Abstract
The mechanism(s) that regulate and coordinate the events of spermiation and blood–testis barrier (BTB) restructuring in the seminiferous epithelium that occur concurrently at stage VIII of the seminiferous epithelial cycle of spermatogenesis are unknown. In this report, fragments derived from the laminin complex composed of laminin α3, β3, and γ3 chains (laminin-333) at the apical ectoplasmic specialization (apical ES) were shown to modulate BTB dynamics directly and/or indirectly via hemidesmosome. Experiments were performed using cultured Sertoli cells with functional tight junction (TJ) barrier and the ultrastructural features of the BTB but not apical ES. Recombinant protein fragments of laminin β3 and γ3 chains were shown to reduce the protein levels of occludin and β1-integrin dose dependently at the Sertoli–Sertoli and Sertoli–basement membrane interface, respectively, thereby destabilizing the BTB permeability function. These results were corroborated by transient overexpression of laminin fragments in Sertoli cells. To further assess the role of β1-integrin in hemidesmosome, knockdown of β1-integrin in Sertoli cells by RNAi was found to associate with occludin redistribution at the Sertoli–Sertoli cell interface, wherein occludin moved away from the cell surface and became associated with endosomes, thereby destabilizing the BTB. In short, an apical ES-BTB-hemidesmosome autocrine regulatory axis was identified in testes, coordinating the events of spermiation and BTB restructuring that occur at the opposite ends of the seminiferous epithelium during spermatogenesis.
Keywords: ectoplasmic specialization, hemidesmosome, seminiferous epithelium, Sertoli cells, tight junction
During spermatogenesis, preleptotene/leptotene spermatocytes at the basal compartment traverse the blood–testis barrier (BTB) at stages ≈VIII-IX of the seminiferous epithelial cycle in adult rat testes, entering the adluminal compartment for further development (1). This event takes place concurrently with spermiation, wherein fully developed spermatids (i.e., spermatozoa) detach from the epithelium at the luminal edge, entering the tubule lumen for their eventual maturation in the epididymis. These morphologic changes, which occur at the opposite ends of the Sertoli cell epithelium, were first described in the 1950s (2). In this report, we provide compelling evidence regarding the mechanism that regulates and coordinates these events. The idea was based on a recent study in which a blockade of the laminin function at the apical ectoplasmic specialization (apical ES) by specific antibodies led to spermatid exfoliation and BTB restructuring (3), even though it was unclear how the anti-laminin γ3 IgG traversed the BTB to reach the apical ES. Nonetheless, this study shows that a disruption of the apical ES may lead to a transient BTB disruption, illustrating a plausible physiological link between these two ultrastructures.
The apical ES is a testis-specific adherens junction (AJ) type that anchors developing spermatids to the Sertoli cell in the epithelium during spermatogenesis (4–6). It has properties of both AJ and focal contacts (4, 7). For instance, many proteins that are restricted to the cell–matrix interface at the focal contacts, such as integrins, laminins, and collagens, are found at the apical ES (4). Indeed, laminin γ3 chain was first identified as a nonbasement laminin at the apical ES in mouse testes (8). Subsequent studies in adult rat testes have shown that laminin γ3, α3, and β3 chains residing in the elongating/elongated spermatids form a protein complex known as laminin-333 (3), which is the bona fide partner of the α6β1-integrin restricted to Sertoli cells (9, 10), constituting the laminin-333/α6β1-integrin adhesion complex at the apical ES. Furthermore, this laminin/integrin complex is associated with proteases: matrix metalloprotease-2 (MMP-2), membrane-type 1 (MT1)-MMP, and tissue inhibitor of metalloproteases-2 (TIMP-2) (11). These findings suggested that the activation of MMP-2 by MT1-MMP and TIMP-2 at the apical ES during spermiation could cleave laminin-333 to generate the biologically active laminin fragments. Indeed, there are reports illustrating that fragments of laminin chains that arise under physiological and pathophysiological conditions are biologically active peptides, regulating cell migration, protein production, inflammatory responses, and others (12, 13). Thus, it is possible that fragments of laminin chains released from the apical ES during spermiation could regulate the BTB in the testis (7).
Although β1-integrin residing in the Sertoli cell is a receptor of laminin-333 at the apical ES (3, 9–11), its precise localization at the basal compartment of the seminiferous epithelium remains unclear. For instance, β1-integrin was reported to be a component of the basement membrane in the seminiferous tubules (14), perhaps at the hemidesmosomes. Others have reported its localization with the actin-based anchoring junction structure (i.e., basal ES) at the BTB (10, 15). It is also not known whether there is cross-talk between β1-integrin in the apical ES, the BTB, and hemidesmosome. We report herein an apical ES-BTB-hemidesmosome axis in the testis that coordinates spermiation and BTB restructuring during spermatogenesis.
Results
Disruptive Effects of Laminin Fragments on the BTB Integrity.
This study was performed by using primary cultures of Sertoli cells (3) with negligible Leydig and germ cell contamination (16) that were shown to form functional BTB (such as a tight junction-permeability barrier) with the ultrastructural features under electron microscopy that mimicked the in vivo barrier (17, 18).
