Abstract
The pathogenesis of chronic joint inflammation remains unclear, although the involvement of pathogen recognition receptors has been suggested recently. In the present article, we describe the role of two members of the NACHT-LRR (NLR) family, Nod1 (nucleotide-binding oligomerization domain) and Nod2 in a model of acute joint inflammation induced by intraarticular injection of Streptococcus pyogenes cell wall fragments. Here, we show that Nod2 deficiency resulted in reduced joint inflammation and protection against early cartilage damage. In contrast, Nod1 gene-deficient mice developed enhanced joint inflammation with concomitant elevated levels of proinflammatory cytokines and cartilage damage, consistent with a model in which Nod1 controls the inflammatory reaction. To explore whether the different function of Nod1 and Nod2 occurs also in humans, we exposed peripheral blood mononuclear cells (PBMCs) carrying either Nod1ins/del or Nod2fs mutation with SCW fragments in vitro. Production of both TNFα and IL-1β was clearly impaired in PBMCs carrying the Nod2fs compared with PBMCs isolated from healthy controls. In line with results in Nod1 gene-deficient mice, PBMCs from individuals bearing a newly described Nod1 mutation produced enhanced levels of proinflammatory cytokines after 24-h stimulation with SCW fragments. These data indicate that the NLR family members Nod1 and Nod2 have different functions in controlling inflammation, and that intracellular Nod1–Nod2 interactions may determine the severity of arthritis in this experimental model. Whether a distorted balance between the function of Nod1 and/or Nod2 is involved in the pathogenesis of human autoinflammatory or autoimmune disease, such as rheumatoid arthritis, remains to be elucidated.
Keywords: innate immunity, rheumatoid arthritis, inflammation, PRR
NACHT-LRR (NLR) receptors are a new family of intracellular microbial sensors that complement the recognition of extracellular pathogen-associated molecular patterns by membrane-bound Toll-like receptors (TLRs) (1, 2). Nod1 and Nod2 are members of the NLR family, which recognize diaminopimelic acid (DAP)-containing muramyl tripeptide or muramyl dipeptide (MDP), from bacterial peptidoglycans (3, 4). Interactions between the intracellular pathways induced by Nods and TLRs have been reported (5, 6). Mutations in the Nod1 and Nod2 genes are associated with inflammatory bowel disease and other inflammatory disorders (7, 8). However, the precise mechanisms through which NOD-mediated recognition of peptidoglycans is involved in the pathogenesis of inflammatory diseases are still unclear, although several mechanisms have been proposed in Crohn's disease, such as reduced induction of antiinflammatory cytokines IL-10 and TGFβ production (9), release of defensins in the gut mucosa (10), negative regulation of TLR2 signals (11), or gain-of-function mutations (12). A better understanding of the intracellular events induced by the interaction between Nod1/Nod2 and peptidoglycans is therefore crucial for both insight into recognition of pathogens by the innate immune system and pathogenesis of chronic inflammation.
Rheumatoid arthritis (RA) is a chronic inflammatory disorder in which the exact pathogenesis is unclear. It is accepted, however, that besides genetic factors, environmental triggers contribute to the pathogenesis of RA. An infectious etiology of RA has been a longstanding hypothesis and, with the discovery of TLR in the synovial tissue of RA patients, the role of bacteria and viruses in the pathogenesis of RA has been invigorated (13, 14). It was recently shown that TLRs are abundant in the synovial lining of RA patients with active disease (15), and it has been suggested that signaling through members of the IL-1R/TLR family plays an important role in inflammatory diseases (16). Because members of the NLR family are also important in the recognition of microorganisms, and bacterial cell-wall components like peptidoglycans are present in the inflamed synovial tissue (17), it is tempting to speculate that Nod1 and Nod2 play a role in the pathogenesis of RA.
In the present study, we investigated the role of Nod1 and Nod2 in experimental arthritis and the role of these NLR-LRR in the modulation of proinflammatory mediators (cytokines) induced by streptococcal cell wall (SCW) components. In addition to Nod1 and Nod2 gene-deficient mice, we investigate the response to SCWs in the cells of individuals bearing Nod1 or Nod2 mutations.
Results
Expression of Nod1 and Nod2 in Synovial Tissue of RA Patients.
