Abstract
Categorical perception is common in humans, but it is not known whether animals perceive continuous variation in their own multidimensional social signals categorically. There are two components to categorical perception: labeling and discrimination. In the first, continuously variable stimuli on each side of a category boundary are labeled. In the second, there is strong discrimination between stimuli from opposite sides of the boundary, whereas stimuli on the same side of the boundary are not discriminated. Here, we show that female túngara frogs respond categorically to complex mating calls that vary simultaneously along multiple dimensions and are within the natural range of signal variation. In response to a transect of synthetic stimuli that varied continuously and systematically in seven dimensions, female túngara frogs label mating calls as either conspecific or not conspecific. For pairs of stimuli that differed by the same magnitude, females discriminate those in different categories but not those in the same category. In addition, latency to respond was significantly shorter when stimuli were in the same versus different categories. Because responses to mating calls are critical in generating species recognition and sexual selection, this finding has implications for both animal perception and the influences of mate choice on the tempo and mode of evolution.
Keywords: animal cognition, animal communication, mate choice, phonotaxis, sexual selection
Animals use only a fraction of available sensory information to assess their worlds, and different animals can have access to different sets of data emanating from the same environmental or social stimuli (1). An animal's perception of the world also can be influenced by how that information is processed. One important factor is whether stimuli are perceived continuously or in discrete categories. Categorical perception in humans is well known in color, speech, and facial discrimination (2–4). Other animals exhibit categorical perception of human phonemes (5, 6). In addition, a few nonhuman animals show categorization of their own signals: song variants in songbirds (7), ultrasonic vocalizations in mouse pups (8), and mating/bat echolocation calls in crickets (9). In these animal studies, and most human studies, however, stimuli varied along a single dimension. It is not known, therefore, if categorical perception extends to the vast majority of communication signals, which covary over multiple dimensions.
Perception of social signals is a critical stage preceding many social decisions. One of the most important decisions an animal makes is deciding with whom to mate. These decisions are usually informed by signals that vary between and within species (10). In most instances, females choose to mate with conspecifics rather than heterospecifics and choose the more attractive males among conspecifics (11, 12). Mating preferences can lead to reproductive isolation between groups, which in turn promotes their divergence into different species. In addition, preferences for certain variants of signal traits among conspecifics can generate sexual selection and lead to the evolution of extreme male traits. Thus the perceptual mechanisms underlying mate choice can play an important role in evolution, but they are often ignored (13).
Many studies investigate how signal variation influences mate choice. One result of such studies is the “preference function,” in which the strength of preference is represented as a function of continuous variation in the mating signal (14–16). The preference function illustrates the nature of selection on male traits and may influence how traits evolve. Contrary to the results of the present study, all studies to date show that a continuous change in a signal parameter covaries with a continuous change in the strength of preference for that signal. Thus females respond to mating signals, whether comparing the same or different species, as being continuously more or less attractive. An alternative perceptual mode that might underlie mate choice is categorical perception, in which stimuli are categorized dichotomously as preferred or nonpreferred. There is no evidence to date for categorical perception of mating signals, although few studies have explicitly tested this hypothesis. We do so here by examining female preferences for mating call variation in the túngara frog, Physalaemus pustulosus.
Most male frogs produce acoustic signals to attract females for mating, and females discriminate call variation between and within species (17). Females exhibit these preferences through phonotaxis, approach toward a mating call (18–21). Although these experiments do not reveal the absolute potential for discriminating among stimuli, i.e., just noticeable differences, they emphasize the more ecologically relevant category of just meaningful differences and are thus more pertinent to the behavioral and evolutionary consequences of perceptual processes (7, 9, 22).
