Abstract
The ≈28,300 species of tetrapods (four-limbed vertebrates) almost exclusively have perennial life spans. Here, we report the discovery of a remarkable annual tetrapod from the arid southwest of Madagascar: the chameleon Furcifer labordi, with a posthatching life span of just 4–5 months. At the start of the active season (November), an age cohort of hatchlings emerges; larger juveniles or adults are not present. These hatchlings grow rapidly, reach sexual maturity in less than 2 months, and reproduce in January–February. After reproduction, senescence appears, and the active season concludes with population-wide adult death. Consequently, during the dry season, the entire population is represented by developing eggs that incubate for 8–9 months before synchronously hatching at the onset of the following rainy season. Remarkably, this chameleon spends more of its short annual life cycle inside the egg than outside of it. Our review of tetrapod longevity (>1,700 species) finds no others with such a short life span. These findings suggest that the notorious rapid death of chameleons in captivity may, for some species, actually represent the natural adult life span. Consequently, a new appraisal may be warranted concerning the viability of chameleon breeding programs, which could have special significance for species of conservation concern. Additionally, because F. labordi is closely related to other perennial species, this chameleon group may prove also to be especially well suited for comparative studies that focus on life history evolution and the ecological, genetic, and/or hormonal determinants of aging, longevity, and senescence.
Keywords: Madagascar, lizard, longevity, semelparity, senescence
Although there are almost limitless theoretical combinations of life history traits, they are remarkably constrained to a continuum of high reproductive rates, rapid growth, and short life spans on one end and the opposite set of traits on the other (1). Because of this, life history theory makes predictions of how traits should evolve for a given set of parameters. For example, organisms experiencing increased adult mortality rates should evolve shorter life spans, and those experiencing increased juvenile mortality rates should evolve longer life spans (2–8). Any change in fecundity, age at maturity, or age-specific mortality that reduces the value of adults and increases the value of juveniles will cause an evolutionary shift from the end of the continuum with slower growth, iteroparity, and longer life span toward the other end with faster growth, semelparity, and shorter life span (7, 9).
Almost all of the nearly 30,000 species of tetrapods (four-limbed vertebrates) have perennial life spans. Within tetrapods, some species with slow growth and delayed maturity exhibit exceptionally long life spans of up to 100+ years (10). At the other extreme, rapid sexual maturity and annual life spans are surprisingly rare. Among endotherms (mammals and birds), near-annual longevity is known only in nine species of marsupials in the families Didelphidae and Dasyuridae, where it is restricted to males, and may also be facultative (11–17). There are no examples of annual amphibians (18). Among reptiles, lizards exhibit the shortest life spans (19), although the shortest-lived are capable of longevity >1 year with multiple clutches per lifetime (20, 21). However, we know very little about taxa such as chameleons, which have proved difficult to study in the field because of poor visibility in forest canopies, compounded by the secretive and cryptic behavior of the animals themselves (22).
Here, based on five seasons of field data, we report the surprising discovery of an annual tetrapod from the arid southwest of Madagascar: the chameleon Furcifer labordi, which has a posthatching life span of just 4–5 months that concludes with synchronous adult population-wide death (Fig. 1). Consequently, F. labordi spends the majority of its lifetime as a developing embryo, and, except for the brief period when adults and their recently laid eggs are both present, the entire population is a single age cohort. This life history is more reminiscent of ephemeral insects than that of a typical tetrapod. We also compare this life history with a sympatric but perennial chameleon, Furcifer verrucosus.
Fig. 1.
Life histories of annual and perennial chameleon species. Shown are the study region climate and life history of two chameleons: the annual F. labordi (Lower) and a hatchling cohort of the perennial F. verrucosus (Upper) over 15 months in southwest Madagascar. Toliara rainfall (blue line) and temperature (red line) are shown (57). Life history phases are: incubating eggs with a suspected diapause (open), juvenile growth (red), courtship (yellow), period of courtship and egg laying overlap (green), egg-laying and senescence (blue), juvenile aestivation (gray). The life span of F. labordi is a single year, with most of this time spent as a developing egg.
