Abstract
At metamorphosis the Xenopus laevis tadpole exocrine pancreas remodels in two stages. At the climax of metamorphosis thyroid hormone (TH) induces dedifferentiation of the entire exocrine pancreas to a progenitor state. The organ shrinks to 20% of its size, and ≈40% of its cells die. The acinar cells lose their zymogen granules and ≈75% of their RNA. The mRNAs that encode exocrine-specific proteins (including the transcription factor Ptf1a) undergo almost complete extinction at climax, whereas PDX-1, Notch-1, and Hes-1, genes implicated in differentiation of the progenitor cells, are activated. At the end of spontaneous metamorphosis when the endogenous TH has reached a low level, the pancreas begins to redifferentiate. Exogenous TH induces the dedifferentiation phase but not the redifferentation phase. The tadpole pancreas lacks the mature ductal system that is found in adult vertebrate pancreases, including the frog. Exocrine pancreases of transgenic tadpoles expressing a dominant negative form of the TH receptor controlled by the elastase promoter are resistant to TH. They do not shrink when subjected to TH. Their acinar cells do not dedifferentiate at climax, nor do they down-regulate exocrine-specific genes or activate Notch-1 and Hes-1. Even 2 months after metamorphosis these frogs have not developed a mature ductal system and the acinar cells are abnormally arranged. The TH-dependent dedifferentiation of the tadpole acinar cells at climax is a necessary step in the formation of a mature frog pancreas.
Keywords: dedifferentiation, thyroid hormone, Thyroid hormone receptor dominant negative, gene expression
The concentration of thyroid hormone (TH) controls amphibian metamorphosis. The TH level increases steadily in tadpoles as they proceed to metamorphosis and then decreases to a base line level after metamorphic climax (1). Metamorphosis in Xenopus laevis involves the remodeling of various organs including skin, brain, intestine, liver, and pancreas (2). Adding TH to the rearing water induces organ remodeling, and antithyroid chemicals like methimazole or perchlorate prevent remodeling. The essential role of thyroid receptors (TRs) has been demonstrated for diverse metamorphic programs by the inhibitory properties of a dominant negative form of the TR (TRDN) on the metamorphosis of various cell types and organs (3–7).
The tadpole exocrine pancreas consists of acinar cells with well developed zymogen granules. Exocrine-specific proteins have been detected as early as stage NF41 (8). The size of the pancreas steadily increases as the tadpole grows up to the climax of metamorphosis, and then at this stage the pancreas loses ≈80% of its volume (9). This pancreatic “regression” at climax is marked by histolysis and cell death (2, 10, 11) and also down-regulation (“extinction”) of exocrine-specific mRNAs and their proteins (11, 12).
We report here that the regression of the tadpole exocrine pancreas involves a TH-controlled dedifferentiation of the acinar cells to a progenitor state. These cells then redifferentiate in the frog to form a typical vertebrate pancreas. A major difference between the tadpole and frog pancreas is the almost complete absence of a recognizable ductal system in the tadpole pancreas. We present evidence that the dedifferentiation of the acinar cells during metamorphic climax is essential for the formation subsequently of a typical adult exocrine pancreas in the frog with a complete set of ducts.
Results
Morphological Changes of the Pancreas During Spontaneous Metamorphosis.
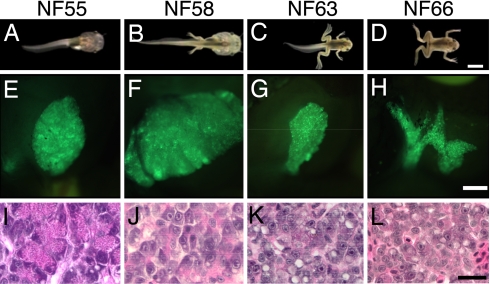
Transgenic tadpoles with the rat elastase promoter driving GFP (pElastase-GFP) were used to observe the change of shape and position of the pancreas during metamorphosis (Fig. 1). The stages of metamorphosis are divided into premetamorphic (up to NF55; Fig. 1A), prometamorphic (NF56–58; Fig. 1B) climax (NF59–65; Fig. 1C) and froglet (NF66; Fig. 1D). The pancreas grows proportionately with the size of the animal to the end of prometamorphosis (NF58; Fig. 1 E and F). The tadpole pancreas is a large flaccid organ that is curled ventrally around the U-shaped duodenum and touches the posterior end of the stomach. During climax the pancreas shrinks dramatically (Fig. 1G), reaching ≈20% of its original volume by NF63. At the end of metamorphosis the frog pancreas is flat and firm having lost its flaccid consistency (Fig. 1H). Throughout premetamorphosis the pancreas is filled with acinar cells that contain characteristic eosinophilic zymogen granules (Fig. 1I). When the tadpole reaches NF58/59, the onset of climax, the acinar cells start to lose their zymogen granules (Fig. 1J). At climax (NF63) most of their cytoplasm is lost and no acinar features remain. Many cells contain cytoplasmic vacuoles (Fig. 1K). Cell debris that is visible by electron microscopy and apoptotic bodies confirmed by TUNEL assay indicate a significant amount of cell death at climax [supporting information (SI) Fig. S1]. From NF58 to NF62 the total DNA content of the pancreas drops by ≈40% (Fig. S2), reflecting the significant cell death that has been reported (10, 11). However, at the same time the total RNA in the remaining cells drops 75% (Fig. S2) as the acinar cells lose their ribosome-rich cytoplasm. Throughout climax the dedifferentiated exocrine cells have prominent nuclei and nucleoli. Later, we will demonstrate that these cells have changed their gene expression profile. At the end of metamorphosis (NF66) regular acinar structures are still not visible (Fig. 1L). They do not reappear until ≈3–4 weeks after stage NF66.