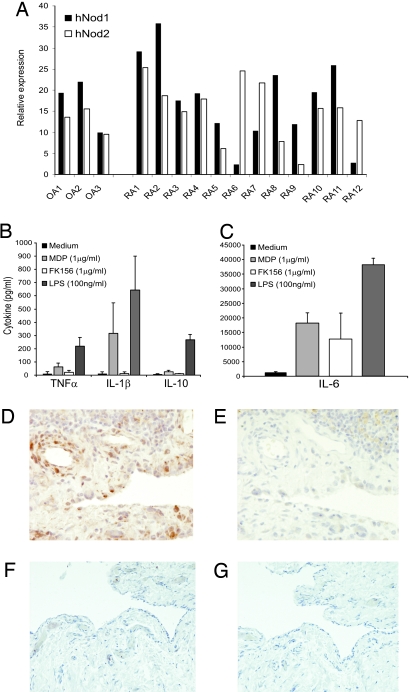
Because it is not known whether both Nod1 and Nod2 were present in the joints of RA patients, we determined the expression of mRNA for Nod1 and Nod2 in synovial tissue samples from RA patients. Fig. 1A shows that RA synovial tissue exhibits marked mRNA expression of both intracellular pathogen recognition receptors Nod1 and Nod2. Of interest, most RA synovia expressed both Nod1 and Nod2, but some RA patients expressed only Nod1 or Nod2. Nod1 and Nod2 mRNA expression was also found in synovial tissue specimens from osteoarthritis (OA) patients. To examine whether Nod1 or Nod2 expression was functional, we exposed RA synovial tissue to specific Nod1 and Nod2 ligands. Fig. 1 B and C show that triggering of both receptors leads to enhanced production of TNFα, IL-1β, IL-6, and IL-10. Of great interest, MDP potently induced IL-1β production in contrast to Nod1. Using immunohistochemistry, we examined the presence of MDP-containing moieties in RA synovial tissue. In line with previous reports, immunoreactive MDP is clearly present in RA synovial tissue samples (Fig. 1D). In contrast to RA, no MDP-containing moieties were present in OA synovial tissue (Fig. 1F). Thus, both the ligand (MDP) and its receptor Nod2 are present in RA joints, which may mean the Nod2 pathway contributes to the pathogenesis of RA.
Fig. 1.
Nod expression in RA synovial tissue. (A) mRNA expression of Nod1 or Nod2 in RA and OA synovial tissue samples expressed as relative expression to the houskeeping gene GAPDH (2−delta Ct value × 1,000). (B and C) Cytokine production of RA synovial tissue after exposure to MDP, FK156, and LPS. (D) MDP-containing fragments in RA synovial tissue showed by immunohistochemical staining of paraffin sections using MoAb 2–4-5 (39). (E) Isotype control. Both synovial lining cells and endothelial cells contain MDP fragments. (D and E) ×200 magnification. (F) MDP staining of OA synovial tissue; note almost no staining. Isotype control. (F and G) ×100 magnification.
SCW-Induced Arthritis in Mice Deficient for Nod1 or Nod2.
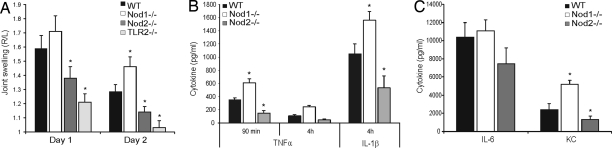
Because Nod1 and/or Nod2 signaling modulates TLR2-activated pathways (5), we investigated the role of Nod1 and Nod2 in SCW-induced arthritis, a model driven by TLR2 activation [Fig. 2A (18)]. Induction of acute arthritis in Nod1 gene-deficient mice led to remarkable enhanced joint swelling on days 1 and 2 (Fig. 2A) (18). In addition, synovial concentrations of TNFα and IL-1β were significantly higher than that of wild-type mice (Fig. 2B). Also, the chemokine KC was elevated in Nod1 gene-deficient mice, which may result in enhanced influx of polymorphonuclear granulocytes (PMN) in inflamed joints (Fig. 2C). Detailed histopathological analysis revealed indeed that inflamed knee joints of Nod1 gene-deficient mice contain more inflammatory cells (PMNs) than the wild-type control. Moreover, early cartilage damage was slightly enhanced in Nod1 gene-deficient mice (Fig. 3 and Table 1). In contrast to the remarkable results in Nod1 gene-deficient mice, induction of SCW-driven arthritis in Nod2 gene-deficient mice showed reduced joint swelling at days 1 and 2 (Fig. 2A). In line with decreased arthritis expression, protein levels of IL-1β, TNFα, and KC were significantly reduced in Nod2 gene-deficient mice when compared with the wild-type control (Fig. 2 B and C). Of great interest, early cartilage damage, determined as cartilage proteoglycan (PG) loss, was almost absent in Nod2 gene-deficient mice (Fig. 3 and Table 1). In addition, we determined synovial mRNA expression of several proinflamatory genes on day 2 of SCW-induced arthritis in both Nod1 and Nod2 gene-deficient mice. Supporting information (SI) Fig. S1A shows that, in line with protein levels measured at the 4-h time point (Fig. 2 B and C), mRNA levels for IL1-β, IL-6, and TNF were enhanced in Nod1 gene-deficient mice and reduced in Nod2 gene-deficient mice when compared with wild-type mice. Similar results were noted for the genes of the proinflammatory enzymes COX-2 and iNOS, which lead to generation of PGE2 and nitric oxide, respectively. Of interest, IL-10 mRNA levels were reduced at 4 h in both Nod1 and Nod2 gene-deficient mice, corresponding with reduced protein concentration of IL-10 in both Nod1 and Nod2 gene-deficient mice after induction of SCW arthritis (Fig. S1B).