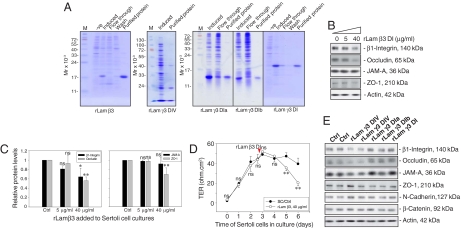
Recombinant proteins corresponding to portions of domain I of laminin β3 and γ3, as well as domain IV of laminin γ3 chains [supporting information (SI) Fig S1 and Table S1] were expressed in Escherichia coli and purified to apparent homogeneity (Fig. 1A). Sertoli cells were cultured alone for 3 days, forming an intact epithelium; thereafter, cells were treated with purified laminin β3 domain I for 3 days with the daily replenishing media containing the same amount of recombinant protein at 0, 5, and 40 μg/ml (Fig. 1B), and the steady-state protein levels of β1-integrin, occludin, and ZO-1, but not JAM-A, were significantly and dose-dependently reduced (Fig. 1 B and C). Since some of these proteins (e.g., occludin and ZO-1) are integral components of the BTB (4, 7), the tight junction (TJ) permeability barrier function was assessed by quantifying the transepithelial electrical resistance (TER) across the Sertoli cell epithelium (17) by treating these cultures with laminin β3 DI recombinant protein, which was added to the apical and basal chambers of the bicameral units on day 2.5 (Fig. 1D, red arrowhead). It was noted that the presence of laminin β3 DI recombinant protein compromised the TJ barrier by inducing a significant decline in TER on days 5 and 6 vs. controls (Fig. 1D). To confirm its specific disruptive effect on BTB function, recombinant proteins of other laminin fragments were also tested under the same conditions as for domain I of laminin β3. It was noted that only domain IV, but not domain I, of laminin γ3 induced a reduction in the protein levels of β1-integrin, occludin, and JAM-A, but not ZO-1, N-cadherin, and β-catenin, which are putative BTB proteins in rat testes (Fig. 1E). Collectively, these data illustrate that the disruptive effects of domain I of laminin β3 and domain IV of laminin γ3 chains on the Sertoli cell BTB were not the results of nonspecific protein effects, and only specific laminin fragments are biologically active peptides to modulate BTB function.
Fig. 1.
A study to assess the effects of different laminin fragments on the steady-state levels of proteins at the BTB and the BTB integrity in primary Sertoli cell cultures. (A) Purification of the recombinant proteins expressed in E. coli. Uninduced (-ve) and induced protein lysates from E. coli, fractions collected from the Ni column (both flow through and wash), and purified proteins were resolved by SDS-PAGE with gels stained by Coomassie blue. (B) The 5 and 40 μg/ml recombinant proteins corresponding to part of domain I of laminin β3 chain (rLam β3) were added to cultures on day 3 after isolation. Recombinant proteins were included in the daily replacement F12/DMEM for an additional 3 days. Cells were terminated on day 6 and lysates were used for immunoblotting, illustrating a dose-dependent inhibition on the production of β1-integrin, occludin, and ZO-1 but not JAM-A. Actin served as a loading control. (C) Immunoblot data were densitometrically scanned and compared. Each bar is the mean ± SD of n = 3, normalized against actin, wherein the control was arbitrarily set at 1, against which one-way ANOVA was performed. (D) Effects of the rLam β3 domain I on the TJ barrier. A total of 40 μg/ml rLam β3 domain I was added on day 2.5 (see red arrowhead), which was also included in the daily replacement F12/DMEM (n = 3). (E) Results of representative immunoblots illustrating the effects of other laminin fragments at 40 μg/ml on Sertoli cell target proteins using the experimental conditions of (B). Ctrl, control; rLam, recombinant laminin; DIV, domain IV; rLam γ3 DIa or DIb, recombinant laminin γ3 chain containing Domain Ia or Domain Ib (Table S1); DI, domain I; ns, not significantly different; ∗, P < 0.05; ∗∗, P < 0.01.
Effects of Overexpression of Laminin Fragments on Sertoli Cell BTB Function.
When DNA constructs containing the same part of the domain I of laminin β3 and γ3, and the domain IV of laminin γ3, as recombinant proteins (Fig. S1) were transiently transfected in Sertoli cells, both mRNAs and proteins of the corresponding laminin fragments were detected by 48 hr thereafter (Fig. 2A). Sertoli cells transfected with vector alone failed to express any laminin chains (Fig. 2A), since laminin-333 is restricted to elongating/elongated spermatids in adult rat testes (3). The effects of overexpression of domain IV of the laminin γ3 chain on the Sertoli cell–TJ barrier were also monitored vs. cells transfected with vector alone. Within ≈24 hr after transfection, a significant decline in TER was detected, which persisted for the next 4 days, illustrating a loss of BTB function (Fig. 2B). This disruptive effect was transient, because the TER was restored by day 7 (Fig. 2B). Significant reduction in the steady-state protein levels of β1-integrin and occludin were detected in Sertoli cells with overexpression of domain IV of γ3 and domain I of β3 chains (Fig. 2 C and D). The results shown in Fig. 2 are consistent with those of Fig. 1. Besides, an activation of ERK (p-ERK) was detected following overexpression of domain IV of laminin γ3 (Fig. 2 C and E).
Fig. 2.