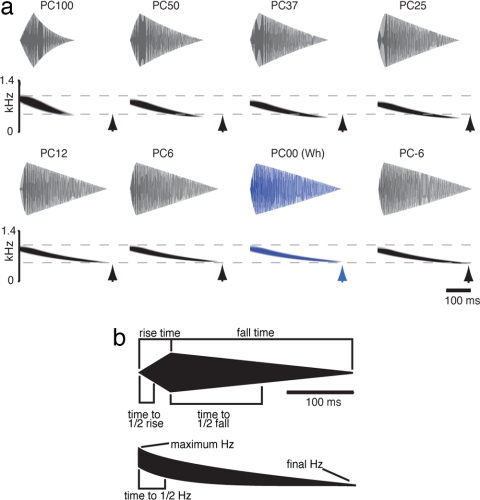
The túngara frog's mating call is a downward frequency sweep, or “whine,” of ≈300 ms (Fig. 1). Males can add “chucks” to the end of this call, which increases its attractiveness. The whine, however, is both necessary and sufficient to elicit phonotaxis from females, and all closely related species have whine-like calls (21). We examined female preferences in response to a series of synthetic calls that varied continuously between the conspecific whine and the call of an allopatric heterospecific, Physalaemus coloradorum. A previous study demonstrated that female túngara frogs recognize the P. coloradorum call but significantly prefer the conspecific call when given a two-choice test (21). Seven call parameters were adjusted by the same percentage to synthesize calls intermediate between each of the species' calls (PC series; Fig. 1); for example, the call PC25 has a fall time of 298 ms, which is 25% different from the P. pustulosus fall time (343 ms) and 75% different from the P. coloradorum fall time [161.7 ms; supporting information (SI) Table S1]. Thus synthetic calls of any “acoustic distance” (the overall summed difference of all seven call parameters) from the conspecific call can be synthesized, and the difference between any pair of stimuli can be easily ascertained (23); e.g., PC6 is 6% different from both the conspecific call (PC00) and the PC12 call. We used nine calls: PC50, PC37, PC31, PC25, PC18, PC12, PC6, PC00, and PC-6. Synthetic advertisement calls have been used previously to explore mate choice in túngara frogs, and among other findings these studies have demonstrated that the synthetic versions of the calls are treated no differently from natural stimuli (24).
Fig. 1.
Acoustic stimuli and parameters used in this study. (a) Oscillograms and spectrograms of the seven synthetic stimuli used in the labeling component of this study and the heterospecific call (PC100) presented for comparison. Dashed lines indicate the beginning and ending frequencies of the conspecific call (PC00, blue) and arrowheads indicate the duration of the PC00. (b) A stylized oscillogram (Upper) and spectrogram (Lower) of the synthetic túngara whine are shown along with the seven acoustic parameters used to construct stimuli in this study.
Reproductively active females were collected in Gamboa, Panamá, and tested with two-choice phonotaxis experiments to assess their preferences. We used two-choice rather than one-choice tests because they better approximate natural mate choice conditions. In addition, two-choice tests are more sensitive to differences between stimuli and are thus conservative when testing for categorical perception (25).
Results
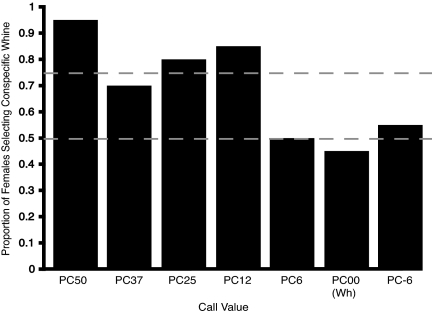
In the first battery of tests, we used the average synthetic version of the conspecific call (PC00) as a referent for exploring categorization. Females discriminated between PC00 and the four most different calls (PC12, P = 0.001; PC25, P = 0.006; PC37, P = 0.057; PC50, P < 0.001; Fig. 2). There was no preference, however, when PC00 was paired with the two most similar calls (PC-6, P = 0.412; PC6, P = 0.588; Fig. 2). A control experiment in which PC00 was paired with itself yielded no preference (PC00, P = 0.412; Fig. 2). The results of these experiments demonstrated labeling; when compared to conspecific calls, the test calls were considered to be either conspecific or not conspecific.
Fig. 2.
Mate choice results for labeling components of study. The whine (call value 00) is the túngara frog call. PC calls vary in the percentage (e.g., 6%, 12%, 18%) in which they differ from the conspecific call relative to the call of P. coloradorum. The proportion of females (n = 20 for each experiment) that prefer the conspecific call to each call variant shows that females label the calls PC-6 through PC6 as conspecific (they do not prefer the conspecific call to these variants) and label calls PC12 through PC50 as not conspecific (they prefer the conspecific call to each of these variants). The dashed lines indicate the null expectation (bottom line) and the critical value for a significant preference (top line).