Fig. 2.
SCW-induced arthritis in Nod1 or Nod2 gene-deficient mice. (A) Joint swelling at days 1 and 2 after induction of SCW arthritis in wild-type, Nod1, Nod2, and TLR2 gene-deficient mice. The joint swelling (99mTechnetium uptake) is expressed as a right-left ratio, and a ratio >1.10 is indicated as inflammation. Joint swelling is strongly reduced in both Nod2 and TLR2 gene-deficient mice. At least seven mice per group were used, and the repeated experiment showed similar results. (B and C) Cytokine concentrations determined 4 h after induction of SCW arthritis in patellae washouts. The cytokines were measured in 50 μl of patellae washout (n = 6 per group), using a multiplex bead array kit. *, P < 0.05, Mann–Whitney U test, compared with wild-type control.
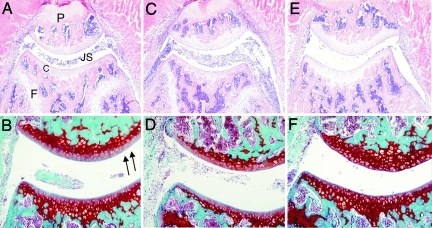
Fig. 3.
Joint pathology in Nod1 or Nod2 gene-deficient mice. (A and B) Knee joint of wild-type mouse, day 2 of SCW arthritis. The inflammatory cells in the joint cavity. (C and D) Nod1−/− mouse showing enhanced joint inflammation. (E and F) Nod2−/− mouse, showing the reduced inflammation in the knee joint. (A, C, and E) Hematoxylin/eosin staining, ×100 magnification. (B, D, and F) Cartilage PG depletion visualized by Safranin O staining (arrows indicate PG depletion), ×200 magnification. P, patella; F, femur; C, cartilage; JS, joint space.
Table 1.
Joint pathology in Nod1- or Nod2-deficient mice
| Influx of inflammatory cells |
Proteoglycan depletion | ||
|---|---|---|---|
| Joint cavity | Synovial tissue | ||
| WT* | 1.3 ± 0.2 | 0.8 ± 0.2 | 0.7 ± 0.2 |
| Nod1−/− | 2.0 ± 0.3† | 1.4 ± 0.03† | 1.6 ± 0.3† |
| Nod2−/− | 0.5 ± 0.03† | 0.3 ± 0.2‡ | 0.3 ± 0.2‡ |
Histology was performed at day 2 of SCW arthritis. Cell influx of both joint cavity and synovial tissue was scored on a scale ranging from 0 to 3. Loss of cartilage matrix proteoglycans was determined on Safranin O-stained sections and scored a scale of 0–3.
*WT, C57Black/six mice.
†P < 0.05, Mann–Whitney U test compared with wild-type mice.
‡P < 0.001, Mann–Whitney U test compared with wild-type mice. At least seven mice per group were included, and the study was repeated once with similar results.
Altered Cytokine Production in Human Peripheral Blood Mononuclear Cells (PBMC) Containing Nod1 or Nod2 Mutations.
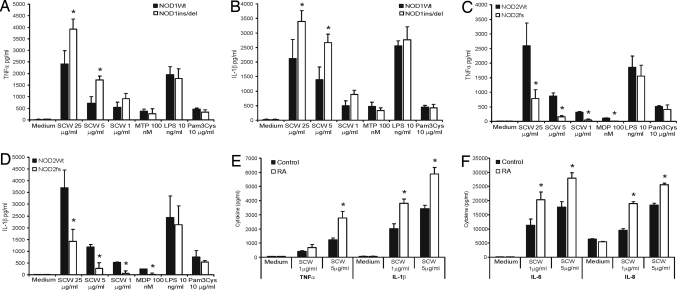
To explore whether these remarkable differential effects of murine Nod1 and Nod2 also occur in humans, we exposed PBMC isolated from individuals bearing Nod1 del/ins or Nod2 3020insC frameshift mutation to SCW fragments and determined the cytokine production. Fig. 4 A and B show that PBMC bearing Nod1 del/ins (ND1-32656) mutation produce significantly more TNFα and IL-1β than control PBMC after exposure for 24 h to SCW fragments. Remarkably, Nod1del/ins and control PBMC produced similar amounts of these cytokines when stimulated with either TLR4 or TLR2 ligands. These findings indicate that loss of Nod1 function does not affect TLR pathways, and that the effects of Nod1 dysfunction on SCW-induced cytokines are likely due to modulation of its Nod2-stimulatory activity. As expected, TNFα and IL-1β release was strongly impaired in PBMCs isolated from Crohn's disease patients carrying the Nod2fs (3020insC) mutation when compared with PBMCs from healthy controls (Fig. 4 C and D). These data corroborate results in Nod2 knockout mice after local injection of SCW fragments (Fig. 2). In line with murine data, IL-10 production of PBMCs from both Nod1del/ins and Nod2fs was impaired after stimulation with SCW fragments, indicating that IL-10 generation depends on both NLR family members (Fig. S1 C and D).