A study by transient expression of laminin fragments in Sertoli cells to assess their effects on the BTB integrity and the steady-state levels of different target proteins. (A) RT-PCR and immunoblot analysis showing the expression of domain IV of laminin γ3 (Left), domain I of laminin γ3 (Center), and domain I of laminin β3 (Right) in Sertoli cells after transfection with laminin γ3 DIV/pCIneo, laminin γ3 DI/pCIneo, and laminin β3 DI/pCIneo expression vectors for 2 and 3 days, respectively, vs. cells transfected with pClneo alone (without laminin cDNA constructs) or normal cells (Ctrl). S16, GAPDH, and actin served as loading controls. (B) Changes in Sertoli cell–TJ barrier by quantifying TER across the cell epithelium in bicameral units (n = 3) where cells were transfected with laminin γ3 DIV/pCIneo vs. pCIneo alone on day 2.5 (arrowhead). (C) The protein levels of β1-integrin, occludin, and ERK/pERK in cells following overexpression with different constructs for 3 days vs. controls. (D–E) Bar graphs summarize results of C. Each bar is a mean ± SD (n = 3) and normalized against actin, wherein cells transfected with pCIneo vector alone were arbitrarily set at 1, against which one-way ANOVA was performed. ns, not significantly different; ∗, P < 0.05; ∗∗, P < 0.01.
Localization of β1-Integrin in the Seminiferous Epithelium of Adult Rat Testes.
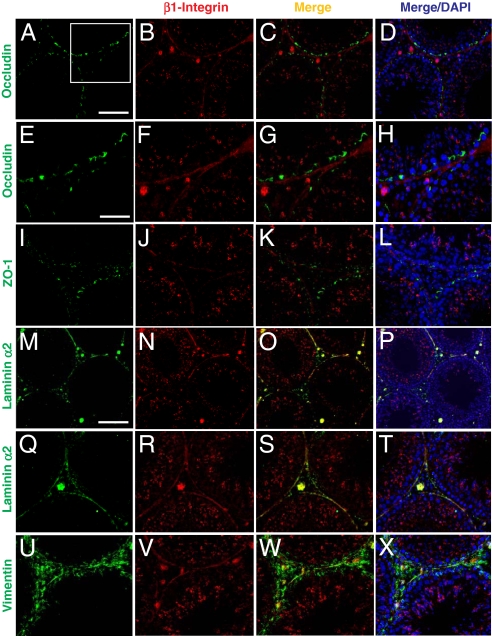
β1-Integrin and α6-integrin form a functional adhesion complex of α6β1-integrin at the apical ES residing in Sertoli cells (9, 10, 15), and α6β1-integrin is the receptor of laminin-333 residing in elongating/elongated spermatids (3, 11). However, the precise localization of β1-integrin at the basal compartment is not well defined. Since specific recombinant laminin chain fragments were shown to perturb the BTB function, we sought to define the precise localization of β1-integrin in the basal compartment. Results of the study were shown in Fig. 3 A–X and Fig. S2. Consistent with the earlier studies, β1-integrin was localized predominantly at the apical ES in stage VIII tubules (Fig. 3 B, J, and N); however, at the basal compartment, relatively little β1-integrin was colocalized with the TJ markers: occludin (Fig. 3 C, G, A–D, and E–H) and ZO-1 (Fig. 3 K and I–L). This thus suggests that β1-integrin is not restricted to the basal ES at the BTB. Instead, β1-integrin was mostly colocalized with a basement membrane marker, laminin α2 (Fig. 3 O, S, M–P, and Q–T) (19), and partially colocalized with intermediate filament component vimentin (Fig. 3 W and U–X). Collectively, these results illustrate that besides its presence at the apical ES, β1-integrin is a component of hemidesmosomes at the Sertoli cell–matrix interface in the basement membrane.
Fig. 3.
A study by immunofluoresent microscopy to examine the localization of β1-integrin in rat testes illustrating its presence at the apical ES and hemidesmosome. (A–D) Occludin (green, FITC) was localized at the BTB in the seminiferous epithelium of normal testes. β1-Integrin (red, Cy3) was predominantly localized at the apical ES, and it was also localized in the basal compartment of the epithelium. However, the signal of β1-integrin in the basal compartment was predominantly found underneath occludin in the merged image (C). (D) DAPI staining of nuclei. Boxed imaged in A was magnified in E–H, illustrating most β1-integrin was localized below the BTB (occludin) (see G and H vs. E and F). (I–L) ZO-1 (green, FITC) was also localized at the BTB above β1-integrin (red, Cy3). (M–P) Laminin α2 (green, FITC, a known basement membrane protein in rodent testes (19) was localized along the basement membrane of the seminiferous tubules as well as Leydig cells in the interstitium. It was shown to colocalize with β1-integrin in the basal compartment, as shown in the merged image (O, see yellowish color). (Q–T) Higher magnification showing the colocalization of β1-integrin with laminin α2 at the hemidesmosomes. (U–X) Vimentin (green, FITC; an intermediate filament structural protein), was localized at both basement membrane as well as at the BTB. β1-integrin (red, Cy3) was found to colocalize with vimentin at the basement membrane (see yellow color in W). (Scale bar in A: 100 μm, which applies to B–D, I–L, and Q–T; in M: 120 μm, which applies to N–P; in E: 50 μm, which applies to F–H and U–X.)
Roles of β1-Integrin on BTB Dynamics.