Results
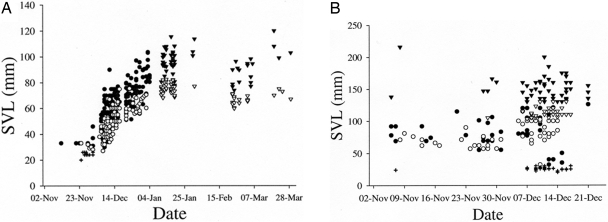
After the cool, dry, inactive season, we observed no F. labordi until the first emergence of hatchlings on November 11, with the onset of the wet season. Over the following 38 days, all F. labordi were a single age cohort of hatchlings and hatchlings-turned-juveniles, with no adults present until December 20 (Table 1). An annual species with synchronous hatching of a single cohort should show a strong, positive correlation between size and date during the growth phase for the entire population; and we found this pattern for F. labordi. Between November 11 and January 3, SVL (snout–vent length) was positively correlated with date in both males (n = 163, r = 0.745, P < 0.001) and females (n = 112, r = 0.759, P < 0.001; Fig. 2A). F. verrucosus differed from F. labordi in that SVL was not significantly correlated with date for either males or females (n = 119, r = −0.024, P = 0.793; n = 96, r = 0.170, P = 0.097, respectively; Fig. 2B). Juvenile F. labordi growth was exceptionally high: marked-recaptured males increased mean body mass by 4.1% daily (n = 24, mean ± 1 SE = 0.32 ± 0.07 g per day) and mean SVL by 1.86% daily (n = 24, 1.36 ± 0.11 mm per day). Female F. labordi also exhibited impressive growth rates during the same portion of the active season: marked-recaptured females increased mean body mass by 2.0% daily (n = 3, 0.09 ± 0.10 g per day) and mean SVL by 1.86% daily (n = 3, 1.26 ± 0.65 mm per day). The maximum growth rate observed in this species was as high as 2.6 mm per day. All posthatching growth was restricted to a period of <60 days.
Table 1.
Population demography of two chameleon species at Ranobe, Madagascar
| Period of active season | n | Percentage of population |
||
|---|---|---|---|---|
| Hatchling | Juvenile | Adult | ||
| F. labordi | ||||
| Nov 11–Nov 29 | 23 | 100 | 0 | 0 |
| Nov 30–Dec 6 | 28 | 32 | 68 | 0 |
| Dec 7–Dec 16 | 146 | 1 | 99 | 0 |
| Dec 17–Dec 29 | 83 | 0 | 24 | 76 |
| Dec 29–Mar 5 | 138 | 0 | 0 | 100 |
| F. verrucosus | ||||
| Nov 5–Nov 30 | 48 | 2 | 79 | 19 |
| Dec 1–Dec 20 | 174 | 29 | 42 | 29 |
| Feb 13–Mar 5 | 104 | 0 | 88 | 13 |
Composite dates for data collected 2003–2006 for the annual species F. labordi and the perennial species F. verrucosus are shown. We were unable to use data for F. verrucosus from December 21–February 12 because of nonrandom sampling of adults only; however, juveniles were present throughout the population for the entire active season. F. labordi adults were either prereproductive (before January 10) or sexually reproductive (after January 10; see Materials and Methods).
Fig. 2.
Cohorts of annual and perennial chameleon species. (A) Composite data (1995, 2003–2006) for SVL and date in the annual F. labordi cohort: unsexed hatchlings <26 mm SVL (+); males (filled symbols); females (open symbols); sexed hatchlings, juveniles, and prereproductive adults (circles); and sexually reproductive adults (triangles). (B) Composite data (2005–2006) for SVL and date for multiple cohorts of the perennial F. verrucosus: hatchlings <30 mm SVL (+); prereproductive (circles) and sexually reproductive (triangles) individuals; males (filled symbols); females (open symbols). Data beyond 21 December are truncated to exclude biased sampling efforts. However, juveniles were present throughout the entire active season (see Table 1).
We first observed reproductive behavior on January 10, and after this date all individuals exhibited adult morphology (Table 1), and growth ceased (Fig. 2A) and was even negative within individuals. Although growth is largely considered irreversible in vertebrates, negative reptile growth has been reported for marine iguanas during periods of poor food availability and stress (23). When reproduction in F. labordi began, we observed negative growth in a small set of marked-recaptured males (n = 6, −0.26 ± 0.30 mm per day). Within the population, growth ceased for both adult males (n = 61, r = −0.085, P = 0.517) and females (n = 55, r = −0.654, P < 0.001; Fig. 2A).