Fig. 4.
TNFα and IL-1β production of PBMC bearing Nod1ins/del or Nod2fs mutation. PBMC were exposed either to a dose range of SCW fragments or to ultrapure TLR2 or TLR4 agonists for 24 h. As positive controls, muramyl-tri-DAP (MTP) and MDP were used. (A and B) TNFα or IL-1β production in control and Nod1ins/del bearing PBMC. (C and D) TNFα and IL-1β production in Nod2fs carrying PBMC. Data represent the mean ± SD of four controls and four frameshift mutations. *, P < 0.05, Mann–Whitney U test, compared with wild-type control.
Enhanced Cytokine Production of PBMC Originated from RA Patients.
To investigate whether PBMC isolated from RA patients respond differently to bacterial components, we exposed RA PBMC to SCW fragments in vitro for 24 h. Thereafter, several proinflammatory cytokines were determined. Fig. 4 E and F clearly demonstrate that RA PBMC produced significantly higher TNFα, IL-1β, IL-6, and IL-8 concentrations when compared with PBMC of healthy controls. These results may indicate that in RA patients, lack of control by Nod1 influences the final production of proinflammatory cytokines after exposure to bacterial components.
Interaction of Nod1-Nod2 Controls Cytokine Production After Stimulation with SCW Fragments.
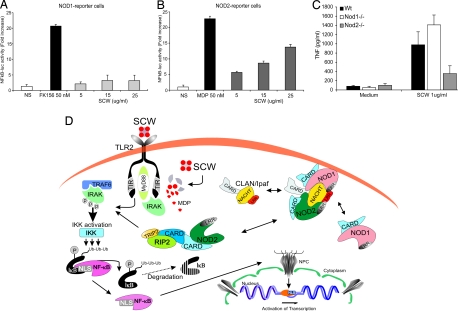
Because it is not known whether Nod1 recognize digested SCW fragments, we exposed Nod1 reporter cells to SCW fragments in vitro. HEK293 cells transiently cotransfected with a Nod1 plasmid and an NF-κB-luciferase construct were exposed to SCW fragments. Fig. 5A shows that Nod1 does not recognize SCW fragments, in contrast to Nod2. HEK293-Nod2 NF-κB-luciferase reporter cells showed a dose-related response when exposed to SCW fragments (Fig. 5B). Peritoneal macrophages isolated from Nod1 or Nod2 gene-deficient mice confirmed the results found in HEK293 transfected cells (Fig. 5C). These data strongly suggest that the enhanced cytokine production in vitro and the deterioration of the joint inflammation observed in Nod1ko mice is not due to a direct interaction of digested SCW fragments and Nod1. More likely there is an intrinsic interaction between Nod1 and Nod2 that inhibits Nod2 signaling and reduce inflammation: these inhibitory signals are not induced by direct interaction of streptococcal peptidoglycan with Nod1. Fig. 5D proposes a model of Nod1–Nod2 interactions as they may occur in TLR2/Nod2-driven signaling cascades.
Fig. 5.
Nod2 is the intracellular receptor for recognition of SCW fragments. (A and B) HEK-293 cells cotransfected with either Nod1 or Nod2 expressing plasmid and an NF-κB-reporter construct. Cells were exposed to a dose range of SCW fragments and positive controls (FK156, MDP). (C) Peritoneal macrophages were stimulated with 1 μg/ml SCW for 24 h; thereafter, TNF was determined by Luminex assay. (D) Schematic representation of the putative Nod1-Nod2 protein complex controlling TLR2 driven pathway. SCW fragments trigger TLR2 pathway directly and after release of MDP from the SCW peptidoglycans, the Nod2 cascade is activated. Enhanced cytokine production will occur by simultaneous activation of TLR2 and Nod2 pathways. Nod1-Nod2 complexes interfere with the latter pathways by inactivation Nod2 receptor.
