Abstract
Apoptotic cells must be rapidly eliminated to avoid harmful inflammatory and autoimmune reactions. Innate immunity is designed/poised to identify dying cells by their unique surface-associated molecular patterns. Here we demonstrate for the first time, to our knowledge, that the human complement protein properdin binds to early apoptotic T cells and initiates complement activation, leading to C3b opsonization and ingestion by phagocytic cells. Properdin binding was facilitated by the glycosaminoglycan chains of surface proteoglycans. Properdin released by activated neutrophils was particularly effective at recognition of apoptotic T cells, whereas the binding activity of properdin in the serum appeared to be inhibited. “Properdin tagging” of apoptotic T cells also induced their uptake by phagocytes independent of complement activation or other complement proteins. Although our findings were made primarily with apoptotic T cells, they suggest that properdin could play a similar role during apoptosis of other cell types.
Keywords: apoptosis, glycosaminoglycan
Apoptosis (programmed cell death) is a fundamental biological process in which harmful or obsolete cells are safely eliminated (1–4). Apoptosis plays critical roles in morphogenesis, cellular homeostasis, regulation of cellular immunity, and the removal of injured and virus-infected cells (1, 3). The prompt clearance of cells at an early stage of apoptosis is essential to avoid the harmful inflammatory and autoimmune reactions that occur when cell integrity is breached during late apoptosis and secondary necrosis (5).
Several pathways ensure the rapid recognition of apoptotic cells (6). During apoptosis, cells express new surface proteins and undergo reorientation of membrane phospholipids resulting in the surface exposure of phosphatidylserine (7, 8). Phagocytes harbor receptors that can interact either directly with the altered apoptotic cell surface (8–10) or via receptors for soluble proteins that bind to apoptotic cells (5, 11, 12). Engagement of phagocyte receptors through these direct or indirect mechanisms induces the uptake of bound apoptotic cells (5, 6).
The complement system quickly identifies potentially harmful targets such as invading pathogens or infected cells, marking them for destruction or removal via opsonization with its major effector molecule, C3b (13, 14). It is therefore not surprising that complement also participates in the recognition of apoptotic cells (5, 12, 15, 16). Complement activation can be triggered via three pathways, the classical pathway (CP), the lectin pathway (LP), and the alternative pathway (AP), each culminating in the formation of the surface-bound C3 convertases and C3b opsonization. According to the current paradigm, recognition of apoptotic cells is initiated through the CP and/or LP, which in turn activates the AP (12, 15, 17–19).
In the longstanding model for the initiation of the AP on target surfaces, nascent C3b generated by the slow fluid phase activation of C3 covalently attaches nonspecifically to nearby targets and forms a starting point for the subsequent assembly of C3bBb, a functional but relatively unstable C3 convertase (13). C3bBb then associates with the serum protein properdin, which stabilizes the complex 5- to 10-fold (20) [supporting information (SI) Fig. S1a]. Recently we described a second mechanism for the assembly of C3bBbP: We showed that properdin could bind certain microbial targets and serve directly as a platform for C3bBbP assembly and function (21, 22) (Fig. S1b). This observation suggested to us that properdin might play a broader role in immune surveillance, and so we asked whether properdin can direct AP activation to other potentially harmful biological targets. In this study we show that human properdin recognizes dying T cells via specific surface proteoglycans and mediates their opsonization and phagocytosis.
Results
Properdin Binds Human Apoptotic CD4+ T Cells.
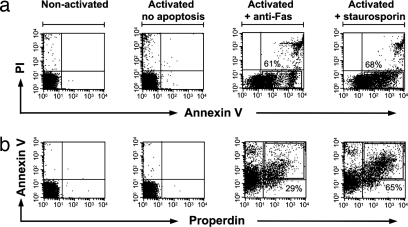
We set out to determine whether properdin recognizes apoptotic cells. To that end we generated three populations of T cells (Fig. 1): (i) primary CD 4+ T cells, isolated directly from blood samples obtained from healthy donors; (ii) activated T cells, derived from primary CD4+ T cells treated with anti-CD3/anti-CD28 mAbs; and (iii) apoptotic T cells, obtained by treating the activated CD4+ T cells further with either anti-Fas mAb or staurosporin and monitoring their apoptotic/necrotic stage via annexin V and propidium iodide (PI) staining (Fig. 1a). We incubated the CD4+ T cell populations with purified properdin at physiological concentrations (5–10 μg/ml) (23). Although purified properdin bound the apoptotic T cells, it bound neither activated (nonapoptotic) nor resting T cells (Fig. 1b), nor did it bind any cells within the freshly purified peripheral blood mononuclear cell (PBMC) populations of healthy donors (data not shown). Properdin binding capacity correlated with annexin V binding capacity, except in the small fraction of late apoptotic/necrotic cells where properdin binding appeared absent. This suggests that properdin might preferentially recognize early and intermediate apoptotic cells.