Fig. 1.
Morphological and histological changes of the X. laevis pancreas at different stages expressing GFP driven by the rat elastase promoter. (A–D) Stages of X. laevis metamorphosis. (E–H) Abdominal view of the pancreas observed by fluorescent microscope. (I–L) H&E-stained sections of pancreases at the corresponding stages. Expression of the elastase-GFP transgene had no effect on normal development and remodeling of pancreas. (Scale bars: A–D, 4 mm; E–H, 1 mm; I–L, 40 μm.)
Dedifferentiation and Redifferentiation of the Pancreas During Metamorphosis.
We examined the expression pattern of exocrine-specific genes by in situ hybridization, real-time RT-PCR, and immunohistochemistry at different stages of metamorphosis. Differentiated exocrine pancreatic cells in tadpole and adult are rich in exocrine-specific product such as amylase, mRNA (Fig. 2 A and D), and protein (Fig. 2 E and H). Dedifferentiation of the exocrine pancreas is characterized by the loss (extinction) of exocrine-specific mRNAs at the climax of metamorphosis (Fig. 2B). Down-regulation of amylase mRNA was also confirmed by real-time RT-PCR (Fig. S3). Reappearance of exocrine mRNAs begins in 1-week-old froglets (data not shown) and gradually increases. Even though the amylase mRNA is lost at climax the amylase protein (leftover protein) remains throughout climax in all of the exocrine cells, confirming that the origin of the undifferentiated cells at climax is the previously differentiated tadpole acinar cells (Fig. 2 F and G). Ptf1a, a transcription factor that is required to express the exocrine-specific genes (13), follows the same down-regulation kinetics as amylase (Fig. 2 I and J), but its mRNA reappears at the end of metamorphosis before the up-regulation of amylase mRNA begins (Fig. 2K).
Fig. 2.
Change in gene expression in the exocrine pancreas during metamorphosis. (A–D and I–L) In situ hybridization with amylase (A–D) and Ptf1a (I–L) probes. Every mRNA encoding a “terminally differentiated” exocrine protein that we have tested is extinguished at metamorphic climax (NF62) to the same extent as these 2 mRNAs (see discussion). (E–H) Although amylase mRNA is extinguished at climax, amylase protein was still detected by immunohistochemistry, confirming the dedifferentiation stages of previously differentiated exocrine cells. (Scale bar: 40 μm.)
The transcription factor PDX-1 is expressed only in β-cells in the adult mammalian pancreas (14). However, both exocrine and endocrine progenitor cells express PDX-1 (15). In the tadpole and frog pancreas PDX-1 mRNA (Fig. 3 A and C) is expressed exclusively in islet cells. At climax PDX-1 expression is activated in the dedifferentiated exocrine cells (Fig. 3B), providing evidence for the progenitor state of the pancreas exocrine cells at climax. Up-regulation of PDX-1 mRNA was also confirmed by real-time RT-PCR (Fig. S3).
Fig. 3.
Change in gene expression in the exocrine pancreas during metamorphosis. (A–C) In situ hybridization with PDX-1 probes. PDX-1 is expressed exclusively in islet cells in the tadpole (A) and frog pancreases (C), and at climax expression is extended to the dedifferentiated exocrine cells (B). (D–I) Notch-1 (D–F) and Hes-1 (G–I) genes are activated at climax, although low level of expression of Notch-1 and Hes-1 has been observed at premetamorphic and adult frogs. (Scale bar: 40 μm.)
Notch signaling in the mammalian pancreas has been implicated in exocrine cell fate and differentiation (16–18). Activated Notch-1 induces Hes-1 expression, and Hes-1 can bind to and inactivate Ptf1a (19), thereby leading to the inhibition of exocrine-specific gene expression and conceivably in the case of amphibian metamorphosis the dedifferentiation of the exocrine pancreas. Notch-1 and Hes-1 mRNAs are up-regulated during metamorphic climax (Fig. 3 E and H and Fig. S3) and then decrease as the pancreas redifferentiates into mature pancreas (Fig. 3 F and I and Fig. S3). TH can dedifferentiate the exocrine pancreas in 48 h of 3,5,3′-l-triiodothyronine (T3) induction by down-regulation of exocrine mRNAs (described below), however, Notch-1 and Hes-1 mRNA are unchanged (Fig. S3). Therefore the activation of the Notch-1 and Hes-1 genes is too late a TH response to account for the first steps of TH-induced dedifferentiation.