Discussion
In the present study, we demonstrate that Nod1 and Nod2 play an important role in the pathogenesis of SCW-induced arthritis. SCW fragments induce inflammation through direct recognition of MDP motifs in streptococcal peptidoglycans by Nod2. In turn, the absence of Nod1 results in an increased activation of cells when Nod2 is engaged, suggesting a regulatory function of Nod1.
Our experiments in Nod2 gene-deficient mice show that Nod2 recognition plays a crucial role in SCW arthritis. Proinflammatory cytokine message and protein, secondary proinflammatory mediators, joint inflammation, and cartilage destruction are impressively reduced in Nod2 gene-deficient mice. From previous studies in our laboratory, we know that the chemokine KC is responsible for influx of PMNs in the joint cavity, and that TNFα and IL-1β are the driving cytokines for initiation of joint swelling and cartilage damage, respectively. The mouse data are consistent with those obtained in humans. Individuals with Crohn's disease homozygous for a loss-of-function frameshift mutation have shown decreased responsiveness to SCW fragments, supporting a role for Nod2 for recognition of SCW. Other studies have demonstrated recognition pathways involving TLR2 and Nod2 for recognition of Streptococcus pneumoniae (19) similarly to our data concerning S. pyogenes. We and others have shown that TLR2 and Nod2 have synergistic effects on cytokine production (5, 6), and this is very likely to contribute to the pathogenesis of SCW-induced arthritis. Whether Nod2 also contributes to the pathogenesis of RA in patients remains to be demonstrated, although the presence of peptidoglycans in the synovial tissue suggests its possible involvement. We demonstrated there is a direct interaction between SCW and Nod2, because HEK293-Nod2 reporter cells respond to stimulation with SCW fragments. Recently, we demonstrated that acute SCW-induced arthritis was partly TLR2/Myd88-dependent, but TLR-independent mechanisms are also likely to be involved (18). Data in Nod2−/− mice demonstrate that the TLR-independent mediation of arthritis by SCW largely depends on Nod2, and it is most likely that synergistic effects between Nod2 and TLR2 drive inflammation in this model. Nod2−/− mice displayed significantly less joint swelling, accompanied by reduced cytokine production and cellular infiltrate (see Fig. 2). The reduced cellular infiltrate is likely mediated by the lower release of KC in Nod2−/− mice, whereas the diminished joint swelling and cartilage destruction was mediated by the decreased release of TNF and IL-1, respectively (20).
One of the most interesting observations of the present study is the increased inflammatory reaction in Nod1−/− mice injected with SCW fragments. When SCW fragments were injected intraarticularly in the joints of Nod1−/− mice, they induced an increased swelling of the joint, more cellular influx, and increased production and release of proinflammatory cytokines and secondary mediators. These data are supported by the response of PBMC isolated from individuals bearing a complex deletion/insertion mutation of Nod1 (22) to SCW, showing that cells from individuals with Nod1 mutation released significantly greater amounts of proinflammatory cytokines compared with wild-type controls stimulated with SCW. That this is not a constitutive repression exerted by Nod1 may be concluded from our observation that there is a normal response to TLR2 and TLR4 stimuli in Nod1 gene-deficient mice and human cells bearing the Nod1ins/del mutation. Thus, both murine and human data argue that, in certain conditions, Nod1 can exert regulatory effects on the interaction between peptidoglycans and Nod2.
We have also investigated the mechanism responsible for the regulatory effects of Nod1 on Nod2 signals. When HEK cells were transfected with Nod1, no direct stimulation was induced by SCW. This demonstrates that the effects of Nod1 on Nod2 are not mediated by a direct recognition of SCW by Nod1. Rather, an interaction between Nod1 and Nod2 is responsible for these effects, because we have shown that Nod2 deficiency also results in reduced Nod1 effects (21). It has been recently demonstrated that CLAN/Ipaf interacts with both Nod1 and Nod2, and this interaction leads to inhibition of Nod2 signals (23). It is therefore tempting to speculate that the regulatory effects of Nod1 on Nod2 signals are mediated through CLAN/Ipaf (see Fig. 5D).
The interaction between SCW and Nod1 is not a direct one, because HEK-Nod1 reporter cells do not respond to SCW. The results obtained in Nod1 gene-deficient mice suggest the possibility that Nod1 and Nod2 pathways interact at intracellular levels, leading to inhibitory effects of Nod1 on Nod2 signaling. This hypothesis is supported by earlier studies showing the role of Nod2 for both stimulation with MDP and the classical Nod1 ligand MTP, strongly suggesting an interaction between Nod1 and Nod2 (21).