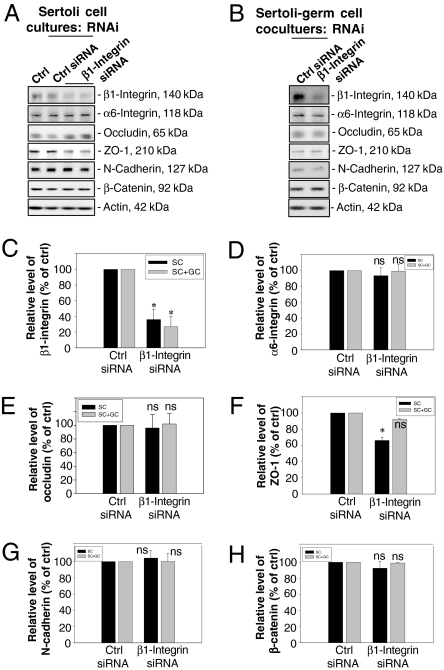
Since fragments of specific laminin chains (Fig. S1) were shown to modulate BTB function and reduce the steady-state level of β1-integrin (Figs. 1 and 2), and β1-integrin was confined mostly to the hemidesmosomes rather than the basal ES at the BTB (Fig. 3), we sought to investigate the functional involvement of β1-integrin in BTB dynamics by using RNA interference (RNAi) techniques with Sertoli cell cultures having functional BTB and cell–matrix junctions, such as hemidesmosomes (Fig. 4A) (18, 20). Sertoli cell cultures seeded on day 0 were subjected to a hypotonic treatment on day 2 (21) to remove any residual germ cells, eliminating all apical ES, such that the β1-integrin studied herein was predominantly restricted to the Sertoli cell–matrix interface. RNAi was performed on day 4 in Sertoli cell epithelium, and cells were terminated on days 7 and 9 (data not shown) by using β1-integrin–specific small interfering RNA (siRNA) (a 21-bp double-stranded RNA) vs. scrambled control siRNA. For Sertoli–germ cell cocultures, total germ cells isolated from adult rat testes on day 4 were plated to the Sertoli cell epithelium to allow the assembly of functional AJ (e.g., apical ES) as verified by light and electron microscopy (6, 18), RNAi was performed on day 5, and cultures were terminated on day 8. The steady-state protein level of β1-integrin in these Sertoli cell cultures following RNAi vs. controls (i.e., Sertoli cells cultured alone and cells treated with nontargeting control siRNA duplex) was monitored by immunoblotting, as shown in Fig. 4 A and C. Cells transfected with β1-integrin siRNA duplex had a ≈70% reduction in β1-integrin protein vs. controls (Fig. 4 A and C) on day 7, which persisted up to day 9 (data not shown). However, there was no significant change in the steady-state levels of TJ (e.g., occludin) and basal ES (e.g., N-cadherin and β-catenin) proteins, nor its putative binding partner α6-integrin (Fig. 4 D and E and G and H) on day 7, which also persisted until day 9 (data not shown), except for a ≈40% decline in the level of ZO-1, a TJ adaptor, on day 7 (Fig. 4F) and day 9 (data not shown). When β1-integrin was knocked down in Sertoli–germ cell cocultures, a significant decline, ≈70%, in β1-integrin was also detected (Fig. 4 B and C), but other markers remained relatively stable, similar to controls (Fig. 4 B–H).
Fig. 4.
A study to assess the effects of β1-integrin knockdown by RNAi on the steady-state levels of junction-associated proteins in primary Sertoli cell and Sertoli–germ cell cocultures. (A–B) Immunoblots showing changes in the steady-state levels of several junction proteins after β1-integrin RNAi in Sertoli cell cultures (A) or Sertoli–germ cell cocultures (B), with actin serving as a loading control. From both sets of cultures, lysates were used for immunoblot analysis as shown in A and B, and data from three experiments were scanned and shown in C–H. Each bar is the mean ± SD (n = 3) and normalized against actin, wherein control cultures were arbitrarily set at 100%, against which one-way ANOVA was performed. ns, not significantly different; ∗, P < 0.01.
A Knockdown of β1-Integrin at the Cell–Matrix Interface by RNAi Leads to a Disruption of Occludin-Based TJ Fibrils at the BTB.
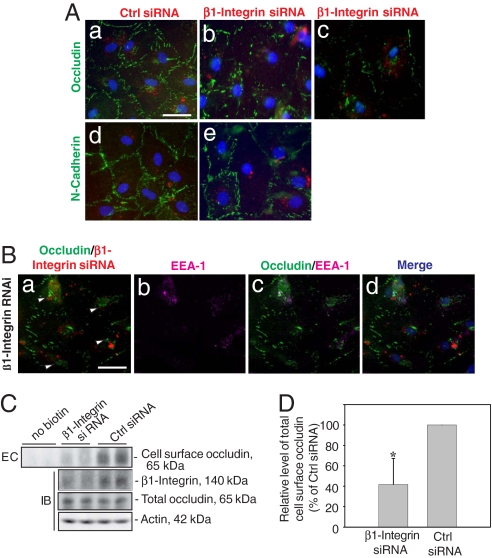
Even though there was no significant alteration in the steady-state protein levels of occludin and N-cadherin at the BTB when ≈70% of β1-integrin was silenced at the cell–matrix interface, a significant decline in ZO-1 was noted (Fig. 4 A and F). We next examined whether there were any changes in the redistribution of proteins at the Sertoli–Sertoli cell interface (Fig. 5 Aa–Ae). When Sertoli cells were transfected with Cy-3-labeled β1-integrin siRNA duplex (red fluorescence in Fig. 5 Ab and Ac), but not control siRNA duplex (red fluorescence in Fig. 5Aa), there was a significant disruption of the immunoreactive “beltlike” structures of occludin (green fluorescence) at the cell–cell interface corresponding to the TJ fibrils, wherein occludin appeared to move away from the cell–cell interface and became cytosol bound (Fig. 5 Ab and Ac vs. Aa). This effect was not detectable when these cells were stained for N-cadherin (Fig. 5 Ae vs. Ad). Occludin vesicles were concentrated around the nucleus (white arrowheads in Fig. 5Ba), apparently as a result of protein endocytosis, and had remarkable tendency to colocalize with an early endosome marker, EEA-1 (Fig. 5 Bc and Bd vs. Ba and Bb). This morphologic observation was further confirmed by the biochemical study shown in Fig. 5 C and D to assess the effects of β1-integrin knockdown on occludin endocytosis. Consistent with the data shown in Fig. 5 A and B, a significant reduction, ≈40%, in total biotinylated Sertoli cell surface occludin was detected (Fig. 5C, top) following a β1-integrin knockdown vs. controls (Fig. 5 C and D), with no change in the steady-state occludin level (Fig. 5C).