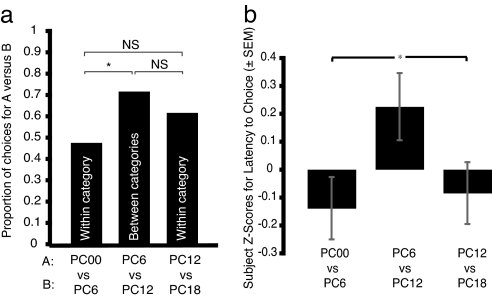
Another hallmark of categorical perception is a lack of discrimination between stimuli within a category and strong discrimination between stimuli of similar difference but in different categories. Given the suggestion of category labeling in the above results, we tested the hypothesis that females should discriminate between two stimuli across categories, but not within each category, even as the stimuli within each pair differed by the same amount. We used a repeated-measures design wherein each female completed three experiments: PC00 versus PC6, PC6 versus PC12, and PC12 versus PC18. We predicted that females would discriminate between PC6 versus PC12 but would not discriminate between the other two pairs even though the calls in each pair differed by 6%. As predicted, there was no preference in the two within-category experiments of PC00 versus PC6 (P = 0.443; Fig. 3a) and PC12 versus PC18, although the data suggested a trend toward a preference (P = 0.059). There was, as predicted, a strong preference in the between-category test, PC6 versus PC12 (P = 0.001). In addition, there was a significant overall Cochran's Q for the three experiments (Q = 6.81, P = 0.03), resulting from a difference in preference between PC00 versus PC6 and between PC6 versus PC12 (Q = 6.54, P = 0.01, Bonferonni corrected). The remaining two pairwise comparisons were not significantly different (Fig. 3a). Further support of categorical perception derives from pairwise tests at the extremes of each category: PC18 versus PC25 (P = 0.412) and PC25 versus PC31 (P = 0.412).
Fig. 3.
Mate choice and latency results for disrimination component of study. (a) Females were tested with pairs of stimuli in which one stimulus (row A on abscissa) is more similar to the conspecific call compared to the alternative (row B). Females do not show a preference between a pair of calls that differ by 6% within the category that is labeled as conspecific (whine versus PC6), whereas they do show a preference between calls that differ by 6% between the categories that are labeled as conspecific and not conspecific (PC6 versus PC12). The difference in the strength of preference between the within- and between-category is statistically significant. There is, however, discrimination between calls that differ by 6% within the not-conspecific category (PC12 versus PC18), but the strength of this discrimination between categories was not statistically significant. NS, not significant. (b) The z scores of the latency to respond from the same phonotaxis tests in a show that females take significantly more time to make a choice in the between-category comparison than in either of the within-category comparisons.
A common finding in categorical perception studies is that response times differ for within- and between-category discriminations (26–28). Pisoni and Tash (26) and Bornstein and Korda (27) found slower response times for evaluating between-category stimuli compared with equivalently different within-category stimuli; this effect, however, was not analyzed statistically. Thus we compared response latencies for the experiments in Fig. 3a. The one-way ANOVA generated a marginally significant overall effect of experiment on latency (F = 2.95, df = 2, P = 0.055; Fig. 3b). Planned independent contrasts showed that the latencies for the between-category comparison were significantly greater than the two within-category comparisons (t147 = 2.406, P = 0.017; Fig. 3b).
Discussion
Our data show that female túngara frogs respond to stimulus variation in a manner that is similar to categorical perception in humans and some other animals. Unlike previous animal studies, we demonstrate that categorical perception can occur in a mate choice context and is not restricted to a single acoustic dimension, as is true in all other studies of categorical perception, but can also emerge in response to acoustic signals that vary in multiple dimensions, a common feature of virtually all social signals. In addition, the synthetic stimuli used in this study comprised a range of acoustic variation that falls within that of the study population's calls as evaluated by using multidimensional scaling (Fig. S1). This natural intraspecific variation has not yet been examined for patterns of continuous or categorical mate choice.
One interpretation of this study is that mate preference and stimulus variation is not always a simple function as is implicit in studies of sexual selection by female choice. If that is the case, then we might expect male traits to evolve in a more punctuated mode (29, 30). It also might suggest reexamination of the notion that females assess continuous quantitative variation in display traits as indicators of male genetic quality (31, 32). Recent studies have investigated in great detail the scaling of male signals in sexual selection (33); our study suggests that scaling of female preferences for these signals is worthy of similar attention.
We suggest that understanding perception of mating signals is critical to a deep understanding of how species recognition evolves and how sexual selection generates some of the most extreme behaviors and morphologies in the animal kingdom.
Materials and Methods
Female frogs were collected between 1900 and 2200 h and tested between 2200 and 0600 h during the summers of 2006 and 2007. After testing, females were toe-clipped to avoid retesting them and returned to their original capture site within 12 h of collection. Collection permits in Panamá were approved by the Autoridad Nacional del Ambiente, and animal care and use approval was done under the University of Texas Institutional Animal Care and Use Committee (6041701). We tested 167 females (410 choice trials) during the course of this study. For the repeated measures component we randomized the order in which the experiments were conducted. The average number of tests that each female participated in was 2.57, and the average number of transects per female was 1.11. Females were highly responsive with successful choices performed in 91.1% of trials.