It has been long hypothesized that microbial components play a role in the initiation and amplification of the inflammation of RA. Chlamydia species, Yersinia species, or mycobacteria have been all suggested to be involved in triggering chronic inflammation in the synovium of RA patients either through persistent infection or by presence of remnants. The recognition of these microorganisms is mediated, at least in part, by TLRs, and TLR-induced pathways are responsible for induction of proinflammatory mediators by their molecular structures (24, 25). In addition to TLRs, Chlamydia spp. are recognized by Nod1 (26, 27), whereas Nod2 is a recognition receptor for mycobacteria (28). Nod1 stimulates cytokine and chemokine production and neutrophil recruitment (29), whereas Nod2 amplifies TLR-induced cytokine production (5, 6). These findings suggest a possible role of Nod1 and/or Nod2 in inflammatory arthritis, and this hypothesis is reinforced by our immunohistochemistry data, which demonstrate the presence of MDP-containing peptidoglycans in the inflamed synovium of RA patients. Moreover, mRNA for both Nod1 and Nod2 are expressed in the joints of patients with RA (Fig. 1). Of high interest, it was very recently shown that Nod2/TLR2 activation programs dendritic cells to promote IL-17-producing memory T cells, which are potentially involved in several autoimmune diseases including RA (30).
From the present study, we conclude that members of the NACHT-LRR receptor family are involved in SCW-induced arthritis in a differential fashion: whereas Nod2 is involved in the recognition of MDP and induction of proinflammatory effects in the inflamed joint, Nod1 has mainly an inhibitory role, exemplified by inhibition of cytokine production and decrease of cell influx. At the integrative level, TLR2 and Nod2 are receptors recognizing SCW fragments and mediating the inflammatory response, whereas Nod1 leads to inhibition of these signals (Fig. 5D). Interestingly, the reduction of IL-10 production upon stimulation in both Nod1 and Nod2 mice suggests that the inhibitory effects of Nod1 are not mediated by endogenous IL-10.
Materials and Methods
Animals.
Male C57BL/6 mice were obtained from Charles River Wiga. Nod1 and Nod2 gene-deficient mice were bred at Institut Pasteur. A breeder pair of TLR2-deficient mice were kindly provided by S. Akira (University of Osaka, Osaka, Japan). Animal experiments were approved by the Radboud University Nijmegen ethics committee.
SCW Preparation and Induction of Chronic SCW Arthritis.
S. pyogenes T12 organisms were cultured overnight in Todd–Hewitt broth. Cell walls were prepared as described in ref. 31. Unilateral arthritis was induced by intraarticular injection of 25 μg of SCW (rhamnose content) in 6 μl of saline into the right knee joint of naïve mice. As control, the left knee joint was injected with 6 μl of saline.
Joint-Swelling Measurement.
Joint inflammation was measured by using the 99mTc uptake method, as described (18). The radioisotope 99mTechnetium (20 μCi per mouse) distributed over the whole body in a few minutes. Because of increased blood flow and edema formation, accumulation of 99mTc in the right (inflamed) joint can be measured with external gamma counting. Joint swelling is expressed as the ratio of the 99mTc uptake in the inflamed over the control joint.
Cytokine Determination.
IL-1β, TNFα, IL-6, and KC were measured 4 h after induction of SCW arthritis. Patellae were isolated from inflamed knee joints and cultured 1 h at room temperature in RPMI medium 1640. The cytokines were measured in 50 μl of patella washouts (18) by using Luminex xMAP technology and Bio-Plex kits (Bio-Rad). Sensitivity was <5 pg/ml. Human TNFα (32), IL-1β, IL-6, and IL-10 were measured by either specific or commercial ELISA kits (R&D Systems) and Pelikine Compact (Sanquin). Sensitivity of both ELISAs was <20 pg/ml.
RNA Isolation and PCR Amplification.
Immediately after cervical dislocation, synovial tissue was isolated from the inflamed knee joints. The synovium samples were immediately stored in N2 until total RNA isolation (33). For details, see SI Text.
Immunohistochemical Staining of MDP-Containing Fragments.
Synovial tissue biopsies were taken from RA patients with active disease (34). A total of 5–10 tissue samples were immediately fixed in 4% formaldehyde embedded in paraffin. MDP-containing fragments were stained as described (35). Serial 7-μM microtome sections were mounted on superfrost slides and incubated with monoclonal mouse anti-MDP (MoAb 2–4, IgG2a) at a concentration of 0.2 μg/ml for 60 min at room temperature followed by biotinylated mouse antimurine IgG for 60 min. Slides were stained with avidin peroxidase, developed with diaminobenzidin (DAB), and counterstained with hematoxylin. As control, sections were stained with irrelevant primary isotype-specific IgG2a (Dako, X0943).
Histological Analysis.