Fig. 1.
Properdin binds to apoptotic human CD4+ T cells. (a) Annexin V and PI staining of nonactivated T cells, activated T cells, and apoptotic T cells (anti-Fas or staurosporin-induced). (b) FACS analysis of properdin bound to T cell populations after their incubation with purified properdin (5 μg/ml in media). Numbers adjacent to the boxes indicate the percentage of annexin V-positive cells (a) or properdin-positive cells within the annexin V-positive population (b). Results shown are representative of five independent experiments.
Properdin Bound to Apoptotic T Cells Initiates C3b Deposition.
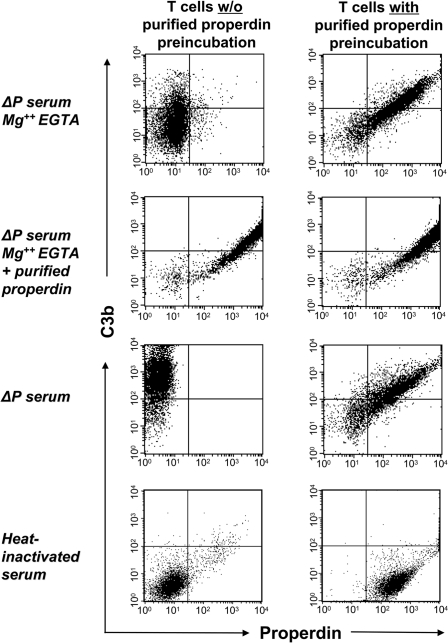
We next assessed whether properdin bound to dying T cells initiates C3b deposition. Early apoptotic T cells were pretreated with purified properdin and incubated with properdin-deficient human serum in the presence of Mg-EGTA, which chelates the calcium required for the classical and lectin pathways but permits the alternative pathway, which requires magnesium. Subsequent analysis by flow cytometry revealed prominent C3b deposition that correlated with the amount of prebound properdin (Fig. 2Right, uppermost panel) and 10-fold greater than that attained by cells that had not been pretreated with properdin [mean fluorescence intensity (MFI) 320 ± 45 versus MFI 35 ± 5] (Fig. 2 Left, uppermost panel). Properdin-mediated C3b deposition achieved maximal levels in 2 min (data not shown). As expected, C3b deposition was also promoted by properdin-deficient serum reconstituted with fluid phase properdin (Fig. 2, second panel) or when calcium was provided to permit the CP and LP to function (Fig. 2, third panel). C3b deposition was negligible when cells were incubated with heat-inactivated (complement-deficient) serum (Fig. 2, lowermost panel). These results demonstrate that properdin that is bound to apoptotic T cells can directly initiate AP activation.
Fig. 2.
Properdin binding to apoptotic T cells initiates C3b deposition. Apoptotic T cells were either left untreated (Left) or preincubated with 5 μg/ml purified properdin (Right). Cells were washed and then incubated for 15 min with 15% properdin-deficient (ΔP) serum diluted in Mg2+ EGTA, ΔP serum diluted in Mg2+ EGTA and reconstituted with purified properdin, 15% ΔP serum diluted in PBS, or 15% heat-inactivated human serum diluted in PBS. Deposition of C3b on apoptotic T cells was measured by FACS analysis (with the following MFIs: uppermost panel in Left, 35 ± 5; uppermost panel in Right, 320 ± 45; second panel in Left, 720 ± 25; second panel in Right, 710 ± 40; third panel in Left, 850 ± 50; third panel in Right, 420 ± 40; lowermost panels in Left and Right, <10). Gates were set at the complete apoptotic cell population, excluding only viable cells. Results shown are representative of three independent experiments.
Properdin Bridges the Association of Apoptotic T Cells and Phagocytes.