Role of TH in Pancreatic Remodeling.
We assessed the role of TH in pancreas remodeling by using two methods. First, we added exogenous TH to the rearing water of premetamorphic tadpoles to induce metamorphic changes. Second, we prepared transgenic animals in which the rat elastase promoter controls a dominant negative form of TRα fused to GFP at its carboxyl terminus (pElastase-TRDN-GFP). Previous experiments demonstrated that expression of this competitive inhibitor blocks a variety of TH-induced programs (3). The protein encoded by the TRDN-GFP transgene is concentrated in the nucleus because of a nuclear localization signal within the TR. The TRDN-GFP transgene is expressed only in exocrine cells and not in insulin-immunoreactive cells (Fig. S4).
A WT premetamorphic pancreas (Fig. 4A) when induced by T3 resembles a normal pancreas at the climax of spontaneous metamorphosis. It shrinks dramatically in size (Fig. 4B), and the acinar cells lose their zymogen granules, and much of their cytoplasmic volume (Fig. 4E). Transgenic tadpoles expressing the TRDN-GFP transgene in their exocrine pancreas are protected from T3-induced alteration (Fig. 4 C and F). In situ hybridization of the T3-exposed premetamorphic control animals demonstrated that T3 induces down-regulation of exocrine-specific markers such as amylase after 4 days of exposure (Fig. 4H). This induced down-regulation of exocrine mRNAs is comparable with that of spontaneous metamorphosis at stage NF63 (Fig. 2B). However, animals expressing the TRDN-GFP transgene failed to down-regulate amylase (Fig. 4I). In some of the transgenic animals protection was not homogenous throughout the pancreas, and patches of cells were not protected against the action of T3. Loss of acinar integrity, loss of zymogen granules, and disappearance of exocrine mRNAs in T3-induced premetamorphic tadpoles along with the protective nature of the TRDN protein against exogenous T3 provides additional proof that TH controls the process of dedifferentiation of the exocrine pancreas.
Fig. 4.
The TRDN-transgene prevents TH-induced exocrine dedifferentiation. (A, D, and G) WT control NF54 tadpole. (B, E, and H) NF54 tadpole after 4 days of 10 nM T3 exposure. (C, F, and I), pElastase-TRDN-GFP NF54 transgenic tadpole after 4 days of 10 nM T3 exposure. (A–C) Abdominal view of the pancreas. The thin white line outlines each pancreas. (D–F) H&E-stained sections. (G–I) In situ hybridization for amylase. Exogenous TH induces the dedifferentiation of the exocrine pancreas, and the TRDN transgene prevents the dedifferentiation process. (Scale bars: A–C, 1 mm; D–I, 40 μm.)
The TRDN Transgene Prevents Remodeling of the Exocrine Pancreas During Spontaneous Metamorphosis.
Transgenic animals were raised through spontaneous metamorphosis. The expression of the TRDN transgene did not affect either tadpole growth or their time to metamorphosis. All external signs of metamorphosis were normal in the transgenic animals. Internal organs like the intestine that do not express the transgene underwent normal remodeling at climax. However, the pancreas did not change its shape during metamorphosis nor did it shrink at climax. Unlike WT (Fig. 5A), the TRDN-transgenic pancreases did not lose their zymogen granules at NF66 (Fig. 5E) nor did they undergo the RNA changes that characterize the normal pancreas at climax. Amylase mRNA was down-regulated in WT froglets (NF66; Fig. 5B) and not in TRDN-transgenic froglets of the same stage (Fig. 5F), and Notch-1 and Hes-1 mRNAs were not activated at climax in these transgenic animals (Fig. 5 G and H). Their protection was not always complete. Some transgenic animals had small islands of cells that responded to spontaneous metamorphosis (data not shown). The growth rates of transgenic and nontransgenic sibling frogs as measured by weighing the animals twice a week for 2 months were the same (data not shown). However, the pancreases in 2-month-old transgenic frogs were abnormal. The acinar cells were disorganized (Fig. 6C), and there were regions of the pancreas that were vacuolated and others with tightly packed nuclei and without proper acini (Fig. S5) that no longer expressed the transgene.
Fig. 5.
The TRDN transgene prevents dedifferentiation of acinar cells in spontaneous metamorphosis. NF66 WT (A–D) and NF66 pElastase-TRDN (E–H) pancreas sections. (A and E) Stained with H&E. (B–D and F–H) In situ hybridization. (B and F) Amylase. (C and G) Notch-1. (D and H) Hes-1. (Scale bar: 40 μm.)
Fig. 6.
Differences in ductal systems between tadpole and frog. (A) Premetamorphic tadpoles have very few ducts compared with (B) adult pancreas. The complex ductal system arises after metamorphosis. (C) Sections of a 2-month-old pElastase-TRDN-GFP frog showing reduced number of ducts. (D) All types of duct cells in control animals (n = 5 per group) were counted in a unit area from H&E-stained sections of NF54 tadpoles (PreMM), 2 month-old frog (2M), and an adult frog. (Scale bar: 40 μm.)