Frogs were tested under infrared light in a sound attenuating chamber (Acoustic Systems) measuring 2.7 × 1.8 × 1.78 m. Female behavior was observed on a video monitor connected to an infrared camera on the chamber ceiling. At the beginning of each phonotaxis test a female was placed under a small cone in the center of the chamber floor. We broadcast the two test stimuli antiphonally from speakers located in the center of the walls at the two poles of the chamber. Stimuli were presented at a rate of one call per 2 s from each speaker, and speakers were calibrated to a peak amplitude of 82 dB sound pressure level (20 μPa). Between successive trials, stimuli were alternated between the two speakers to avoid a stimulus × side bias. We concurrently tested for side bias by using identical stimuli. Those results and an analysis of the present data strongly demonstrate the lack of a side bias in this study.
Stimuli were presented for 3 min while the female was under the cone. Then the cone was raised and a phonotactic choice was scored if a female approached one of the two stimuli within a 10-cm radius without simply following the wall. A female failed to exhibit a phonotactic choice if she was motionless for the initial 5 min after the cone was raised or during any 2-min interval thereafter. Finally, a female did not exhibit phonotaxis if she failed to make a choice within 15 min after the cone was raised. In addition to measuring choice we recorded latency to choice.
We used a binomial test (one-tailed) to evaluate each experiment against the null prediction of no preference (P = 0.5); there was the a priori expectation that females prefer the conspecific call to alternatives (20). For repeated measures experiments we also used the nonparametric Cochran's Q test to evaluate each female's response under one experiment compared with the other two experiments. Response latencies for repeated-measures experiments were converted to subject z scores and analyzed with a one-way ANOVA.
Supplementary Material
Acknowledgments.
We thank the assistants who participated in these experiments; the Smithsonian Tropical Research Institute for logistical support; and R. Diehl, S. Phelps, D. McFadden, and two anonymous reviewers for comments on the manuscript. Funding was provided by National Science Foundation Grants IOB 0544096 (to M.J.R.) and 0517328 (to M.J.R. and R.C. Taylor).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802201105/DCSupplemental.
References
- 1.von Uexküll J. Environment [Umwelt] and inner world of animals. In: Burghardt GM, editor. Foundations of Comparative Ethology. New York: Van Nostrand Reinhold; 1909/1985. pp. 222–245. [Google Scholar]
- 2.Harnad S. Categorical Perception: The Groundwork of Cognition. New York: Cambridge Univ Press; 1987. [Google Scholar]
- 3.Diehl RL, Lotto AJ, Holt LL. Speech perception. Annu Rev Psych. 2004;55:149–179. doi: 10.1146/annurev.psych.55.090902.142028. [DOI] [PubMed] [Google Scholar]
- 4.Webster MA, Kaping D, Mizokami Y, Duhamel P. Adaptation to natural facial categories. Nature. 2004;428:557–561. doi: 10.1038/nature02420. [DOI] [PubMed] [Google Scholar]
- 5.Kuhl PK. Discrimination of speech by nonhuman animals, basic auditory sensitivities conducive to the perception of speech sound categories. J Acous Soc Am. 1981;70:340–349. [Google Scholar]
- 6.Kluender KR, Diehl RL, Killeen PR. Japanese quail can learn phonetic categories. Science. 1987;237:1195–1197. doi: 10.1126/science.3629235. [DOI] [PubMed] [Google Scholar]
- 7.Nelson DA, Marler P. Categorical perception of a natural stimulus continuum: Birdsong. Science. 1989;244:976–978. doi: 10.1126/science.2727689. [DOI] [PubMed] [Google Scholar]
- 8.Ehret G, Haack B. Categorical perception of mouse pup ultrasounds by lactating females. Naturwissenschaften. 1981;68:208–209. doi: 10.1007/BF01047208. [DOI] [PubMed] [Google Scholar]
- 9.Wyttenbach RA, May ML, Hoy RR. Categorical perception of sound frequency by crickets. Science. 1996;273:1542–1544. doi: 10.1126/science.273.5281.1542. [DOI] [PubMed] [Google Scholar]