Fig. 5.
A study to assess changes in the distribution of occludin following RNAi of β1-integrin in Sertoli cell cultures. Sertoli cell epithelium was transfected with siRNA duplex on day 4 and cultured for two additional days before being used for fluorescent microscopy. (Aa and Ad) Sertoli cells were transfected with Cy3-labeled nontargeting control siRNA (red fluorescence in cells) and stained for occludin (green, FITC) and N-cadherin (green, FITC), which was restricted mostly at the cell–cell interface. (Ab, Ac, and Ae) Cells were transfected with Cy3-labeled β1-integrin siRNA duplex, illustrating a disappearance of occludin, but not N-cadherin, from the cell surface (Aa–Ac vs. Ad–Ae), and occludin appeared to become internalized (see Ab and Ac from two different experiments vs. Aa). (Scale bar: Aa, 10 μm, which applies to Ab–Ae.) (Ba) A loss of occludin (green) from the cell–cell interface was noted where cells were transfected with Cy3-labeled β1-integrin siRNA (red; see Ba vs. Aa). Most internalized occludin proteins (green) that appeared in cell cytosol (white arrowheads; Ba) associated with an early endosome marker (EEA-1, purple, Cy5; Bb), and the merged images are shown in Bc; Bd shows the merged images of Ba–Bc and with DAPI staining for Sertoli cell nuclei. (Scale bar: Ba, 10 μm, which applies to Bb–Bd.) (C) Endocytosis (EC) assay (see SI Methods) to assess the effects of β1-integrin knockdown on occludin internalization vs. controls. Significantly less biotinylated occludin was detected at the cell surface after β1-integrin RNAi vs. control (Top), whereas the total cellular occludin did not change considerably when assessed by immunoblotting (IB) (Bottom) wherein the endogenous β1-integrin level was reduced following RNAi (Middle). Actin served as the loading control. (D) A bar graph that summarizes results of C. Each bar is the mean ± SD (n = 3) wherein vehicle control was arbitrarily set at 100%. ∗, P < 0.01 by Student t test.
Discussion
The apical ES and the BTB are two ultrastructures in the seminiferous epithelium that are located at the opposite ends of the adjacent Sertoli cells (Fig. 6). Junctional complexes at these two sites undergo restructuring concurrently at stages VIII–IX of the epithelial cycle to facilitate (i) spermiation and (ii) the transit of preleptotene/leptotene spermatocytes across the BTB. In adult rat testes, one of the major cell adhesion complexes at the apical ES is the laminin-333/α6β1 integrin complex (4, 5, 7). In the present study, fragments of laminin-333 were shown to regulate BTB function near the basal lamina. First, recombinant proteins corresponding to specific domains of the laminin chains were shown to perturb the Sertoli cell–TJ barrier in vitro. These proteins also down-regulated the steady-state levels of occludin at the BTB as well as β1-integrin at the Sertoli cell–basement membrane interface, perhaps at the hemidesmosomes. It is important to note that primary Sertoli cell cultures are an established in vitro system to monitor TJ barrier function that mimics the BTB in vivo morphologically and physiologically based on earlier reports (18, 22, 23), illustrating the significance of these findings. Second, these results are in agreement with data from overexpression experiments. Furthermore, laminin fragments apparently mediated the effects via the ERK signaling pathway. Third, to delineate the functional significance of the laminin fragment–mediated reduction in the β1-integrin level, primary Sertoli cell cultures were transfected with β1-integrin-specific siRNA to confirm the functional linkage along the apical ES-BTB-hemidesmosome axis. Indeed, when β1-integrin was knocked down by RNAi, a redistribution of occludin at the Sertoli–Sertoli cell interface was detected, moving away from the TJ fibrils, undergoing enhanced endocytosis, and associating with early endosomes via its interaction with EEA-1.