We observed no aestivation behavior by adults of F. labordi: none emerging at the beginning of the active season and none entering aestivation at the end of the active season. In contrast, we have observed both emerging and aestivation behavior multiple times in F. verrucosus and other arid-adapted chameleons. Additional museum specimens with known collecting dates and field records (see Materials and Methods) support these conclusions. Adult F. labordi have never been found in the field between May and November, whereas F. verrucosus have been collected in all months except July and August. In the perennial F. verrucosus, we observed adults, juveniles, and hatchlings frequently throughout the beginning of the active season (Fig. 2B and Table 1). Based on size classes, F. verrucosus comprises at least three age cohorts once hatchlings emerge: (i) hatchlings, (ii) subadults and adults from the previous year's hatch, and (iii) older adults (Fig. 2B).
The frequency of gravid F. labordi females peaked from late January to late February. We observed a radio-tracked female excavating a nest and depositing a clutch of 11 eggs on February 3: mean egg length was 11.7 mm (15.2% of her SVL), and total clutch mass was 4.4 g (36.7% of her preoviposition mass) (24). We did not observe any gravid females after March 2. We estimate that egg laying in F. labordi occurs mostly in February, and incubation spans 8–9 months with hatching in November, similar to the 10-month incubation period observed in captivity (25, 26). No species in the genus Furcifer are known to have incubation periods shorter than 8 months. These long incubation periods that are common among chameleons are the result of embryos being in diapause at the time of oviposition. Diapause terminates after several months, but development remains arrested by cold torpor until nest temperature increases as the wet season approaches (27–31). Consequently, the eggs resume development, and hatchlings synchronously emerge at the onset of the wet season in November (Fig. 1). Some other reptile species may hatch before emergence and overwinter inside the nest as hatchlings (32). However, in chameleons, this scenario appears unlikely because the group is mostly characterized by long incubation periods; delayed nest emergence, after hatching, has never been observed for any captive chameleon (26, 28).
In 2004, the last active adults (both species) were found on February 11, but in 1995 active adults were collected as late as March 28 (see Materials and Methods). Thus, the termination of the active season likely varies among years, falling approximately between February and April. After egg laying, the active season for F. labordi concludes with senescence and population-wide adult death. Developing eggs (incubating for 8–9 months) represent the entire population during the prolonged dry season, which is considerably longer than the posthatching life span of 4–5 months (Fig. 1). However, adult and subadult F. verrucosus aestivate over the dry season.
Discussion
No other tetrapod species, including short-lived marsupials (11, 12, 17, 33) and lizards, are known to have a life history similar to that of F. labordi. Previous reviews of other lizards (20, 21, 34) report 11 species as putatively annual. However, in all species in which survivorship was quantified by the original authors (cf. extrapolated from anecdotal literature), maximal postembryonic longevity was actually greater than 1 year, and none exhibited obligate annual population turnover. Our review of longevity in tetrapods, which included >1,700 species and 194 publications (available on request from the corresponding author), did not find obligate annual population turnover for any other tetrapod species, nor did we find any other tetrapod with a postembryonic life span of only 4–5 months. F. labordi is also unique among tetrapods in that it spends the majority of its life cycle inside the egg, a life history more reminiscent of ephemeral insects or aquatic vertebrates than of other terrestrial tetrapods.
At the end of the active season, radio-tracked and marked-recaptured F. labordi exhibited worsening body condition, including physical characteristics typical of senescence such as reduced mass, slower locomotion, and reduced strength when gripping branches. For example, males lost an average of 0.30 ± 0.16 g per day at the end of the breeding season (n = 6), and we also observed multiple instances of radio-tracked chameleons falling from trees, for unknown reasons, during normal locomotor activity. Additionally, from January 20—February 10, two of seven radio-tracked individuals were found dead of unknown causes but with no signs of mutilation. We also found several non-radio-tracked and unmarked dead F. labordi in a similar unmutilated condition toward the end of the active season. Conversely, for F. verrucosus, individuals continued to appear robust and healthy at the end of the breeding season, and none were found dead unmutilated.
