fitted crystal structures of tubulin and kinesin-1 (11). Atomic models of bovine tubulin (21) and human kinesin-1 (20) (dark blue ribbon) were fit into map density using the real-space docking function “Fit Model in Map” from UCSF Chimera. Sites for photocross-linking experiments are rendered in colored van-derWaals (VDW) spheres, where red indicates that specific cross-links were found, and green indicates they were not. ADP is rendered in VDW spheres. The head–tail contact at Switch I, where a magenta–yellow boundary occurs on the isosurface, is circled. (B) Side view, similar to A but rotated 90° about the vertical axis, with tail–microtubule contacts visible. (C) Reconstruction of nucleotide-free kinesin-1 bound to microtubules, from ref. 11. View, rendering, and color scheme are matched to A and B, and view orientation is intermediate between those of A and B. Switch I is out of density in this structure, as it is for several others at this resolution. (D) Kinesin-1 head–tail reconstruction with the same orientation, view, rendering, and color scheme. Switch I from the x-ray crystal structure (PDB ID code 1mkj) is within the cryoEM density in this reconstruction, consistent with the tail holding Switch I in a “solution-like” state. See text for discussion.
We performed the B4M photocross-linking experiments as described above using either monomeric or dimeric head constructs containing Cys 188, in combination with each of the tail constructs shown in Fig. 1A. SDS/PAGE gels of all of these photocross-linking reactions revealed similar results. A sharp band appears on the gel with an apparent molecular weight equal to head and tail combined (Fig. 1C). The experiment using dimeric heads and tails containing residues 823–963 (left gel) shows two cross-linked products that differ in size by about 1.5 kDa. Both products (indicated by the double arrows) were excised and analyzed separately by mass spectrometry, and both contained peptides from both the head and tail. Therefore, we conclude that the smaller of these two products is due to cross-linking of the observed proteolytic product of this tail fragment. The size of this product is consistent with proteolysis occurring between residues 944 and 963. Previous experiments have shown that proteolysis of the kinesin-1 tail between residues 944 and 963 is common, and that residues C-terminal to 944 are not necessary for tail-mediated regulation of the heads' ATPase activity (2). Thus, we used either the tail construct containing residues 823–944 or the 27-mer tail peptide for all subsequent experiments of this study. Tail proteolysis is substantially reduced in the tail construct containing residues 823–944 (Fig. 1C, center gel), and completely eliminated in the 27-mer tail peptide (Fig. 1C, right gel), both of which still cross-link efficiently to heads containing Cys 188.
That monomeric heads cross-link efficiently to the 27-mer tail peptide (Fig. 1C, right gel) indicates the monomeric kinesin-1 head and 27-mer tail peptide can associate directly, albeit weakly, despite the fact that they lack the majority of the interacting neck and tail coiled-coil residues that stabilize kinesin-1's folded conformation (Fig. S1). This result is consistent with previous data indicating that short peptides containing the QIAKPIRP sequence have regulatory activity, and residues C-terminal to 944 are not necessary for the tail to bind to the head (2, 5, 10).
Identification of Head–Tail Cross-Linked Products by MALDI-MS.
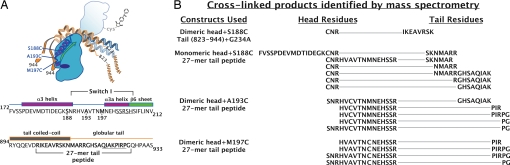
To identify the exact locations on the tail that cross-linked to the head, we excised cross-linked head + tail bands from Coomassie-stained gels from the above experiments (Fig. 1C), digested with trypsin and analyzed the resulting peptides by MALDI-MS. Fig. 2A shows a diagram model of a cross-linked product of a kinesin-1 head and tail, with sequences corresponding to cross-linked regions in the head and tail below. The break between the coiled-coil tail and globular tail is shown to occur at residue 910, as predicted by the COILS program (17).
Fig. 2.