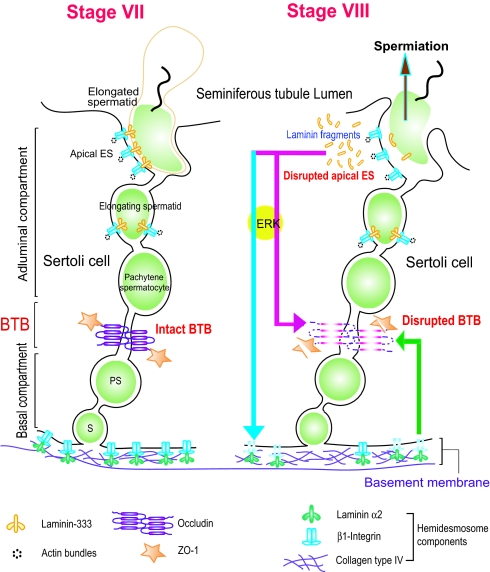
Fig. 6.
A schematic drawing illustrating an autocrine regulatory loop in adult rat testes. At spermiation, biologically active laminin fragments released from the laminin/integrin complex at the apical ES, (e.g., via MMP-2), significantly reduce the level of occludin at the BTB (red arrow). These fragments can also reduce the level of β1-integrin at the hemidesmosomes (blue arrow), destabilizing the BTB via a feedback mechanism (green arrow). Collectively, these combined effects facilitates preleptotene spermatocyte transit at the BTB. This autocrine regulatory mechanism thus coordinates spermiation and BTB restructuring that occur at the opposite ends of the Sertoli cell epithelium.
The mechanism by which fragments of the laminin chains are formed in the seminiferous epithelium in vivo remains unknown. However, recent studies have illustrated that the laminin γ3 chain is a substrate of matrix metalloprotease 2 (MMP-2) (11). Also, membrane-type 1 (MT1)-MMP, and tissue inhibitor of metalloproteases-2 (TIMP-2), which are known to activate MMP-2 (24), are localized at the apical ES and are associated with the β1-integrin-laminin γ3 complex (11). It is plausible that at spermiation the activation of MMP-2 by the MT1-MMP/TIMP-2 complex may lead to proteolytic cleavage of specific laminin chains at the apical ES. This would generate the biologically active fragments, which in turn regulate the steady-state levels of the integral membrane proteins at the BTB and/or mediated via its effects on the hemidesmosomes as supported by the results reported herein (Fig. 6). The hypothetical scheme shown in Fig. 6 presents a model for the mechanism that coordinates the events of spermiation and BTB restructuring that occur concurrently at stage VIII of the epithelial cycle. This hypothesis is further supported by two lines of evidence. First, proteolytic fragments of laminin chains are known biologically active peptides and are capable of regulating cell migration, protein production, activation of signal transducers, organelle formation, and others (for reviews, see 4,7). For instance, recombinant LG4 module of the laminin α3 chain in laminin-5 (laminin-332) and a 19–amino acid residue synthetic peptide within this LG4 domain were shown to stimulate the expression of MMP-1 in keratinocytes and fibroblasts (13). Second, the migration of monocytes across the blood–brain barrier was recently shown to be associated with an activation of proteases, such as MMPs. This activation led to proteolytic breakdown of occludin at the blood–brain barrier to facilitate monocyte migration (25), analogous to the possible cleavage of laminin-333 by MMP-2 at the apical ES during spermiation, and the transit of spermatocytes across the BTB facilitated by laminin fragments. Also, occludin possesses a putative extracellular MMP cleavage site (26), and the endothelial cells that constitute the blood–brain barrier express high levels of MMP-2 and MMP-9 (27). Again, this is comparable with the spatial relationship of the laminin 333–α6β1-integrin complex and MMP-2, MT1-MMP, and TIMP-2 (4), and the presence of proteases/protease inhibitors at the BTB (7). Furthermore, the model depicted in Fig. 6 offers an explanation for the earlier observation that local administration of anti-laminin α3 or γ3 IgG to the testis perturbed apical ES and BTB function (3), even though they might not have reached the apical ES by traversing the BTB. For instance, laminin fragments generated at the apical ES and translocated to the site of hemidesmosomes and BTB could have been “trapped” and “immobilized” by the antibodies (3). This thus disrupts the local regulatory loop and the BTB.
Although β1-integrin was localized predominantly at the apical ES, some β1-integrin was found at the BTB as previously reported (10). However, most of the β1-integrin staining in the epithelium outside the apical ES was at the hemidesmosomes. Since the primary Sertoli cell cultures used for our studies had negligible germ cell contamination (16), the laminin fragment-induced β1-integrin reduction in these cultures can be attributed mostly to the loss of β1-integrin at the hemidesmosomes instead of at the apical or basal ES. In short, these findings illustrate the presence of a functional apical ES-BTB-hemidesmosome axis in the seminiferous epithelium, which is used to coordinate the events of spermiation and BTB restructuring during spermatogenesis (see Fig. 6).
Materials and Methods
Animals.
The use of animals was approved by The Rockefeller University Laboratory Animal Use and Care Committee with protocol numbers 03017 and 06018.
Primary Sertoli Cell Cultures and Treatment of Cells with Recombinant Proteins.
Sertoli cells were isolated from 20-day-old Sprague–Dawley rat testes (3). Functional BTB that mimicked the in vivo barrier was formed in these cultures within ≈3 days when assessed by quantifying the TER across the cell epithelium (17) and by electron microscopy (18). These cultures were used to assess the effects of purified recombinant proteins corresponding to different fragments of the laminin chains (SI Methods).
Transient Overexpression of Laminin Fragments in Sertoli Cells.