Presently, it is unclear why F. labordi exhibits such a bizarre and extreme life history compared with other tetrapods. One hypothesis is that the harsh environment with extreme seasonality contributes to life history extremes. For example, short-lived, annual killifish deposit eggs in the mud, and they survive the harsh, dry season by entering a diapause, an adaptation to the highly fluctuating environment. Annualism is likely the ancestral character state; however, it has been evolutionarily lost by lineages found in more stable environments (35). The climate of Madagascar is highly variable (36): environmental unpredictability is much greater than other tropical areas, especially in the southwest, which exhibits unusually high interannual variability in rainfall. In response to stochastic climate fluctuations, many mammals of Madagascar differ from close relatives in more stable environments in that the Malagasy species exhibit more extreme versions of either “short-lived” or “long-lived” life histories (36). Dewar and Richard (36) suggested that both responses are possible “solutions” to the same evolutionary “problem.” Concordant with life history theory, the best solution depends on how environmental instability affects age-specific mortality (7, 8). Among several Malagasy mammals (carnivores, primates, tenrecs, and rodents), reduced juvenile survivorship due to environmental variability resulted in the evolution of longer life spans (37), whereas stochastic climatic variables that reduced adult survivorship resulted in the evolution of shorter life spans (36, 38). If environmental unpredictability differentially affected age-specific survivorship in chameleons, this may help explain why F. labordi is annual whereas other sympatric chameleons are perennial.
An alternative, and not mutually exclusive, explanation may emerge at the interface between life history theory and hormone–behavior relationships. High adult mortality rates can drive the evolution of rapid growth and earlier age of reproduction (2–7), with the cost being decreased longevity, often as a result of a trade-off between resources allocated to somatic cell maintenance compared with reproduction (6, 7, 10, 39–41). Hormones can control these trade-offs (42). For example, increased androgens in both natural populations and by experimental manipulation can be correlated with mating success (43, 44) but are also known to contribute to traits typically associated with increased adult mortality rates (e.g., reduced survival, increased parasite loads, increased energetic expenditure) (45–50). It seems possible that a change in the social structure in ancestral F. labordi, to a social system characterized by increased androgen levels or sensitivity, could contribute to increased intrinsic and/or extrinsic adult mortality. Indeed, F. labordi is characterized by physically intense combat and agonistic courtship (unpublished data). A similar mode of evolutionary selection appears to have played a role in the evolution of semelparity in at least one other tetrapod, the marsupial Phascogale calura (33). Accounting for hormonal regulation of physiology and behavior is critical to a comprehensive understanding of life history evolution (1, 42). Although our hypothesis is plausible, the role of hormones, and even behavior to a lesser extent, is unexplored in chameleons. Our hypothesis can be tested by quantifying seasonal hormone profiles, social systems, and sexual selection within a phylogenetic comparative framework.
Mortality is high during the brief mating phase of the active season: four of seven radio-tracked individuals died from predation or unknown causes from January 20 to February 10. The physically intense social system of this species, the harsh and unpredictable environment it inhabits, with a brief active season and where adult mortality is already high, may exacerbate the compression of life into such a brief period. In accordance with life history theory, the result would be evolutionary selection for reduced life span, smaller body size, and earlier age of reproduction (2–8). This may be advantageous for two reasons. First, because F. labordi is sympatric with other larger, but closely related (51, 52), perennial chameleons, a shift in body size and age of reproduction may alleviate some dimensions of niche overlap. Second, the metabolic theory of ecology states that smaller organisms have more resources to allocate to reproduction than their larger-bodied counterparts, relative to body mass, producing new individuals and genes at faster rates (53).
F. labordi has a life history like no other tetrapod, but it still conforms to predictions of life history theory: it experiences high adult mortality, is the smallest chameleon within a closely related group, exhibits rapid growth, and has an early age of reproduction. What makes this species unique among tetrapods is how extreme it has compressed its suite of life history traits into a single, brief season and that it spends the majority of its life cycle in a more benign and predictable environment: the egg. In fact, its entire life span is shorter than the age of sexual maturity in many other chameleons (26). Our findings suggest that the notorious rapid death of chameleons in captivity may, for some species, actually represent the natural adult life span. Consequently, a new appraisal may be warranted concerning the viability of chameleon breeding programs, which could have special significance for species of conservation concern. Additionally, if chameleons become a better studied group, it will also be possible to construct life history tables to test empirically the evolution of semelparity in some species and why iteroparity is present in others. Because F. labordi is closely related to other perennial species, this chameleon group may prove also to be especially well suited for comparative studies that focus on life history evolution and the ecological, genetic, and/or hormonal determinants of aging, longevity, and senescence.