Pancreatic Duct Maturation During Spontaneous Metamorphosis.
H&E-stained sections identified the pancreatic ducts by their morphology (20) and were confirmed by antibody staining against carbonic anhydrase II (CAII) (data not shown), which stains all types of ductal cells (21). Like other vertebrates the mature frog pancreas has a complex ductal system consisting of intercalated, lobular, main ducts (Fig. 6B) and extra-pancreatic ducts that connect with the bile duct and attach to the intestine (Fig. S6). The premetamorphic pancreas has simple ducts with a single cuboidal epithelial cell lining (Fig. 6A). These structures are visible at all stages of metamorphosis sparsely distributed in the exocrine pancreas. By the end of metamorphosis larger lobular and collecting ducts have begun to form. We have quantified the presence of ductal components at different developmental stages by counting the number of duct cells. There is a large increase in the number of duct cells in the postmetamorphic frog pancreas compared with the premetamorphic pancreas (Fig. 6D). The intercalated ducts that connect the acini to the lobular duct were not detected in the premetamorphic pancreas but appear in postmetamorphosis along with the redifferentiation of the acinar cells (4-week-old froglets; data not shown). The extra-pancreatic ducts that connect the pancreas to the intestine are present at all stages of development. However, these larger ducts also undergo maturation. They have a single layer of epithelial cells in the tadpole with very little collagen or smooth muscle matrix (Fig. S6). The surrounding matrices appear after metamorphosis. In the frog these ducts are larger in diameter and have a thick collagen matrix surrounding them. The pancreatic ductal system in a 2-month-old TRDN frog was underdeveloped compared with the control sibling (Fig. 6). No intercalated ducts were observed and the lobular ducts were highly disorganized and were scarce (Fig. 6C and Fig. S5). However, the maturation of extra-pancreatic ducts was not affected in the TRDN frogs (Fig. S6), as the transgene was never expressed in those duct cells.
Discussion
Remodeling Begins with a TH-Dependent Dedifferentiation of the Exocrine Pancreas.
The premetamorphic tadpole pancreas is a large flaccid structure with well developed acini (Fig. 1) that are rich in zymogen granules and contain high levels of the proteins that characterize vertebrate exocrine pancreases. The dramatic reduction in pancreas volume that occurs at metamorphic climax or can be induced by exogenous TH has been described for Hyla (22), Bufo (10), Xenopus (22), and Rana (23). Organ shrinkage at climax has been estimated to be between 70% and 90%. Janes (22) concluded that the pancreas undergoes “partial degeneration” during metamorphosis followed by “regeneration.” Cellular “necrosis” and “dehydration” have been held responsible for the decrease in pancreatic mass (11, 22, 23). Apoptosis of pancreatic cells is elevated at climax (10). We have also observed increased apoptosis and the accumulation of cellular debris at the climax of metamorphosis by electron micrography (Fig. S1). The cell loss in the X. laevis pancreas during metamorphosis measured by the total DNA content of the pancreas was ≈40% (Fig. S2). However, this amount of cell death accounts for just part of the organ shrinkage. After just 4 days of TH induction or at the climax of metamorphosis, all of the exocrine cells lost their zymogen granules, >70% of their total RNA, and most of their cytoplasmic volume (Fig. 4). Every terminally differentiated pancreas-specific gene whose mRNA we measured was down-regulated at climax. The list includes carboxypeptidase A and B, α amylase, lipase, chymotrypsin, trypsin, zymogen granule membrane protein, DNase I, elastase I, and protein disulfide isomerase (data not shown). Ptf1a is a key transcription factor that is required for the expression of exocrine-specific genes in X. laevis (24, 25) as it is in mammals (26). TH-induced down-regulation of Ptf1a mRNA is not rapid enough to cause the drop of the exocrine-specific mRNAs (data not shown). However, this gene is reactivated before the pancreatic enzyme genes at the end of metamorphosis (Fig. 2) and might play an important role in the redifferentiation of the exocrine pancreas.
PDX-1, Notch-1, and Hes-1 are expressed in exocrine progenitor cells (18, 27, 28). These three genes are up-regulated at climax (Fig. 3). PDX-1 is expressed presumably exclusively in the islet cells in the differentiated tadpole and frog pancreas (Fig. 3) as it is in the mature mammalian pancreas (14). However, PDX-1 expression is activated in progenitor exocrine cells (15, 29), and we show that there is a similar pervasive activation of PDX-1 in the dedifferentiated exocrine cells at climax (Fig. 3). These same cells up-regulate Notch-1 and Hes-1 (Fig. 3) at climax. Blocking the TH response by overexpressing a TRDN inhibits the dedifferentiation of the pancreas at climax of spontaneous metamorphosis (Fig. 5) and that induced by exogenous TH (Fig. 4). At the end of metamorphosis these transgenic animals still have a tadpole-like pancreas with acinar cells containing zymogen granules. The exocrine-specific mRNA, amylase, is not down-regulated at climax nor do these transgenic pancreases up-regulate Notch-1 or Hes-1 mRNAs at climax (Fig. 5).