Mass spectrometry analysis of cross-linked products. (A) Diagram model and partial sequences of kinesin-1 head and tail. Coloring and relative positions of head and tail elements are as in Fig. 1B, except that positions of cysteine mutations used for cross-linking (S188C, A193C, and M197C) in the head are indicated, α3/Switch I are in purple, and the β6 sheet immediately after Switch I is in green. Below, sequences of head (residues 172–212) and tail (residues 894–933) near the identified B4M cross-links are shown. Residues 188, 193, and 197 of the head sequence are in bold, Switch I residues are bracketed, and the kinesin superfamily conserved SSRSH sequence in Switch I is underlined. The predicted break between the tail coiled-coil and globular tail is shown in the tail sequence (17). The 27-mer peptide sequence is bracketed, and the conserved regulatory QIAKPIRP sequence is underlined. (B) Cross-linked products of heads and tails identified by MALDI-MS. The cys mutation in the head and the constructs used for B4M photocross-linking reactions are identified in the left column. Specific head-tail cross-links found in each reaction are shown in the two right columns, with a line connecting the head and tail peptide fragments found within each cross-linked product.
Fig. 2B summarizes the results of several B4M cross-linking experiments analyzed by MALDI-MS. Head and tail constructs used for each cross-linking experiment are shown on the left. Cross-linked, trypsinized peptides identified by MALDI-MS that contained both head and tail sequences are indicated for each reaction on the right, joined by a line. For each of these products, the position of the cross-link on the head is at the added cysteine residue, and the position on the tail is within the peptide sequence displayed. The sequence of the cross-linked product of the dimeric head+G234A+S188C and the dimeric tail containing residues 823–944 was confirmed by sequencing using tandem mass spectrometry as described in ref. 18. All other cross-linked products occurred in “families,” that is, several similar masses were identified that result from slight variations in the tryptic digest pattern. Families of cross-linked products are grouped together in Fig. 2B. The existence of these families serves as positive identification of a cross-link (18).
Monomeric and dimeric head constructs containing Cys 188 cross-linked to similar locations in the 27-mer tail peptide and the dimeric tail construct. These data show that our head and tail constructs can associate specifically, regardless of whether the interaction of the neck coiled-coil and tail coiled-coil is intact. The locations of the single cysteine addbacks at residues 188, 193, and 197 on the kinesin-1 structure roughly form a line on the side of kinesin-1 leading from α3 (Cys 188) into Switch I (Cys 193 and 197) (Fig. 2A). The cross-links follow a pattern such that the region of the tail that cross-links to Cys 188 is immediately N-terminal to the Cys 193 and Cys 197 cross-links. Notably, Cys 193 and Cys 197 in Switch I cross-link directly to the conserved QIAKPIRP sequence of the tail. Because Switch I controls the rate of microtubule-stimulated ADP release by kinesin motors (14), an interaction of the QIAKPIRP sequence with Switch I is consistent with the fact that the sequence inhibits microtubule-stimulated ADP release approximately 80-fold (2).
Visualization of Head–Tail Interactions by CryoEM.
To gain a detailed structural picture of the regulatory head–tail interaction, we performed cryoEM on microtubules decorated with head–tail products that were first photocross-linked at Cys 188 as before, then enriched using TALON affinity purification (see Methods). Although this material is enriched for the cross-linked product, some non-cross-linked material was present. For these experiments, we used G234A, a head mutant that binds tightly to microtubules regardless of nucleotide state (19). The cross-linked product of monomeric G234A heads and the tail protein containing residues 823–944 binds tightly to microtubules in the presence of ADP. We expect that the G234A mutation enables the heads to remain tightly bound to the microtubule while the tail prevents ADP release. It is possible that the Switch I–tail contact with wild-type kinesin-1 is primarily formed in solution, but by using the G234A mutant, we have imaged the interaction of the head and tail using cryoEM on microtubules.
In our 8-Å cryoEM map, shown in Fig. 3, density for tubulin and kinesin-1 closely resembles that reported for the 8-Å nucleotide-free kinesin-1–microtubule complex (11). Considerable additional density corresponds to the cross-linked tail (yellow density in Fig. 3). The tail appears as an elongated density parallel to the microtubule axis and makes simultaneous contacts with the kinesin-1 head (Fig. 3A) and the microtubule (Fig. 3B). Although we cannot deduce the precise structure of the tail from this map, direct and specific head–tail contacts are readily apparent. These are described in detail below.