Materials and Methods
The study site, Ranobe forest (23°01′30″ S, 43°36′36″ E), was located in southwestern Madagascar, ≈30 km north of Toliara. The forests of the southwest are spiny, and vegetation was typically xerophyllous thickets that included the family Didiereaceae and the genus Euphorbia (54). The region is classified as a “desert and xeric shrubland ecoregion” (55) and is the driest region in Madagascar, including during the wet, active season. Most rainfall is attributed to the brief and sporadic passage of tropical storms over the Indian Ocean (56). The mean annual rainfall of Toliara is 420 mm, with the wet season typically from December to February (56): mean monthly precipitation for these months is 89.9 mm (57). Mean annual temperature is 24.2°C. Like most arid environments, daily (day vs. night) and seasonal (wet vs. dry) temperature differences are high. Data were collected over four field seasons: February 22–March 5, 2003; December 20, 2003–January 30, 2004; December 5–December 16, 2005; and November 4–December 12, 2006. We reviewed historical precipitation data for Toliara (1951–2005) and found that the available data from the same periods as our study were within expected rainfall amounts in this dry region (i.e., the years we collected life history data were not characteristic of excessive drought compared with normal).
F. labordi and F. verrucosus are sexually dimorphic chameleons, inhabit arid regions of Madagascar (58), and are seasonally active only during the wet season. Both species have secondary sexual characters, but they are more exaggerated in F. labordi. F. verrucosus males possess large cranial casques, whereas females do not. Male F. labordi have proportionately larger cranial casques than F. verrucosus (unpublished data) and large rostral appendages; these typically “male” traits are also present in females, but to a lesser degree.
All specimens were collected by hand at night; they often sleep within 2 m of the ground. Upon capture, we marked locations, placed lizards individually in cloth, mesh bags, and transported them to a base camp where they experienced the same environmental conditions as they would in the forest. We suspended the mesh bags from narrow cord to prevent predation from arboreal, nocturnal snakes. The following morning, we measured body size (SVL) and total length (TL) by holding each chameleon so that maximal extension was apparent (i.e., no observable curvature to the body or tail). These measurements were made to the nearest 0.1 mm with calipers. We measured mass by using spring scales to the nearest 0.1 g (≤10 g) or 1 g (>10 g). We returned all lizards to their point of capture within 24 h and observed no adverse signs of social or handling stress in any individuals. Because of the brief period in which we possessed these individuals and because these chameleons do not drink except during sporadic periods of rain, we did not provide any supplemental food or water.
We gave all individuals a permanent identification by toe-clipping the most distal phalanx in a three-toe combination, with only one toe clipped per foot. We observed no adverse side effects of this marking procedure on the behavior and survivability of individuals, nor did we observe any partial or full-phalanx regrowth to confuse individual markings. Radio transmitters weighing <10% of the animal's body mass were affixed to the dorsal ridge of seven F. labordi with liquid adhesive. We located each lizard daily and made brief (<30-min) focal observations, three or four times per day.
For both species, we classified each individual as hatchling, juvenile, or adult. However, because F. labordi is unique in comprising a single cohort that transitions from juveniles to adults that are not sexually active initially, we also classified adults in this species as either prereproductive or sexually reproductive. Prereproductive adults were those exhibiting fully developed secondary sexual characters and noticeably larger body size relative to the rest of the population, but were present before courtship started in the population (January 10). After January 10, adult females exhibited sexually receptive coloration and were sexually reproductive. All adult males exhibited hemipenal bulges. Hatchlings lacked secondary sexual characters (casque and dorsal crest, plus rostral appendage in F. labordi), and juveniles represented all other individuals.
We queried additional museum collections for localities and collecting dates of F. labordi. Our search yielded 8 specimens at the University of Michigan Museum of Zoology (UMMZ) collected by C.J.R. March 18–28, 1995, at Ranobe, and 34 others at UMMZ collected from throughout the species' range. No other specimens with usable locality and/or collection date data were found in other collections.
Acknowledgments.