The final redifferentiation phase of remodeling occurs when the endogenous TH has returned to a baseline level after the climax of metamorphosis (1), giving us reason to believe that TH is not involved in the redifferentiation of the pancreas after metamorphosis in the frog.
Plasticity of the Pancreas.
The adult mammalian exocrine pancreas exhibits plasticity and has been shown to dedifferentiate under several conditions. Duct ligation is an established technique to cause tissue damage, and the acini become metaplastic and transdifferentiate into tubular duct-like structures (30). Mature acinar cells lose their identity and subsequently dedifferentiate and redifferentiate in chronic pancreatitis (31) upon exposure to the pharmacological agent, cerulein (32, 33) and when placed into cell culture (34).
In view of the mammalian experiments we considered whether injury to the pancreatic duct at climax in X. laevis might play a role in TH-induced dedifferentiation. The extensive intestinal remodeling that occurs at the same time (35) might secondarily damage the pancreatic duct. However, the elastase TRDN transgenic animals undergo normal or exogenous TH-induced intestinal remodeling while their pancreases are inhibited from dedifferentiation (Fig. 4). Therefore, in Xenopus, the dedifferentiation process is an organ autonomous event mediated directly by TH. Differentiation of the exocrine pancreas follows a well studied sequence of gene activation events in progenitor cells, including Notch signaling activation (27, 29). Redifferentiation in spontaneous metamorphosis is marked by the activation of the Notch-1 pathway at about the same time as PDX-1. The period of exocrine expression of PDX-1 ends at around NF66, whereas the Notch pathway remains activated for at least 4 weeks after metamorphosis. Notch pathway activation has also been observed after duct ligation (36) and after caerulein exposure (33) in mammalian models. Notch-1 activation is known to induce dedifferentiation of the pancreas presumably by inducing Hes-1 that binds to the critical exocrine transcription factor Ptf1a (19). However, TH induction does not activate Notch-1 or Hes-1 in the first 48 h when down-regulation of exocrine-specific mRNAs has begun. Possibly the low level of Notch-1 protein in tadpoles is activated by protease cleavage (37) and that initiates dedifferentiation.
Redifferentation of acinar cells into endocrine and ductal cells has been reported by several authors (34, 36, 38). In a separate study, we have shown that the dedifferentiated exocrine cells do not give rise to insulin-producing cells during the redifferentiation phase (S.M. and D.D.B., unpublished data). The TRDN transgenic frogs have a greatly reduced ductal system with no evidence of intercalated duct as late as 2 months after metamorphosis, further indicating that the intercalated ducts originate from dedifferentiated acinar cells.
The Difference Between the Tadpole and the Frog Pancreas.
The most striking feature of the tadpole pancreas is its underdeveloped duct system. The only readily identifiable ducts are an occasional simple tube that consists of a single layer of small cuboidal cells without supporting matrix (Fig. 6). These small ducts do not change their size or abundance during metamorphosis. In the tadpole the major pancreatic duct that joins the bile duct and then attaches to the duodenum has a simple epithelial wall that is one cell thick with no collagen support matrix (Fig. S6). All sizes of ducts are plentiful in the frog pancreas, and the larger ones have a thick collagen matrix (Fig. 6). The tadpole acinar cells synthesize a high level of the same digestive enzymes as the frog acinar cells, but it is not clear how the tadpole exocrine products are collected for delivery into the intestine considering the paucity of ducts. One explanation for TH's role in maturation of the pancreas at metamorphosis is to induce the exocrine cells, which are almost exclusively acinar in the tadpole, to revert to a progenitor state that then redifferentiates into a more traditional pancreas containing not only acini but a complete ductal system. The animals expressing the TRDN transgene cannot dedifferentiate their pancreas at climax nor form a normal duct system even 2 months after metamorphosis (Fig. 6 and Fig. S5). These animals grow normally to sexual maturity.
These studies with spontaneous metamorphosis and TH induction in WT and transgenic animals confirm the essential role of TH in pancreatic dedifferentiation at metamorphic climax and the importance of this temporary return to a progenitor state to form a mature vertebrate pancreas after metamorphosis. The proteins that are expressed in the tadpole acinar cells do not survive long enough to be lineage tracers for the new ductal system in the frog. Yet all facts indicate that these duct cells are derived from previously differentiated tadpole acinar cells. A schematic representation of exocrine pancreas architecture and the gene expression changes with development is illustrated in the Fig. 7. We report that pancreatic dedifferentiation and redifferentiation as a normal part of an animal's developmental program and life cycle. The fact that dedifferentiation of the tadpole pancreas is controlled completely by a simple hormone increases its value as a model system for studying pancreas development.
Fig. 7.