To identify specific residues involved in tail interactions with the head and the microtubule, we fitted the crystal structures of monomeric human kinesin-1 (20) and bovine tubulin (21) into our map. Residue 188 is within 5 Å of the tail density, consistent with the fact that the heads and tails used for this cryoEM reconstruction were cross-linked together by B4M at this location. The most significant contact between the head and tail in our map encloses residues 193 and 197 in Switch I (Fig. 3A, magenta). That residues 188, 193, and 197 all appear within or very near the tail density in our cryoEM map is consistent with the specific cross-linking of the tail to these residues, shown in Fig. 2. Also consistent with our photocross-linking data, locations that did not cross-link to the tail (328, 330, and 333) appear on the opposite side of the kinesin-1 head from the tail density in our cryoEM map (Fig. 3A, Movie S1). Looking at two adjacent kinesin-1 head–tail complexes in this 8-Å reconstruction, a distinct gap can be seen between the side of a kinesin-1 head where residues 328, 330, and 333 are located, and the tail corresponding to the adjacent head (Fig. S3). Thus, we conclude that the tail interacts with the side of the kinesin-1 head near Switch I, and it does not interact with the side of the head near the neck linker.
The most prominent contact made by the tail in our map is with Switch I of the head. This is an extensive interaction, because it is maintained even if the cryoEM reconstruction is rendered at isocontour levels where no other head–tail and head–microtubule interactions are evident (Movie S1). To demonstrate that this tail–Switch I contact is specific, we produced an asymmetric microtubule density map by reconstructing our data without averaging the 13 protofilaments together (Fig. S4).
Although the asymmetric map shown in Fig. S4 is of lower resolution compared with our fully averaged 8-Å map (16 Å; see Methods), it preserves the specific geometry of the 13-protofilament microtubule, including the seam. The 40-Å offset of α and β tubulin subunits at the seam disrupts the usual position of the heads immediately to the right side of the seam relative to tubulin subunits immediately to the left side of the seam. Therefore, if the Switch I–tail contact simply reflected a convenient crevice in the kinesin-1–microtubule interface for the tail to dock into, it would be absent at the microtubule seam, because the crevice formed by the kinesin-1 heads to the right of the seam and tubulin subunits to the left of the seam is different from everywhere else on the microtubule (Fig. S4A). However, the asymmetric reconstruction shows that the Switch I–tail contact is clearly preserved and appears strong for tails lying along the seam (Fig. S4C). This would be seen only for a specific contact between the tail and Switch I.
The Tail Induces the Head to Adopt a Conformation with High ADP Affinity.
The presence of the tail in our cryoEM reconstruction confers unique structural features to the head that are consistent with the tail's regulatory function. Fig. 3C shows the microtubule-bound nucleotide-free kinesin-1 structure (11) for comparison to our tail-bound structure (Fig. 3D). The entire Switch I element from the x-ray crystal structure of ADP-bound kinesin-1 [1mkj, dark blue (20)] fits into the electron density assigned to Switch I in our cryoEM reconstruction of the head–tail complex on microtubules (Fig. 3D, magenta). This is in stark contrast to the cryoEM reconstruction of nucleotide-free kinesin-1 without bound tail (Fig. 3C), in which the majority of the Switch I element from the x-ray crystal structure is well outside the cryoEM density. Indeed, the Switch I conformations seen in x-ray crystal structures of ADP-bound kinesin-1 (20, 22) are different from those found in cryoEM reconstructions of kinesin-1-microtubule complexes in various nucleotide states (ref. 11; C.V.S. and K.H.D., unpublished data). These data are consistent with the hypothesis that Switch I moves when kinesin-1 binds to microtubules. By analogy to the known movement of Switch I in myosin motors upon actin activation, the movement of Switch I may weaken kinesin-1's ADP affinity, allowing for microtubule-stimulated ADP release (14). Conversely, because the conformation of Switch I found in x-ray crystal structures of ADP-bound kinesin-1 fit into Switch I density in our cryoEM reconstruction of the tail-bound kinesin-1–microtubule complex (Fig. 3D), we hypothesize that the tail inhibits ADP release by binding to Switch I and locking the head into a “solution-like” conformation with very high ADP affinity.
A “Paused” State for Kinesin-1 on Microtubules.
In our map, the tail makes simultaneous contacts with the microtubule and Switch I. Through these two contacts, the tail could potentially pause the head on the microtubule, so it would be bound through the microtubule–tail contact while the QIAKPIRP sequence of the tail simultaneously shuts down the head by interacting with Switch I. Tail–microtubule contacts are observable between the tail and both the α and β tubulin subunits in the H10/S9 loop (Fig. 3B). The region of the tail that contacts β-tubulin is near the Cys 188 cross-link (Fig. 2, approximately residues 913–915), consistent with previous data showing that residues 901–911 of the tail have microtubule-binding activity (2). Residues 907–916 of the tail also constitute a portion of the binding site for the Fez-1 protein that activates cargo transport (23). Our hypothesized “paused state” appears to contradict the main function of tail-mediated regulation, which is to prevent kinesin-1 from binding to microtubules. However, the C-terminal residues that were not present in our truncated dimeric tail construct, the Fez-1 protein, or other factors, may reversibly mask the tail–microtubule interaction. This would enable regulatory partners of kinesin-1 to fully control its motile properties by inducing transitions between its regulated state in solution, our hypothesized paused state on microtubules, and the actively moving state on microtubules.