Field studies in Madagascar were made possible by the assistance of the Ministries des Eaux et Forêts, the Association Nationale pour la Gestion des Aires Protégées, and the Université d'Antananarivo, Département de Biologie Animale. We thank D. Rakotondravony and O. Ramilijaona for assistance with the project and H. Thomas and D. Kidney for valuable logistical advice. We thank M. Lovern, T. A. Baird, M. Palmer, R. Voss, R. Lehtinen, R. A. Nussbaum, and G. Schneider for their contributions. Research was supported by National Science Foundation Grant DEB 99-84496 (to C.J.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Ricklefs RE, Wikelski M. The physiology/life-history nexus. Trends Ecol Evol. 2002;17:462–468. [Google Scholar]
- 2.Austad SN, Fischer KE. Mammalian aging, metabolism, and ecology: Evidence from the bats and marsupials. J Gerontol. 1991;46:B47–B53. doi: 10.1093/geronj/46.2.b47. [DOI] [PubMed] [Google Scholar]
- 3.Holmes DJ, Austad SN. Fly now, die later: Life-history correlates of gliding and flying in mammals. J Mammal. 1994;75:224–226. [Google Scholar]
- 4.Hughes KA, Reynolds RM. Evolutionary and mechanistic theories of aging. Annu Rev Entomol. 2005;50:421–445. doi: 10.1146/annurev.ento.50.071803.130409. [DOI] [PubMed] [Google Scholar]
- 5.Ricklefs RE. Evolutionary theories of aging: Confirmation of a fundamental prediction, with implications for the genetic basis and evolution of life span. Am Nat. 1998;152:24–44. doi: 10.1086/286147. [DOI] [PubMed] [Google Scholar]
- 6.Ricklefs RE. Embryo development and ageing in birds and mammals. Proc R Soc London Ser B. 2006;273:2077–2082. doi: 10.1098/rspb.2006.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stearns SC. The Evolution of Life Histories. Oxford: Oxford Univ Press; 1992. [Google Scholar]
- 8.Roff DA. Life History Evolution. Sunderland, MA: Sinauer Associates; 2002. [Google Scholar]
- 9.Young TP. Evolution of semelparity in Mount Kenya lobelias. Evol Ecol. 1990;4:157–171. [Google Scholar]
- 10.Kirkwood TBL. In: Handbook of the Biology of Aging. Finch CE, Schneider EL, editors. New York: Van Nostrand Reinhold; 1985. [Google Scholar]
- 11.Bradley AJ, McDonald IR, Lee AK. Stress and mortality in a small marsupial (Antechinus stuartii, Macleay) Gen Comp Endocrinol. 1980;40:188–200. doi: 10.1016/0016-6480(80)90122-7. [DOI] [PubMed] [Google Scholar]
- 12.Cockburn A. In: Marsupial Biology: Recent Research New Perspectives. Saunders N, Hinds L, editors. Berlin: Springer; 1997. pp. 28–40. [Google Scholar]
- 13.Cockburn A, Scott MP, Scotts DJ. Inbreeding avoidance and male-biased natal dispersal in Antechinus spp (Marsupialia, Dasyuridae) Anim Behav. 1985;33:908–915. [Google Scholar]
- 14.de Magalhaes JP, Toussaint O. The evolution of mammalian aging. Exp Gerontol. 2002;37:769–775. doi: 10.1016/s0531-5565(02)00008-6. [DOI] [PubMed] [Google Scholar]
- 15.Dickman CR, Braithwaite RW. Postmating mortality of males in the dasyurid marsupials, Dasyurus and Parantechinus. J Mammal. 1992;73:143–147. [Google Scholar]
- 16.Karr JR, Nichols JD, Klimkiewicz MK, Brawn JD. Survival rates of birds of tropical and temperate forests: Will the dogma survive? Am Nat. 1990;136:277–291. [Google Scholar]
- 17.Kraaijeveld K, Kraaijeveld-Smit FJL, Adcock GJ. Does female mortality drive male semelparity in dasyurid marsupials? Proc R Soc London Ser B. 2003;270:S251–S253. doi: 10.1098/rsbl.2003.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanco MA, Sherman PW. Maximum longevities of chemically protected and nonprotected fishes, reptiles, and amphibians support evolutionary hypotheses of aging. Mech Ageing Dev. 2005;126:794–803. doi: 10.1016/j.mad.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Shine R, Charnov EL. Patterns of survival, growth, and maturation in snakes and lizards. Am Nat. 1992;139:1257–1269. [Google Scholar]