- 10.Ryan MJ, Bernal XE, Rand AS. Patterns of mating call preferences in túngara frogs, Physalaemus pustulosus. J Evol Biol. 2007;20:2235–2247. doi: 10.1111/j.1420-9101.2007.01420.x. [DOI] [PubMed] [Google Scholar]
- 11.Kirkpatrick M, Ryan MJ. The paradox of the lek and the evolution of mating preferences. Nature. 1991;350:33–38. [Google Scholar]
- 12.Andersson M. Sexual Selection. Princeton: Princeton Univ Press; 1994. [Google Scholar]
- 13.Ryan MJ, Akre KL, Kirkpatrick M. Primer: Mate choice. Curr Biol. 2007;17:313–316. doi: 10.1016/j.cub.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Ritchie MG. The shape of female mating preferences. Proc Natl Acad Sci USA. 1996;93:14628–14631. doi: 10.1073/pnas.93.25.14628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner WE., Jr Measuring female mating preferences. Anim Behav. 1998;55:1029–1042. doi: 10.1006/anbe.1997.0635. [DOI] [PubMed] [Google Scholar]
- 16.Kirkpatrick M, Rand AS, Ryan MJ. Mate choice rules in animals. Anim Behav. 2006;71:1215–1225. [Google Scholar]
- 17.Gerhardt HC, Huber F. Acoustic Communication in Insects and Anurans. Chicago: University of Chicago Press; 2002. [Google Scholar]
- 18.Ryan MJ. Female mate choice in a neotropical frog. Science. 1980;209:523–525. doi: 10.1126/science.209.4455.523. [DOI] [PubMed] [Google Scholar]
- 19.Ryan MJ. The Túngara Frog, A Study in Sexual Selection and Communication. Chicago: University of Chicago Press; 1985. [Google Scholar]
- 20.Ryan MJ, Fox JH, Wilczynski W, Rand AS. Sexual selection for sensory exploitation in the frog Physalaemus pustulosus. Nature. 1990;343:66–67. doi: 10.1038/343066a0. [DOI] [PubMed] [Google Scholar]
- 21.Ryan MJ, Rand AS. Female responses to ancestral advertisement calls in túngara frogs. Science. 1995;269:390–392. doi: 10.1126/science.269.5222.390. [DOI] [PubMed] [Google Scholar]
- 22.Nelson DA, Marler P. The perception of birdsong and an ecological concept of signal space. In: Stebbins WC, Berkeley MA, editors. Comparative Perception: Complex Signals. New York: Wiley; 1990. pp. 443–478. [Google Scholar]
- 23.Ryan MJ, Rand W, Hurd PL, Phelps SM, Rand AS. Generalization in response to mate recognition signals. Am Nat. 2003;161:380–394. doi: 10.1086/367588. [DOI] [PubMed] [Google Scholar]
- 24.Rand AS, Ryan MJ, Wilczynski W. Signal redundancy and receiver permissiveness in acoustic mate recognition by the túngara frog, Physalaemus pustulosus. Am Zool. 1992;32:15–17. [Google Scholar]
- 25.Phelps SM, Rand AS, Ryan MJ. A cognitive framework for mate choice and species recognition. Am Nat. 2006;167:28–42. doi: 10.1086/498538. [DOI] [PubMed] [Google Scholar]
- 26.Pisoni DB, Tash J. Reaction times to comparisons within and across phonetic categories. Percept Psychophys. 1974;15:285–290. doi: 10.3758/bf03213946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bornstein MH, Korda NO. Discrimination and matching within and between hues measured by reaction times: Some implications for categorical perception and levels of information processing. Psych Res. 1984;46:207–222. doi: 10.1007/BF00308884. [DOI] [PubMed] [Google Scholar]
- 28.Campanella S, et al. Right N170 modulation in a face discrimination task: An account for categorical perception of familiar faces. Psychophysiology. 2000;37:796–806. [PubMed] [Google Scholar]
- 29.Gould SJ. The Structure of Evolutionary Theory. Cambridge, MA: Harvard Univ Press; 2002. [Google Scholar]
- 30.Turner JRG. Why we need evolution by jerks. New Sci. 1984;101:34–35. [Google Scholar]
- 31.Zahavi A, Zahavi A. The Handicap Principle. New York: Oxford Univ Press; 1997. [Google Scholar]
- 32.Kokko H, Brooks R, Jennions MD, Morley J. The evolution of mate choice and mating biases. Proc R Soc London Ser B. 2003;270:653–664. doi: 10.1098/rspb.2002.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kodric-Brown A, Sibly RM, Brown JH. The allometry of ornaments and weapons. Proc Natl Acad Sci USA. 2006;1003:8733–8738. doi: 10.1073/pnas.0602994103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.