Switch I Is a Common Regulatory Target for Kinesin-1 and Small GTPases.
Kinesins and small GTPases share a common core structure and mechanism by which nucleotide binding and hydrolysis result in enzyme activation. It is therefore intriguing that the interaction of the conserved QIAKPIRP sequence in the kinesin-1 tail with Switch I is analogous to the manner in which several small GTPases are regulated. In light of the structural, enzymatic, and now regulatory, similarities between small GTPases and kinesin-1 it is tempting to suggest that targeting Switch I may be a common means of regulation for other members of the kinesin superfamily.
Methods
Constructs Used (Fig. 1A).
Untagged cysteine-light monomeric and dimeric head constructs of human kinesin-1 heavy chain were received from R. Vale (University of California, San Francisco). These constructs were expressed and purified as described (24). Tail constructs were created by PCR by using a full-length human kinesin-1 clone from R. Vale and purified by using TALON resin (Clontech). Full details on cloning and purification of tail constructs are described SI Text. The 27-mer tail peptide (residues 901–927 with an N-terminal biotin tag) was purchased from GenScript. The biotin tag was not used for any specific purpose in this study.
Photocross-linking with B4M.
Heads were dialyzed into labeling buffer (25 mM Hepes, pH 7.5; 100 mM NaCl; 2 mM MgCl2; 1 mM EGTA; 200 μM Tris(2-carboxyethyl) phosphine hydrochloride, 50 μM ADP) at 4°C. A 5-fold molar excess of B4M (Invitrogen) was added to 1–2 mg/ml head and reacted for 12 h in the dark. The reaction was quenched with 25 mM 3-mercaptoethanol (βME) and excess label removed by repeated spin concentration in centrifugal filter devices (Millipore) into binding buffer (50 mM K-acetate; 10 mM Tris-acetate, pH 7.0; 4 mM MgSO4; 20 mM imidazole; 5 mM βME; 40 μM ADP) plus 300 mM NaCl. Approximately 90% of kinesin-1 heads reacted with B4M under these conditions, as measured by 5,5′-dithiobis 2-nitrobenzoic acid (Pierce) assay. TALON-purified tails were added to yield an approximate ratio of five tails:one head. The combined proteins were dialyzed into binding buffer for 3 h, spun 10 min at 355,000 × g to remove any aggregates, then irradiated for 5 min with 254-nm UV light. Approximately 25% of monomeric heads or 35% of dimeric heads cross-linked to tails or the 27-mer peptide.
Mass Spectrometry Analysis of Photocross-Linked Products.
Sample preparation and trypsin digestion procedures for mass spectrometry and mass spectral data analysis were essentially as described (18). Some of the digests were applied to a C18 PepMap 100 column (Dionex) and chromatographed using a gradient from 2% acetonitrile, 0.1% formic acid, to 80% acetonitrile, 0.08% formic acid on an UltiMate 3000 Nano LC System (Dionex) attached to a Probot microfraction collector (LC Packings) spotting sample directly to a MALDI plate in 7 mg/ml α-cyano-4-hydroxy-cinnamic acid supplemented with 2% (wt/wt) ammonium citrate in 75% acetonitrile. Internal standards were insulin B-chain and angiotensin 1–7 clip. The sequence of the dimeric head + G234A + S188C cross-linked to the tail construct containing residues 823–944 was confirmed by tandem mass spectrometry as described (18).
Protein Preparation, Grid Preparation, and Data Analysis for CryoEM.