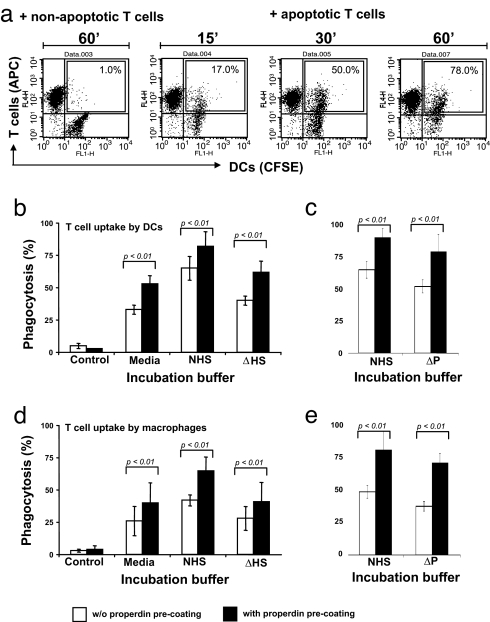
We next determined whether properdin-directed C3b opsonization of apoptotic T cells promotes their ingestion by phagocytes. To that end, T cells prelabeled with allophycocyanin (APC)-conjugated anti-CD4 mAb were incubated in 15% normal human serum with mature confluent CFSE-labeled dendritic cells (DCs) that had been grown in microtiter wells. Noningested T cells were removed by washing, and the DCs were detached with trypsin/EDTA and analyzed by flow cytometry. Using this experimental design, CFSE+ DCs that had ingested the APC+ apoptotic T cells would now be detected as CFSE+/APC+. After 60 min, ≈80% of the DCs incubated with apoptotic T cells had become APC+. In contrast, <5% of the DCs incubated with control nonapoptotic T cells were APC+ (Fig. 3 a and b). As expected, maximal uptake of apoptotic T cells by DCs was dependent on the presence of normal human serum. Uptake was diminished to 65% when cells were incubated in the presence of heat-inactivated (complement-deficient) serum and to 55% in serum-free media (Fig. 3b). Performing the phagocytosis assays in properdin-depleted serum decreased uptake to 80% compared with normal human serum (Fig. 3c). Pretreatment of apoptotic T cells with properdin enhanced their association with the phagocytes under each of the analyzed conditions: in media alone and in the presence of normal human serum, heat-inactivated serum, and properdin-depleted serum (Fig. 3 b and c). Comparable results were obtained by using immature DCs (data not shown) or macrophages in place of mature DCs (Fig. 3 d and e). Properdin-dependent uptake was not expected in the absence of serum components. Maximum uptake in serum-free media occurred between 15 and 30 min of incubation (Fig. S2 a and b), and at 30 min T cells, DCs, and macrophages had not stained positively for C3b, thus excluding the possible participation of C3 that might have been synthesized by the phagocytes (data not shown). These findings suggest most simply that macrophages/DCs can recognize properdin-tagged targets without C3b receptor (CR3) engagement (17). This possibility is consistent with our subsequent observation that differentiated macrophages and DCs can bind purified properdin (Fig. S3a).
Fig. 3.
Quantification of properdin-mediated uptake of apoptotic T cells by macrophages and DCs. (a) FACS-based assay for the measurement of ingested T cells. DCs were grown to confluence and labeled with CFSE. Apoptotic T cells were labeled with an APC-conjugated anti-CD4 mAb and added to the DCs in media containing 15% NHS. At indicated time points, nonbound/ingested T cells were aspirated and DCs were detached. The percentage of CFSE/APC-positive DCs (CFSE-labeled DCs that ingested apoptotic APC-labeled T cells during the incubation period) was determined by FACS analysis and is shown within the boxes. (b and c) Properdin increases uptake of apoptotic T cells by DCs. Apoptotic, APC-labeled T cells were either incubated with purified properdin (5 μg/ml media) or left untreated. T cells were washed and then incubated with DCs in media, media with NHS, media with ΔHS (b), or media with properdin-depleted serum (ΔP) (c) for 60 min, and T cell uptake was measured by FACS. (d and e) Properdin increases uptake of apoptotic T cells by macrophages. Experiments were performed as in b and c but with macrophages in place of DCs. Shown are the mean ± SD values of three separate experiments. Statistical difference between the ingestion of T cells either with or without properdin preincubation by DCs and macrophages was P < 0.01 in all cases.
We confirmed and extended our FACS analysis with light microscopy: As above, differentiated human macrophages generated ex vivo from purified monocytes were incubated with apoptotic T cells that had been pretreated with properdin or with control untreated apoptotic T cells. After the incubation, noningested T cells were removed by washing and the remaining cells were examined by light microscopy (Fig. S4). As in the FACS experiments, macrophages ingested apoptotic T cells more readily than nonapoptotic control cells, uptake was largely dependent on the presence of normal human serum, and uptake was greatest when apoptotic T cells were first pretreated with properdin. Once again, pretreatment of apoptotic T cells with properdin alone enhanced their phagocytosis even in the absence of complement activation.