Whole knee joints were removed and fixed in 4% formaldehyde for 5 days before decalcification in 5% formic acid and processing for paraffin embedding. Tissue sections (7 μm) were stained either with haematoxylin/eosin (cell influx) or saffranin O (cartilage PG loss). Histopathological changes in the knee joints were scored in the patella/femur region on five semiserial sections, spaced 70 μm apart. Scoring was performed by using the following parameters: in the haematoxylin/eosin-stained slides, the amount of cells infiltrating the synovial lining and the joint cavity was scored from 0 to 3. PG depletion was scored in the safranin O-stained slides on a scale from 0 to 3, ranging from stained cartilage to fully destained cartilage (33).
Stimulation of HEK293-Nod1-, HEK293-Nod2- Reporter Cells, and Peritoneal Macrophages by SCW Fragments.
HEK293 cells were cultured and cotransfected with 75 μg of NF-κB–luciferase reporter and Nod1 or Nod2 expression plasmid. Transfected HEK293 cells were exposed for 24 h to a dose range of SCW fragments, or a positive control and luciferase activity was determined (see SI Text).
Genotyping of Nod1 Variants.
DNA was isolated according the standard procedure by using Nucleospin Blood Quick Pure kit (Bioke). PCR amplification of Nod1 gene fragments bearing the insertion-deletion polymorphism (ND1 + 32656) was performed in a 5-μl reaction volume containing 2.5–10 μg of DNA template (22). The insertion-deletion (ND1 + 32656) polymorphism was analyzed by conventional PCR, by using primers and conditions described in ref. 22. We selected one homozygote and three heterozygote individuals for the insertion-deletion polymorphism that were further used for cytokine studies. As control, four wild-type controls were included in the study.
Genotyping of Nod2 Variants.
PCR amplification of Nod2 gene fragments containing the polymorphic site 3020insC was performed as described (5).
Isolation of Mononuclear Cells and Stimulation of Cytokine Production.
Isolation of PBMCs was performed as described (5); see SI Text.
In Vitro Exposure of RA Synovial Tissue to Nod1 or Nod2 ligands.
Synovial tissue biopsies (3 mm in diameter) of RA patients were cultured for 24 h in medium alone, MDP (1 μg/ml), or FK156 (1 μg/ml) or LPS (100 ng/ml). Thereafter, production of TNFα, IL-1β, IL-6, and IL-10 was determined by ELISA. Three biopsies per ligand and two RA patients were included in this set of experiments.
Statistical Analysis.
The data are expressed as mean ± SD unless mentioned otherwise. Differences between experimental groups were tested by using the Mann–Whitney U test performed on GraphPad Prism 4.0 software (GraphPad). P values of ≤0.05 were considered significant.
Supplementary Material
Acknowledgments.
M. M. A. Helsen and Birgitte Oppers-Walgreen are acknowledged for support in in vivo studies and histological analysis. This work was supported by the Dutch Arthritis Association (Grants 03-1-301 and 06-1-301). M.G.N. was supported by a VIDI Grant from the Netherlands Organization for Scientific Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710445105/DCSupplemental.
References
- 1.Inohara N, Chamaillard M, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 2.Kufer TA, Fritz JH, Philpott DJ. NACHT-LRR proteins (NLRs) in bacterial infection and immunity. Trends Microbiol. 2005;13:381–388. doi: 10.1016/j.tim.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Girardin, et al. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 4.Girardin SE, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 5.Netea MG, et al. Nod2 modulates specific Toll-like receptor pathways for the induction of cytokine release. J Immunol. 2005;174:6518–6523. doi: 10.4049/jimmunol.174.10.6518. [DOI] [PubMed] [Google Scholar]
- 6.van Heel DA, et al. Association of Nod2 leucine-rich repeat variants with susceptibility to Crohn's disease. Lancet. 2005;365:1794–1796. [Google Scholar]
- 7.Hugot J-P, et al. Muramyl dipeptide and toll-like receptor sensitivity in Nod2-associated Crohn's disease. Nature. 2001;411:599–603. doi: 10.1016/S0140-6736(05)66582-8. [DOI] [PubMed] [Google Scholar]
- 8.Ogura Y, et al. A frameshift mutation in Nod2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 9.Netea MG, et al. Nod2 mediates induction of the antiinflammatory signals induced by TLR2-ligands: implications for Crohn's disease. Eur J Immunol. 2004;34:2052–2059. doi: 10.1002/eji.200425229. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi KS, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe T, Kitani A, Murray PJ, Strober W. Nod2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–808. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 12.Maeda S, et al. Nod2 mutation in Crohn's disease potentiates NF-κB activity and IL-1beta processing. Science. 2005;307:734–738. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 13.Andreakos E, Sacre S, Foxwell BM, Feldmann M. The toll-like receptor-nuclear factor kappaB pathway in rheumatoid arthritis. Front Biosci. 2005;10:2478–2488. doi: 10.2741/1712. [DOI] [PubMed] [Google Scholar]