Schematic representation of morphological and gene expression changes in the exocrine pancreas during X. laevis metamorphosis. The adult frog pancreas has all of the types of ducts that have been described for adult vertebrates. Tadpoles lack the intercalated ducts. At the climax of metamorphosis, TH induces dedifferentiation of the exocrine pancreas to a progenitor state that has extinguished the mRNAs encoding terminally differentiated proteins (represented by amylase). PDX-1, Notch-1, and Hes-1 are up-regulated. Late in climax Ptf1a is reactivated followed by the differentiation of acinar and duct cells. Abbreviations: ac-acinar; ld-lobular duct, icd-intercalated duct. Figure is not drawn to scale.
Materials and Methods
Plasmid Constructs and Transgenesis.
Transgenic Xenopus were prepared by using a 205-bp rat elastase promoter (39, 40) driving either GFP (a gift from M. Horb, Clinical Research Institute of Montreal, Canada) or a dominant negative (TRDN) form of the TRα fused to GFP (5). TRDN is the coding sequence of X. laevis TRα lacking 36 bp from its C terminus fused by a 15-bp linker to GFP. This TRDN-flex-GFP was prepared from the construct pCS2+[teto]TRDN-GFP3 (5) by PCR using a primer that incorporates an EcoRI site at the 5′ end and a SmaI site at the 3′ end. The PCR product was purified, cut with EcoRI and SmaI, and ligated into linearized pElastase-GFP that had been cleaved with the same restriction enzymes. Both pElastase-GFP and pElastase-TRDN-GFP were linearized with NotI for transgenesis.
Transgenic animals were prepared by restriction enzyme-mediated integration nuclear transplantation (41) with some modifications (42). The linearized pElastase-TRDN-GFP was always coinjected with a linearized γ-crystaline-GFP construct (43). Successful transgenesis was confirmed from tail tip DNA by PCR. Only animals with visible GFP expression in their pancreas confirmed by fluorescent stereomicroscopy and through immunohistochemistry (described below) of cryosections with a GFP antibody were used for experiments. The promoter drives expression only in the acinar cells of NF54 tadpoles (S.M. and D.D.B., unpublished work). This is true for both the GFP and the TRDN-GFP reporters. The former GFP protein is localized in the cytoplasm, and the latter fused protein is in the nucleus. In these experiments we used F1 progeny of pElastase-GFP males and F0 pElastase-TRDN-GFP animals.
Raising and Treatment of Premetamorphic Tadpoles.
Tadpoles were raised in a flow-through system at 22°C with a 12-h light-dark cycle. X. laevis tadpoles were staged according to Nieuwkoop and Faber (44). Tadpoles and frogs were anesthetized with ice-cold MS-222 (3-aminobenzoic acid ethyl ester; Sigma) before their pancreases were isolated for direct visualization with a fluorescent microscope (Leica) or for tissue fixation. In TH-induced metamorphosis study premetamorphic (NF54) tadpoles (at ≤5 animal per liter; n = 5/6 per group) were reared in 4 liters of 0.1× MMR (10 mM NaCl, 0.2 mM KCl, 0.1 mM MgCl2, 0.2 mM CaCl2, and 0.5 mM Hepes, pH 7.5). A final concentration of 10 nM T3 was added to the rearing water, and duration of exposure was for 4 days. The water with T3 was changed every 2 days.
Histology, Immunohistochemistry, and In Situ Hybridization.
Pancreases were fixed in Bouin's fixative, embedded in paraffin, sectioned, and stained with H&E. For immunohistochemistry samples were fixed in 4% paraformaldehyde, embedded in OCT compound, cryosectioned, and immunostained as described (6). Primary antibodies used for immunohistochemistry were guinea pig anti-insulin (Linco Research) at 1:500 dilution, rabbit anti-GFP (Torrey Pines Biolabs) at 1:500 dilution, CAII (Chemicon International) at 1:200 dilution and mouse anti-amylase (Santa Cruz Biotechnology) at 1:50 dilution. Amylase staining was done in paraffin-embedded sections, and antigen-unmasking techniques was used according to the manufacturer's protocol (Vector Laboratories). Fluorescence-conjugated secondary antibodies (Molecular Probes) were used at a dilution of 1:400. Nuclei were counterstained with 0.5 μg/ml DAPI.
Intercalated and lobular ducts (both intralobular and interlobular ducts) were identified by their characteristic morphology (20) from the H&E-stained sections and confirmed by immunostaining with the antibody against CAII (data not shown) (21). Nuclei for each kind of duct were counted by digital micrography from premetamorphic tadpoles, 2-month-old control, and adult frog pancreases (n = 5 per group). The area of each section was estimated by digital imaging using ImageJ software (National Institutes of Health). The number of each ductal cell type is expressed per square millimeter of pancreas.
Total pancreatic RNA and DNA were isolated and quantified at different developmental stages after extraction with TRIzol (Invitrogen). In situ hybridization was performed on 4% paraformaldehyde fixed and cryosectioned tissues according to the protocol established in our laboratory (6). The digoxigenin-labeled antisense probes were prepared for amylase (GenBank accession no. BF048382), Ptf1a (GenBank accession no. AY372268), Notch-1 (GenBank accession no. M33874), Hes-1 (GenBank accession no. AF383160), and PDX-1 (GenBank accession no. X16849) as described (45). Images were taken with a Nikon Eclipse E800 microscope and SPOT RT digital camera (Diagnostic Instruments). In each group, 5–10 animals were used for in situ hybridization, immunostaining, and histology. Identical results were obtained for samples from the same stage. Real-time RT-PCR was used to quantify mRNA in both spontaneous and T3-induced pancreatic samples (SI Text).