Properdin Binds Nonapoptotic Malignant T Cells.
Given that properdin binds apoptotic T cells, we speculated that properdin might recognize other potentially dangerous T cells. We examined the binding activity of properdin to Jurkat cells, a derivative of a human T cell leukemia line. Indeed, purified properdin binds nonapoptotic Jurkat cells (Fig. S5a). In contrast, neither C1q nor MBL, two other complement proteins involved in the recognition of apoptotic cells, recognizes the malignant T cell line (Fig. S5a). Pretreatment of Jurkat cells with properdin before incubation in properdin-depleted serum led to a >20-fold increase in AP-dependent C3b deposition over nonpretreated control cells (Fig. S5b). Properdin also bound nonapoptotic HUT 78 cells (a T cell leukemia derivative) but not nonapoptotic Raji cells (a B cell Burkett's lymphoma derivative) (data not shown).
Properdin Binds Glycosaminoglycan (GAG) Chains Associated with Cell Surface Proteoglycans.
Previous studies have shown that properdin can bind heparin, a prevalent GAG (24). Because protein:GAG conjugates (proteoglycans) are present on most mammalian cells and can direct protein:surface interactions through their specific GAG structures (25), we hypothesized that properdin might bind apoptotic T cells via surface proteoglycans.
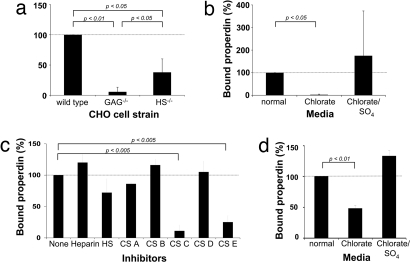
Wild-type and GAG-defective type Chinese hamster ovary (CHO) cells have been used extensively to study protein:GAG interactions (26). We observed that properdin binds wild-type CHO cells, known to harbor both heparan sulfate (HS) and chondroitin sulfate (CS) surface proteoglycans (Fig. 4a). Average properdin binding was decreased to 38 ± 22%) of the wild type in CHO cells deficient in HS (26) and to 6 ± 8% of wild type in CHO cells deficient in both HS and CS GAGs (Fig. 4a) (27). These observations indicate that properdin can bind CHO cells via both HS and CS chains. Additional experiments showed that the recognition of CHO cells by properdin depends on the presence of sulfate groups within the GAG chains: Properdin did not bind to cells pretreated with sodium chlorate, conditions that reduce GAG sulfation (28). Properdin binding was recovered, however, if cells were pretreated with both sodium chlorate and sodium sulfate, conditions that permit GAG sulfation (Fig. 4b).
Fig. 4.
Sulfated GAGs mediate the binding of properdin to apoptotic T cells. (a) Wild-type, GAG-deficient, and HS-deficient CHO cells were incubated with purified properdin and bound properdin measured via FACS. (b) Wild-type CHO cells were cultured in chlorate-containing media to prevent sulfation of GAGs and then analyzed for their ability to bind properdin. The addition of sodium sulfate (SO4) reversed the inhibitory effect of chlorate on GAG sulfation. (c) Apoptotic T cells were preincubated at 20 μg/ml in buffer/media with heparin (predominantly alternating IdoA2S-GlnNS6S), HS (an undersulfated form of heparin), CS-A (chondroitin sulfate-A; GlcA-GalNAc4S), CS-B (dermatan sulfate; IdoA-GalNAc), CS-C (GlcA-GalNAc6S), CS-D (GlcA2S-GalNAc6S), or CS-E (GlcA-GalNAc4, 6S). Purified properdin was then added to all samples, and properdin deposition on the T cell surface was assessed. (d) T cells were activated in normal media or media containing chlorate or chlorate and SO4, and deposition of purified properdin was measured after apoptosis induction. All values shown are the mean ± SD values derived from a minimum of three separate experiments.