- 20.Tinkle DW, Wilbur HM, Tilley SG. Evolutionary strategies in lizard reproduction. Evolution. 1970;24:55–74. doi: 10.1111/j.1558-5646.1970.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 21.Tinkle DW. Concept of reproductive effort and its relation to evolution of life histories of lizards. Am Nat. 1969;103:501–516. [Google Scholar]
- 22.Raxworthy CJ. Reptiles, rainforest and conservation in Madagascar. Biol Conserv. 1988;43:181–211. [Google Scholar]
- 23.Wikelski M, Thom C. Marine iguanas shrink to survive El Niño: Changes in bone metabolism enable these adult lizards to reversibly alter their length. Nature. 2000;403:37–38. doi: 10.1038/47396. [DOI] [PubMed] [Google Scholar]
- 24.Karsten KB, Andriamandimbiarisoa LN. Furcifer labordi (Labord's chameleon), reproduction. Herpetol Rev. 2008 in press. [Google Scholar]
- 25.Kohler G. Incubation of Reptile Eggs. Malabar, FL: Krieger; 2005. [Google Scholar]
- 26.Nečas P. Chameleons: Nature's Hidden Jewels. Frankfurt am Main: Chimaira; 1999. [Google Scholar]
- 27.Andrews RM, Donoghue S. Effects of temperature and moisture on embryonic diapause of the veiled chameleon (Chamaeleo calyptratus) J Exp Zool. 2004;301A:629–635. doi: 10.1002/jez.a.56. [DOI] [PubMed] [Google Scholar]
- 28.Blanc F. Table de développement de Chamaeleo lateralis Gray, 1831. Ann Embryol Morphol. 1974;7:99–115. [Google Scholar]
- 29.Díaz-Paniagua C, Cuadrado M. Influence of incubation conditions on hatching success, embryo development and hatchling phenotype of common chameleon (Chamaeleo chamaeleon) eggs. Amphib Reptil. 2003;24:429–440. [Google Scholar]
- 30.Díaz-Paniagua C, Cuadrado M, Blázquez MC, Mateo JA. Reproduction of Chamaeleo chamaeleon under contrasting environmental conditions. Herpetol J. 2002;12:99–104. [Google Scholar]
- 31.Ferguson GW, Murphy JB, Ramanamanjato JB, Raselimanana AP. 2004. The Panther Chameleon: Color Variation, Natural History, Conservation, and Captive Management. (Krieger, Malabar, FL, p 10, 81) [Google Scholar]
- 32.Ultsch GR. The ecology of overwintering among turtles: Where turtles overwinter and its consequences. Biol Rev. 2006;81:339–367. doi: 10.1017/S1464793106007032. [DOI] [PubMed] [Google Scholar]
- 33.Bradley AJ. Reproduction and life history in the red-tailed phascogale, Phascogale calura (Marsupialia: Dasyuridae): The adaptive-stress senescence hypothesis. J Zool. 1997;241:739–755. [Google Scholar]
- 34.Clobert J, Garland T, Barbault R. The evolution of demographic tactics in lizards: A test of some hypotheses concerning life history evolution. J Evol Biol. 1998;11:329–364. [Google Scholar]
- 35.Murphy WJ, Collier GE. A molecular phylogeny for aplocheiloid fishes (Atherinomorpha, Cyprinodontiformes): The role of vicariance and the origins of annualism. Mol Biol Evol. 1997;14:790–799. doi: 10.1093/oxfordjournals.molbev.a025819. [DOI] [PubMed] [Google Scholar]
- 36.Dewar RE, Richard AF. Evolution in the hypervariable environment of Madagascar. Proc Natl Acad Sci USA. 2007;104:13723–13727. doi: 10.1073/pnas.0704346104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richard AF, Dewar RE, Schwartz M, Ratsirarson J. Life in the slow lane? Demography and life histories of male and female sifaka (Propithecus verreauxi verreauxi) J Zool. 2002;256:421–436. [Google Scholar]
- 38.Gould L, Sussman RW, Sauther ML. Demographic and life-history patterns in a population of ring-tailed lemurs (Lemur catta) at Beza Mahafaly Reserve, Madagascar: A 15-year perspective. Am J Phys Anthropol. 2003;120:182–194. doi: 10.1002/ajpa.10151. [DOI] [PubMed] [Google Scholar]