Photocross-linked protein was batch bound to TALON resin (Clontech) for 1 h at 4°C. Resin was washed with TALON buffer (50 mM phosphate buffer, pH 7.0; 20 mM imidazole; 2 mM MgCl2; 5 mM βME; 40 μM ADP) plus 300 mM NaCl, eluted with TALON buffer plus 300 mM NaCl plus 500 mM imidazole, and spin-concentrated as described above. This step enriched the cross-linked product ≈3-fold, so that ≈65% of heads were cross-linked to tails. Protein was dialyzed for 1.5 h each into EM buffer (2.5 mM Pipes, pH 6.8; 2 mM MgCl2; 1 mM EGTA; 5 mM βME; 40 μM ADP) plus 10 mM NaCl plus 10 mM Imidazole, then EM buffer plus 5 mM NaCl plus 5 mM imidazole, and shipped overnight on ice for cryoEM. Microtubules were prepared as described (11) but with no added kinesin-1 or apyrase; after the final ultracentrifuge step, the microtubules were resuspended in a final volume of 7.5 μl in a buffer of 25 mM Pipes, pH 6.8; 25 mM KCl; 1 mM EGTA; 1 mM MgCl2. For each specimen, 0.4 μl of microtubules was added to 3 μl of distilled water and applied to a nonglow-discharged homemade holey carbon grid. After wicking away the excess fluid with filter paper, 2 μl of head–tail cross-linked product was applied; after 60 sec of incubation, the grid was blotted and plunge-frozen in liquid ethane. Data collection and image processing were performed as described in ref. 11, using the nucleotide-free kinesin–1-microtubule complex of that work as an initial reference. Approximately 400,000 particles were incorporated into the final dataset. Six cycles of reference matching and volume reconstruction were carried out as described (11), yielding a nominal resolution of 8 Å as reported by the program RMEASURE (25), in agreement with Fourier Shell Correlation comparisons using two independent sets of particles (26).
Supplementary Material
Acknowledgments.
We thank R. Vale for constructs; M. Seeger, S. McBrayer, M. Wallace, D. Quilici, and R. Woolsey for assistance; Park Packing Company for supplying material for tubulin preparation; and the COBRE Proteomics core facility supported by COBRE Grant 1P20RR018751. K.A.D. is supported by National Institutes of Health Grant T32 GM008382, S.E.R. is supported by National Institutes of Health Grant 5 R01 GM072656-02, and C.R.C. by National Institutes of Health Grant 5 RO1 AR040917-18. C.V.S. and K.H.D. are supported by National Institutes of Health Grants GM46033 and GM51487 and by the U.S. Department of Energy under contract no. DE-AC02-05CH11231. The mass spectral work was performed at the Nevada Proteomics Center at the University of Nevada, Reno, funded by National Institutes of Health Grant P20 RR-0164-05 from the Nevada IDeA Network of Biomedical Research Excellence (INBRE) Program of the National Center for Research Resources.
Footnotes
The authors declare no conflict of interest.
Data deposition: The cryo-EM map reported in this paper has been deposited into the Electron Microscopy Database (EMBD), no. EMD-5011.
¶Stock MF, Hackney DD, 48th Annual Biophysical Society Meeting, February 14–18, 2004, Baltimore, MD.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803575105/DCSupplemental.
References
- 1.Cai D, Hoppe AD, Swanson JA, Verhey KJ. Kinesin-1 structural organization and conformational changes revealed by FRET stoichiometry in live cells. J Cell Biol. 2007;176:51–63. doi: 10.1083/jcb.200605097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hackney DD, Stock MF. Kinesin's IAK tail domain inhibits initial microtubule-stimulated ADP release. Nat Cell Biol. 2000;2:257–260. doi: 10.1038/35010525. [DOI] [PubMed] [Google Scholar]
- 3.Verhey KJ, et al. Light Chain-dependent Regulation of Kinesin's Interaction with Microtubules. J Cell Biol. 1998;143:1053–1066. doi: 10.1083/jcb.143.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman DS, Vale RD. Single-molecule analysis of kinesin motility reveals regulation by the cargo-binding tail domain. Nat Cell Biol. 1999;1:293–297. doi: 10.1038/13008. [DOI] [PubMed] [Google Scholar]
- 5.Stock MF, et al. Formation of the compact confomer of kinesin requires a COOH-terminal heavy chain domain and inhibits microtubule-stimulated ATPase activity. J Biol Chem. 1999;274:14617–14623. doi: 10.1074/jbc.274.21.14617. [DOI] [PubMed] [Google Scholar]