- 14.Corr M. The tolls of arthritis. Arthritis Rheum. 2005;52:2233–2236. doi: 10.1002/art.21214. [DOI] [PubMed] [Google Scholar]
- 15.Roelofs MF, et al. The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 2005;52:2313–2322. doi: 10.1002/art.21278. [DOI] [PubMed] [Google Scholar]
- 16.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 17.Van der Heijden IM, et al. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum. 2000;43:593–598. doi: 10.1002/1529-0131(200003)43:3<593::AID-ANR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Joosten LAB, et al. Toll-like receptor 2 pathway drives streptococcal cell wall-induced joint inflammation: critical role of myeloid differentiation factor 88. J Immunol. 2003;171:6145–6153. doi: 10.4049/jimmunol.171.11.6145. [DOI] [PubMed] [Google Scholar]
- 19.Opitz B, et al. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J Biol Chem. 2004;35:36426–36432. doi: 10.1074/jbc.M403861200. [DOI] [PubMed] [Google Scholar]
- 20.Kuiper S, et al. Different roles of tumour necrosis factor alpha and interleukin 1 in murine streptococcal cell wall arthritis. Cytokine. 1998;10:690–702. doi: 10.1006/cyto.1998.0372. [DOI] [PubMed] [Google Scholar]
- 21.Netea MG, et al. The frameshift mutation in Nod2 results in unresponsiveness not only to Nod2- but also Nod1-activating peptidoglycan agonists. J Biol Chem. 2005;280:35859–35867. doi: 10.1074/jbc.M504924200. [DOI] [PubMed] [Google Scholar]
- 22.Hysi P, et al. Nod1 variation, immunoglobulin E and asthma. Hum Mol Genet. 2005;14:935–941. doi: 10.1093/hmg/ddi087. [DOI] [PubMed] [Google Scholar]
- 23.Damiano JS, Oliveira V, Welsh K, Reed JC. Heterotypic interactions among NACHT domains: implications for regulation of innate immune responses. Biochem J. 2004;381:213–219. doi: 10.1042/BJ20031506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Netea MG, et al. Non-LPS components of Chlamydia pneumoniae stimulate cytokine production through Toll-like receptor 2-dependent pathways. Eur J Immunol. 2002;32:1188–1195. doi: 10.1002/1521-4141(200204)32:4<1188::AID-IMMU1188>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 25.Means TK, et al. Human Toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–3927. [PubMed] [Google Scholar]
- 26.Opitz B, et al. Nod1-mediated endothelial cell activation by Chlamydophila pneumoniae. Circ Res. 2005;96:319–326. doi: 10.1161/01.RES.0000155721.83594.2c. [DOI] [PubMed] [Google Scholar]
- 27.Welter-Stahl L, et al. Stimulation of the cytosolic receptor for peptidoglycan, Nod1, by infection with Chlamydia trachomatis or Chlamydia muridarum. Cell Microbiol. 2006;8:1047–1057. doi: 10.1111/j.1462-5822.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- 28.Ferwerda G, et al. Nod2 and Toll-Like Receptors are two non-redundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 2005;1:279–285. doi: 10.1371/journal.ppat.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masumoto J, et al. Nod1 acts as an intracellular receptor to stimulate chemokine production and neutrophil recruitment in vivo. J Exp Med. 2006;203:203–213. doi: 10.1084/jem.20051229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Beelen AJ, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:1–10. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Cromartie WJ, Craddock JG, Schwab JH, Anderle SK, Yang C. Arthritis in rats after systemic injection of streptococcal cell or cell wall. J Exp Med. 1977;146:1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Netea MG, et al. Low-density lipoprotein receptor-deficient mice are protected against lethal endotoxemia and severe gram-negative infections. J Clin Invest. 1996;97:1366–1372. doi: 10.1172/JCI118556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joosten LAB, et al. An IFN-gamma-independent proinflammatory role of IL-18 in murine streptococcal cell wall arthritis. J Immunol. 2000;165:6553–6558. doi: 10.4049/jimmunol.165.11.6553. [DOI] [PubMed] [Google Scholar]
- 34.Joosten LAB, et al. Association of interleukin-18 expression with enhanced levels of both interleukin-1beta and tumour necrosis factor alpha in knee synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:339–347. doi: 10.1002/art.10814. [DOI] [PubMed] [Google Scholar]
- 35.Hoijer MA, Melief MJ, van Helden-Meeuwsen CG, Eulderink F, Hazenberg MP. Detection of muramic acid in a carbohydrate fraction of human spleen. Infect Immun. 1995;63:1652–1657. doi: 10.1128/iai.63.5.1652-1657.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.