Laminin fragments corresponding to part of domain I of laminin β3 and γ3 chains and domain IV of laminin γ3 chain (Fig. 1) were cloned into pCI-neo mammalian expression vector (Promega) at the restriction enzyme sites between NheI and EcoRI by using specific primers (Table S1). The authenticity of these clones was confirmed by direct nucleotide sequencing (Genewiz). On day 2 after Sertoli cell isolation, ≈1 or 0.5 μg of DNA of each construct was transfected to Sertoli cells plated on Matrigel-coated bicameral units or 12-well plates at a cell density of 1.2 × 106 or 0.45 × 106, respectively, by using Effectene Transfection reagent (Qiagen). Transfection mixture was removed 24 hr later and replaced with fresh F12/DMEM. RNA and protein lysates were extracted 2 and 3 days thereafter, respectively. The TJ barrier integrity after transient expression of laminin γ3 domain IV vs. pCIneo vector alone was also assessed by TER measurement as described (17).
Sertoli Cell-Specific Knockdown of β1-Integrin by RNAi.
On day 2 after isolation, cultures were subjected to a hypotonic treatment to remove residual germ cells. This step also eliminated any remaining β1-integrin at the apical ES between Sertoli cells and elongating/elongated spermatids, of which β1-integrin is a putative component (4, 5, 7). Thus, β1-integrin detected in these Sertoli cell-only cultures was derived mostly at the cell–matrix interface, because relatively little β1-integrin was detected at the Sertoli–Sertoli cell interface at the basal ES (Fig. 3 and Fig. S2), which is also part of the BTB (4). On day 4, Sertoli cells were transfected with 100 nM ON-TARGETplus 21-mer double-stranded siRNAs: sense 5′-GGAUAGGUCCAACGGCUUAUU-3′ and antisense: 5′-PUAAGCCGUUGGACCUAUCGUU-3′ (cat. no. J-089600-10), targeting rat β1-integrin (NM-017022; Dharmacon, Inc.) by TransIT-TKO (cat no. MIR 2150; Mirus) according to the manufacturer's instructions. Transfection mixture was replaced 24 hr after transfection with F12/DMEM supplemented with growth factors (3), and cells were then terminated 3 and 5 days thereafter (i.e., on days 7 and 9). In all RNAi experiments, negative controls were included in which cells were transfected with equal amounts of ON-TARGETplus siCONTROL nontargeting siRNA 5′-UGGUUUACAUGUCGACUAA-3′ (cat no. D-001810-01; Dharmacon). Selected experiments were conducted by using Sertoli–germ cell cocultures (18), wherein total germ cells isolated from 90-day-old rat testes were added to the Sertoli cell epithelium on day 4 by using a Sertoli–germ cell ratio of 1:2. Cells were then transfected with siRNA (100 nM) on day 5 after anchoring junctions (e.g., apical ES) were formed when examined by electron microscopy (18). Cocultures were terminated on day 8.
Dual-Labeled Immunofluorescent Analysis.
The distribution of integral membrane proteins in cells after β1-integrin knockdown was examined by fluorescent microscopy. In brief, Sertoli cells were isolated and cultured on Lab-TekChamber Slide System (Nalge Nunc International) coated with Matrigel at a cell density of ≈0.1 × 106 cells per 1.8 cm2. To ascertain that cells were indeed transfected with the siRNA when distribution of integral membrane proteins at the cell–cell interface were examined, siRNA was labeled with Cy3 by the Label IT siRNA tracker intracellular localization kit (cat. no. MIR 7212; Mirus) 1 day before transfection. On day 4, cells were transfected with 100 nM fluorescently labeled siRNA by TransIT-TKO transfection reagent. Transfection mixture was removed 24 hr thereafter, cells were then cultured for an additional 24 hr and fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100. After blocking with 10% normal goat serum (vol/vol), cells were incubated with 1:100 rabbit anti-occludin (cat. no. 71-1500, lot 51202542; Invitrogen, Zymed) and 1:100 mouse anti-early endosomal antigen 1 (EEA-1; cat. no. 610456, lot 76250; BD Biosciences). Following overnight incubation, secondary antibodies conjugated with FITC or Cy-5 (Zymed) diluted in PBS at 1:50 were incubated with cells on slides for ≈30 min. Cells were mounted in Vectashield Hardset, and fluorescent micrographs were obtained (3).
General Methods.
Endocytosis assay, RT-PCR, immunoblot analysis, fluorescent microscopy, Sertoli cell TJ-barrier function monitored by TER measurement, and statistical analyses were performed as described (3,17) (Table S1, SI Methods).
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grants NICHD U01 HD045908 and R03 HD051512, The Contraceptive Research and Development Program (Consortium for Industrial Collaboration in Contraceptive Research Grant CIG-01-72) and Hong Kong Research Grants Council HKU7599/06M.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711264105/DCSupplemental.