Supplementary Material
Acknowledgments.
We thank Drs. Marko Horb, Steven Leach, and Tomas Pieler for their valuable advice and gifts of plasmids and Rejeanne Juste for expert technical assistance. The research was funded by grants from the National Institutes of Health and the G. Harold and Leila Y. Mathers Charitable Trust.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803569105/DCSupplemental.
References
- 1.Leloup J, Buscaglia M. Triiodothyronine: Metamorphosis hormone of the amphibians. C R Acad Sci. 1977;284:2261–2263. [Google Scholar]
- 2.Dodd MHI, Dodd JM. The Biology of Metamorphosis. New York: Academic; 1976. [Google Scholar]
- 3.Schreiber AM, Das B, Huang H, Marsh-Armstrong N, Brown DD. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. Proc Natl Acad Sci USA. 2001;98:10739–10744. doi: 10.1073/pnas.191361698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marsh-Armstrong N, Cai L, Brown DD. Thyroid hormone controls the development of connections between the spinal cord and limbs during Xenopus laevis metamorphosis. Proc Natl Acad Sci USA. 2004;101:165–170. doi: 10.1073/pnas.2136755100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das B, Schreiber AM, Huang H, Brown DD. Multiple thyroid hormone-induced muscle growth and death programs during metamorphosis in Xenopus laevis. Proc Natl Acad Sci USA. 2002;99:12230–12235. doi: 10.1073/pnas.182430599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai L, Brown DD. Expression of type II iodothyronine deiodinase marks the time that a tissue responds to thyroid hormone-induced metamorphosis in Xenopus laevis. Dev Biol. 2004;266:87–95. doi: 10.1016/j.ydbio.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Brown DD, et al. Thyroid hormone controls multiple independent programs required for limb development in Xenopus laevis metamorphosis. Proc Natl Acad Sci USA. 2005;102:12455–12458. doi: 10.1073/pnas.0505989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horb ME, Slack JM. Expression of amylase and other pancreatic genes in Xenopus. Mech Dev. 2002;113:153–157. doi: 10.1016/s0925-4773(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 9.Bollin E, Jr, Carlson CA, Kim KH. Anuran pancreas development during thyroxine-induced metamorphosis of Rana catesbeiana: RNA metabolism during the regressive phase of pancreas development. Dev Biol. 1973;31:185–191. doi: 10.1016/0012-1606(73)90329-1. [DOI] [PubMed] [Google Scholar]
- 10.Milano EG, Chimenti C. Morphogenesis of the pancreas of Bufo bufo during metamorphosis. Gen Comp Endocrinol. 1995;97:239–249. doi: 10.1006/gcen.1995.1023. [DOI] [PubMed] [Google Scholar]
- 11.Leone F, Lambert-Gardini S, Sartori C, Scapin S. Ultrastructural analysis of some functional aspects of Xenopus laevis pancreas during development and metamorphosis. J Embryol Exp Morphol. 1976;36:711–724. [PubMed] [Google Scholar]
- 12.Shi YB, Brown DD. Developmental and thyroid hormone-dependent regulation of pancreatic genes in Xenopus laevis. Genes Dev. 1990;4:1107–1113. doi: 10.1101/gad.4.7.1107. [DOI] [PubMed] [Google Scholar]
- 13.Lin JW, et al. Differential requirement for ptf1a in endocrine and exocrine lineages of developing zebrafish pancreas. Dev Biol. 2004;270:474–486. doi: 10.1016/j.ydbio.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 14.Guz Y, et al. Expression of murine STF-1, a putative insulin gene transcription factor, in β-cells of pancreas, duodenal epithelium, and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121:11–18. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- 15.Habener JF, Stoffers DA. A newly discovered role of transcription factors involved in pancreas development and the pathogenesis of diabetes mellitus. Proc Assoc Am Physicians. 1998;110:12–21. [PubMed] [Google Scholar]
- 16.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hald J, et al. Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Dev Biol. 2003;260:426–437. doi: 10.1016/s0012-1606(03)00326-9. [DOI] [PubMed] [Google Scholar]
- 18.Esni F, et al. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213–4224. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh B, Leach SD. Interactions between Hairy/Enhancer of Split-related proteins and the pancreatic transcription factor Ptf1–p48 modulate function of the PTF1 transcriptional complex. Biochem J. 2006;393:679–685. doi: 10.1042/BJ20051063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egerbacher M, Bock P. Morphology of the pancreatic duct system in mammals. Microsc Res Tech. 1997;37:407–417. doi: 10.1002/(SICI)1097-0029(19970601)37:5/6<407::AID-JEMT5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 21.Lohr M, et al. Immortalized bovine pancreatic duct cells become tumorigenic after transfection with mutant k-ras. Virchows Arch. 2001;438:581–590. doi: 10.1007/s004280100397. [DOI] [PubMed] [Google Scholar]