- 39.Kirkwood TBL. Evolution of ageing. Mech Ageing Dev. 2002;123:737–745. doi: 10.1016/s0047-6374(01)00419-5. [DOI] [PubMed] [Google Scholar]
- 40.Parsons PA. The ecological stress theory of aging and hormesis: An energetic evolutionary model. Biogerontology. 2007;8:233–242. doi: 10.1007/s10522-007-9080-z. [DOI] [PubMed] [Google Scholar]
- 41.Sgro CM, Partridge L. A delayed wave of death from reproduction in Drosophila. Science. 1999;286:2521–2524. doi: 10.1126/science.286.5449.2521. [DOI] [PubMed] [Google Scholar]
- 42.Zera AJ, Harshman LG. The physiology of life history trade-offs in animals. Annu Rev Ecol Syst. 2001;32:95–126. [Google Scholar]
- 43.Borgia G, Wingfield JC. Hormonal correlates of bower decoration and sexual display in the satin bowerbird (Ptilonorhynchus violaceus) Condor. 1991;93:935–942. [Google Scholar]
- 44.Denardo DF, Sinervo B. Effects of steroid hormone interaction on activity and home-range size of male lizards. Horm Behav. 1994;28:273–287. doi: 10.1006/hbeh.1994.1023. [DOI] [PubMed] [Google Scholar]
- 45.Klukowski M, Nelson CE. Ectoparasite loads in free-ranging northern fence lizards, Sceloporus undulatus hyacinthinus: Effects of testosterone and sex. Behav Ecol Sociobiol. 2001;49:289–295. [Google Scholar]
- 46.Marler CA, Moore MC. Evolutionary costs of aggression revealed by testosterone manipulations in free-living male lizards. Behav Ecol Sociobiol. 1988;23:21–26. [Google Scholar]
- 47.Wikelski M, Lynn S, Breuner C, Wingfield JC, Kenagy GJ. Energy metabolism, testosterone and corticosterone in white-crowned sparrows. J Comp Physiol A. 1999;185:463–470. [Google Scholar]
- 48.Wikelski M, Steiger SS, Gall B, Nelson KN. Sex, drugs and mating role: Testosterone-induced phenotype-switching in Galapagos marine iguanas. Behav Ecol. 2005;16:260–268. [Google Scholar]
- 49.Wingfield JC, Lynn SE, Soma KK. Avoiding the “costs” of testosterone: Ecological bases of hormone–behavior interactions. Brain Behav Evol. 2001;57:239–251. doi: 10.1159/000047243. [DOI] [PubMed] [Google Scholar]
- 50.Marler CA, Walsberg G, White ML, Moore M. Increased energy expenditure due to increased territorial defense in male lizards after phenotypic manipulation. Behav Ecol Sociobiol. 1995;37:225–231. [Google Scholar]
- 51.Raxworthy CJ, Forstner MRJ, Nussbaum RA. Chameleon radiation by oceanic dispersal. Nature. 2002;415:784–787. doi: 10.1038/415784a. [DOI] [PubMed] [Google Scholar]
- 52.Townsend T, Larson A. Molecular phylogenetics and mitochondrial genomic evolution in the Chamaeleonidae (Reptilia, Squamata) Mol Phylogenet Evol. 2002;23:22–36. doi: 10.1006/mpev.2001.1076. [DOI] [PubMed] [Google Scholar]
- 53.Brown JH, Sibly RM. Life-history evolution under a production constraint. Proc Natl Acad Sci USA. 2006;103:17595–17599. doi: 10.1073/pnas.0608522103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koechlin J. In: Biogeography and Ecology in Madagascar. Battistini R, Richard-Vindard G, editors. The Hague: W Junk; 1972. pp. 145–190. [Google Scholar]
- 55.Olson DM, et al. Terrestrial ecoregions of the world: A new map of life on earth. Bioscience. 2001;51:933–938. [Google Scholar]
- 56.Jury MR. In: The Natural History of Madagascar. Goodman SM, Benstead JP, editors. Chicago: Univ of Chicago Press; 2003. pp. 75–87. [Google Scholar]
- 57.Vose RS, et al. ORNL/CDIAC-53, CDIAC NDP-041. Oak Ridge, TN: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory; 1992. The Global Historical Climatology Network: Long-Term Monthly Temperature, Precipitation, Sea Level Pressure, and Station Pressure Data. Available at ftp://ftp.ncdc.noaa.gov/pub/data/ghcn/v1/ [Google Scholar]
- 58.Brygoo ER. Reptiles Sauriens Chamaeleonidae: Genre Chamaeleo. Faune Madagascar. 1971;33:1–318. [Google Scholar]