- 6.Bathe F, et al. The complex interplay between the neck and hinge domains in kinesin-1 dimerization and motor activity. Mol Biol Cell. 2005;16:3529–3537. doi: 10.1091/mbc.E04-11-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackney DD, Levitt JD, Suhan J. Kinesin undergoes a 9S to 6S conformational transition. J Biol Chem. 1992;267:8696–8701. [PubMed] [Google Scholar]
- 8.Hisanaga S, et al. The molecular structure of adrenal medulla kinesin. Cell Motil Cytoskeleton. 1989;12:264–272. doi: 10.1002/cm.970120407. [DOI] [PubMed] [Google Scholar]
- 9.Seiler S, et al. Cargo binding and regulatory sites in the tail of fungal conventional kinesin. Nat Cell Biol. 2000;2:333–338. doi: 10.1038/35014022. [DOI] [PubMed] [Google Scholar]
- 10.Yonekura H, et al. Mechanism of tail-mediated inhibition of kinesin activities studied using synthetic peptides. Biochem Biophys Res Commun. 2006;343:420–427. doi: 10.1016/j.bbrc.2006.02.169. [DOI] [PubMed] [Google Scholar]
- 11.Sindelar CV, Downing KH. The beginning of kinesin's force-generating cycle visualized at 9-A resolution. J Cell Biol. 2007;177:377–385. doi: 10.1083/jcb.200612090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Himmel DM, et al. Crystallographic findings on the internally uncoupled and near-rigor states of myosin: Further insights into the mechanics of the motor. Proc Natl Acad Sci USA. 2002;99:12645–12650. doi: 10.1073/pnas.202476799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willard FS, Kimple RJ, Siderovski DP. Return of the GDI: The GoLoco motif in cell division. Annu Rev Biochem. 2004;73:925–951. doi: 10.1146/annurev.biochem.73.011303.073756. [DOI] [PubMed] [Google Scholar]
- 14.Kull FJ, Endow SA. Kinesin: Switch I and II and the motor mechanism. J Cell Sci. 2002;115:15–23. doi: 10.1242/jcs.115.1.15. [DOI] [PubMed] [Google Scholar]
- 15.Kimple RJ, Kimple ME, Betts L, Sondek J, Siderovski DP. Structural determinants for GoLoco-induced inhibition of nucleotide release by G alpha subunits. Nature. 2002;416:878–881. doi: 10.1038/416878a. [DOI] [PubMed] [Google Scholar]
- 16.Dorman G, Prestwich GD. Benzophenone photophores in biochemistry. Biochemistry. 1994;33:5661–5673. doi: 10.1021/bi00185a001. [DOI] [PubMed] [Google Scholar]
- 17.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 18.Salzameda B, Facemyer KC, Beck BW, Cremo CR. The N-terminal lobes of both regulatory light chains interact with the tail domain in the 10 S-inhibited conformation of smooth muscle myosin. J Biol Chem. 2006;281:38801–38811. doi: 10.1074/jbc.M606555200. [DOI] [PubMed] [Google Scholar]
- 19.Rice S, et al. A structural change in the kinesin motor protein that drives motility. Nature. 1999;402:778–783. doi: 10.1038/45483. [DOI] [PubMed] [Google Scholar]
- 20.Sindelar CV, et al. Two conformations in the human kinesin power stroke defined by X-ray crystallography and EPR spectroscopy. Nat Struct Biol. 2002;9:844–848. doi: 10.1038/nsb852. [DOI] [PubMed] [Google Scholar]
- 21.Lowe J, Li H, Downing KH, Nogales E. Refined structure of alpha beta-tubulin at 3.5 A resolution. J Mol Biol. 2001;313:1045–1057. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- 22.Sack S, et al. X-ray structure of motor and neck domains from rat brain kinesin. Biochemistry. 1997;36:16155–16165. doi: 10.1021/bi9722498. [DOI] [PubMed] [Google Scholar]
- 23.Blasius TL, Cai D, Jih GT, Toret CP, Verhey KJ. Two binding partners cooperate to activate the molecular motor Kinesin-1. J Cell Biol. 2007;176:11–17. doi: 10.1083/jcb.200605099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naber N, et al. EPR spectroscopy shows a microtubule-dependent conformational change in the kinesin Switch 1 domain. Biophys J. 2003;84:3190–3196. doi: 10.1016/S0006-3495(03)70043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sousa D, Grigorieff N. Ab initio resolution measurement for single particle structures. J Struct Biol. 2007;157:201–210. doi: 10.1016/j.jsb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 26.van Heel M. Similarity measures between images. Ultramicroscopy. 1986;21:95–100. [Google Scholar]
- 27.Pettersen EF, et al. UCSF chimera–A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.