- 22.Janes RG. Studies on the amphibian digestive system II. Comparative histology of the pancreas, following early larval development, in certain species of anura. J Morphol. 1937;61:581–611. [Google Scholar]
- 23.Race JJ, Robinson C, Terry RJ. The influence of thyroxine on the normal development of pancreas in Rana pipens larvae. J Exp Zool. 1966;162:181–192. [Google Scholar]
- 24.Jarikji ZH, et al. Differential ability of Ptf1a and Ptf1a-VP16 to convert stomach, duodenum, and liver to pancreas. Dev Biol. 2007;304:786–799. doi: 10.1016/j.ydbio.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afelik S, Chen Y, Pieler T. Combined ectopic expression of Pdx1 and Ptf1a/p48 results in the stable conversion of posterior endoderm into endocrine and exocrine pancreatic tissue. Genes Dev. 2006;20:1441–1446. doi: 10.1101/gad.378706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cockell M, Stevenson BJ, Strubin M, Hagenbuchle O, Wellauer PK. Identification of a cell-specific DNA-binding activity that interacts with a transcriptional activator of genes expressed in the acinar pancreas. Mol Cell Biol. 1989;9:2464–2476. doi: 10.1128/mcb.9.6.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen J, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 28.Habener JF, Kemp DM, Thomas MK. Minireview: Transcriptional regulation in pancreatic development. Endocrinology. 2005;146:1025–1034. doi: 10.1210/en.2004-1576. [DOI] [PubMed] [Google Scholar]
- 29.Apelqvist A, et al. Notch signaling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 30.Wang RN, Kloppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia. 1995;38:1405–1411. doi: 10.1007/BF00400600. [DOI] [PubMed] [Google Scholar]
- 31.Tezel E, et al. REG I as a marker for human pancreatic acinoductular cells. Hepatogastroenterology. 2004;51:91–96. [PubMed] [Google Scholar]
- 32.Strobel O, et al. β-Cell transdifferentiation does not contribute to preneoplastic/metaplastic ductal lesions of the pancreas by genetic lineage tracing in vivo. Proc Natl Acad Sci USA. 2007;104:4419–4424. doi: 10.1073/pnas.0605248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen JN, et al. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Means AL, et al. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005;132:3767–3776. doi: 10.1242/dev.01925. [DOI] [PubMed] [Google Scholar]
- 35.Schreiber AM, Cai L, Brown DD. Remodeling of the intestine during metamorphosis of Xenopus laevis. Proc Natl Acad Sci USA. 2005;102:3720–3725. doi: 10.1073/pnas.0409868102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rooman I, et al. Expression of the Notch signaling pathway and effect on exocrine cell proliferation in adult rat pancreas. Am J Pathol. 2006;169:1206–1214. doi: 10.2353/ajpath.2006.050926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawey ET, Johnson JA, Crawford HC. Matrix metalloproteinase 7 controls pancreatic acinar cell transdifferentiation by activating the Notch signaling pathway. Proc Natl Acad Sci USA. 2007;104:19327–19332. doi: 10.1073/pnas.0705953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lardon J, Bouwens L. Metaplasia in the pancreas. Differentiation. 2005;73:278–286. doi: 10.1111/j.1432-0436.2005.00030.x. [DOI] [PubMed] [Google Scholar]
- 39.Beck CW, Slack JM. Gut-specific expression using mammalian promoters in transgenic Xenopus laevis. Mech Dev. 1999;88:221–227. doi: 10.1016/s0925-4773(99)00217-8. [DOI] [PubMed] [Google Scholar]
- 40.Kruse F, Rose SD, Swift GH, Hammer RE, MacDonald RJ. An endocrine-specific element is an integral component of an exocrine-specific pancreatic enhancer. Genes Dev. 1993;7:774–786. doi: 10.1101/gad.7.5.774. [DOI] [PubMed] [Google Scholar]
- 41.Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- 42.Huang H, Marsh-Armstrong N, Brown DD. Metamorphosis is inhibited in transgenic Xenopus laevis tadpoles that overexpress type III deiodinase. Proc Natl Acad Sci USA. 1999;96:962–967. doi: 10.1073/pnas.96.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smolich BD, Tarkington SK, Saha MS, Stathakis DG, Grainger RM. Characterization of Xenopus laevis γ-crystallin-encoding genes. Gene. 1993;128:189–195. doi: 10.1016/0378-1119(93)90562-h. [DOI] [PubMed] [Google Scholar]
- 44.Nieuwkoop PD, Faber J. Normal tables of Xenopus laevis (Daudin) Amsterdam: North Holland; 1956. [Google Scholar]
- 45.Berry DL, Schwartzman RA, Brown DD. The expression pattern of thyroid hormone response genes in the tadpole tail identifies multiple resorption programs. Dev Biol. 1998;203:12–23. doi: 10.1006/dbio.1998.8974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.