References
- 1.Russell LD. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat. 1977;148:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- 2.LeBlond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann NY Acad Sci. 1952;55:548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- 3.Yan HHN, Cheng CY. Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem. 2006;281:17286–17303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- 4.Yan HHN, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: A friend or a foe of spermatogenesis? BioEssays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogl AW, Pfeiffer DC, Mulholland D, Kimel G, Guttman J. Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol. 2000;63:1–15. doi: 10.1679/aohc.63.1. [DOI] [PubMed] [Google Scholar]
- 6.Mruk DD, Cheng CY. Sertoli–Sertoli and Sertoli–germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 7.Yan HHN, Mruk DD, Cheng CY. Junction restructuring and spermatogenesis: The biology, regulation, and implication in male contraceptive development. Curr Top Dev Biol. 2007;80:57–92. doi: 10.1016/S0070-2153(07)80002-0. [DOI] [PubMed] [Google Scholar]
- 8.Koch M, et al. Characterization and expression of the laminin γ3 chain: a novel, non-basement membrane-associated, laminin chain. J Cell Biol. 1999;145:605–618. doi: 10.1083/jcb.145.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palombi F, Salanova M, Tarone G, Farini D, Stefanini M. Distribution of β1 integrin subunit in rat seminiferous epithelium. Biol Reprod. 1992;47:1173–1182. doi: 10.1095/biolreprod47.6.1173. [DOI] [PubMed] [Google Scholar]
- 10.Salanova M, Stefanini M, De Curtis I, Palombi F. Integrin receptor α6β1 is localized at specific sites of cell-to-cell contact in rat seminiferous epithelium. Biol Reprod. 1995;52:79–87. doi: 10.1095/biolreprod52.1.79. [DOI] [PubMed] [Google Scholar]
- 11.Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2004;70:945–964. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- 12.Koshikawa N, et al. Proteolytic processing of laminin-5 by MT1-MMP in tissues and its effects on epithelial cell morphology. FASEB J. 2004;18:364–366. doi: 10.1096/fj.03-0584fje. [DOI] [PubMed] [Google Scholar]
- 13.Utani A, et al. Laminin α3 LG4 module induces matrix metalloproteinase-1 through mitogen-activated protein kinase signaling. J Biol Chem. 2003;278:34483–34490. doi: 10.1074/jbc.M304827200. [DOI] [PubMed] [Google Scholar]
- 14.Giebel J, Loster K, Rune GM. Localization of integrin β1, α1, α5, and α9 subunits in the rat testis. Int J Androl. 1997;20:3–9. doi: 10.1046/j.1365-2605.1997.d01-105.x. [DOI] [PubMed] [Google Scholar]
- 15.Mulholland DJ, Dedhar S, Vogl AW. Rat seminiferous epithelium contains a unique junction (ectoplasmic specialization) with signaling properties both of cell/cell and cell/matrix junctions. Biol Reprod. 2001;64:396–407. doi: 10.1095/biolreprod64.1.396. [DOI] [PubMed] [Google Scholar]
- 16.Lee NPY, Mruk DD, Conway AM, Cheng CY. Zyxin, axin, and Wiskott–Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl. 2004;25:200–215. doi: 10.1002/j.1939-4640.2004.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee NPY, Cheng CY. Regulation of Sertoli cell tight junction dynamics in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/3′,5′-cyclic guanosine monophosphate/protein kinase G signaling pathway: An in vitro study. Endocrinology. 2003;144:3114–3129. doi: 10.1210/en.2002-0167. [DOI] [PubMed] [Google Scholar]
- 18.Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki T, Giltay R, Talts U, Timpl R, Talts JF. Expression and distribution of laminin α1 and α2 chains in embryonic and adult mouse tissues: An immunochemical approach. Exp Cell Res. 2002;275:185–199. doi: 10.1006/excr.2002.5499. [DOI] [PubMed] [Google Scholar]
- 20.Grima J, Zhu L, Cheng CY. Testin is tightly associated with testicular cell membrane upon its secretion by Sertoli cells whose steady-state mRNA level in the testis correlates with the turnover and integrity of inter-testicular cell junctions. J Biol Chem. 1997;272:6499–6509. doi: 10.1074/jbc.272.10.6499. [DOI] [PubMed] [Google Scholar]
- 21.Galdieri M, Ziparo E, Palombi F, Russo MA, Stefanini M. Pure Sertoli cell cultures: A new model for the study of somatic–germ cell interactions. J Androl. 1981;5:249–259. [Google Scholar]
- 22.Janecki A, Jakubowiak A, Steinberger A. Effect of cadmium chloride on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment cultures—a new model for toxicological investigations of the “blood–testis” barrier in vitro. Toxicol Appl Pharmacol. 1992;112:51–57. doi: 10.1016/0041-008x(92)90278-z. [DOI] [PubMed] [Google Scholar]
- 23.Byers S, Hadley MA, Djakiew D, Dym M. Growth and characterization of epididymal epithelial cells and Sertoli cells in dual environment culture chambers. J Androl. 1986;7:59–68. doi: 10.1002/j.1939-4640.1986.tb00871.x. [DOI] [PubMed] [Google Scholar]
- 24.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reijerkerk A, et al. Diapedesis of monocytes is associated with MMP-mediated occludin disappearance in brain endothelial cells. FASEB J. 2006;20:E1901–E1909. doi: 10.1096/fj.06-6099fje. [DOI] [PubMed] [Google Scholar]
- 26.Bojarski C, et al. The specific fates of tight junction proteins in apoptotic epithelial cells. J Cell Sci. 2004;117:2097–2107. doi: 10.1242/jcs.01071. [DOI] [PubMed] [Google Scholar]
- 27.Harkness KA, et al. Dexamethasone regulation of matrix metalloproteinase expression in CNS vascular endothelium. Brain. 2000;123:698–709. doi: 10.1093/brain/123.4.698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.