We next examined whether properdin also recognizes apoptotic T cells via GAGs. Because GAG mutations were not available for the human T cell system, we first examined the effects of a variety of fluid phase GAGs on properdin:cell interactions. Apoptotic T cells were preincubated with the indicated fluid phase GAGs, properdin was then added, and the cells were analyzed for properdin deposition. HS, a partially sulfated GAG composed primarily of alternating IdoA2S and GlnNS6S, reduced properdin binding to the T cells by 30 ± 8%, chondroitin C (CS-C, GlcA-GalNAc6S) reduced binding by 75 ± 12%, and CS-E (GlcA-GalNAc4, 6S) showed the highest impact, reducing properdin binding by 85 ± 2% (Fig. 4c). These results indicated that properdin recognized apoptotic T cells via GAG-binding site(s) and suggested that certain GAG sulfate groups may be involved. Thus, we treated apoptotic T cells with chlorate to prevent/decrease GAG sulfation. As with the case of CHO cells, binding of properdin to the surface of apoptotic T cells was decreased >50% by the treatment (Fig. 4d).
We also examined the effects of soluble GAGs on the binding of properdin to Jurkat cells, DCs, and macrophages. In the case of Jurkat cells, properdin binding was inhibited 88 ± 8% by heparin (a highly sulfated form of HS), 84 ± 12% by CS-C, and 97 ± 2% by CS-E (Fig. S5c), but not inhibited by HS. In the case of the phagocytes, preincubation of immature or mature DCs with CS-C and CS-E together decreased properdin binding from ≈85 ± 3% to 5 ± 2% (Fig. S3b). Similar results were obtained when DCs were incubated with either CS-C or CS-E alone, indicating that these GAGs have comparable inhibitory properties (data not shown). Preincubation of macrophages with CS-C and/or CS-E, however, did not reduce properdin binding (Fig. S3b). Accordingly, the addition of CS-C and CS-E during the incubation of DCs with properdin-covered apoptotic T cells abrogated T cell phagocytosis, whereas the GAGs had no inhibitory effect on the phagocytosis of apoptotic T cells by macrophages (Fig. S3c).
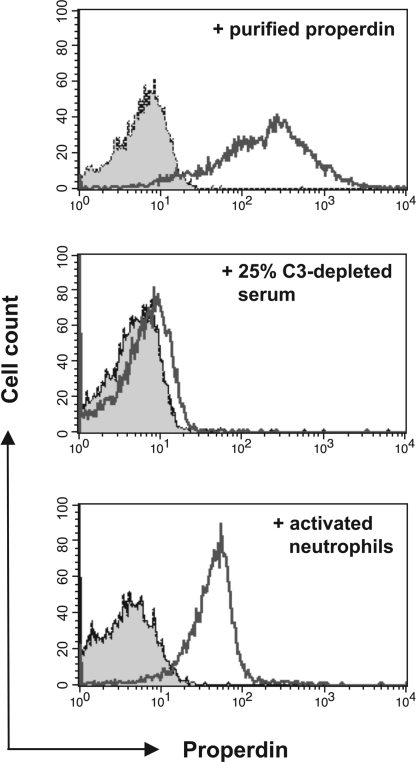
Properdin Newly Released from Neutrophils Binds Apoptotic T Cells.
Although properdin is found in plasma (23), it is also stored in neutrophil granules and rapidly released upon cell stimulation (29). It has been proposed that the release of properdin from neutrophils is a major determinant of local AP activity (30). We therefore used apoptotic T cells to compare the target-binding activity of endogenous serum properdin to that of properdin released from activated neutrophils.
We incubated apoptotic T cells with C3-depleted serum, which was used to prevent properdin binding to preformed surface-deposited C3b. Surprisingly, we saw only minimal properdin binding under these conditions (Fig. 5 Top and Middle). We did observe properdin bound to the apoptotic T cells in a parallel control experiment wherein the cells were pretreated with purified properdin before serum exposure (data not shown). Thus, we conclude that properdin circulating in serum does not freely bind to apoptotic T cells. In contrast, we readily detected properdin bound to apoptotic T cells that had been incubated with freshly activating neutrophils (Fig. 5 Bottom). Importantly, properdin binding was independent of complement activation because concomitant C3b deposition on either the T cells or neutrophils after their coincubation was not observed (data not shown). We detected only minimal properdin deposited on nonapoptotic T cells incubated with activated neutrophils or on apoptotic T cells that had been incubated with nonactivated neutrophils (data not shown). These results indicate that properdin freshly expelled by degranulating neutrophils freely binds apoptotic T cells whereas endogenous serum properdin is subject to stringent regulation.
Fig. 5.
Neutrophil-derived properdin binds to apoptotic T cells. (Top) FACS analysis of properdin bound to apoptotic T cells after their incubation with purified properdin. (Middle) Measurement of bound properdin on apoptotic T cells after their incubation for 15 min with 25% C3-depleted human serum diluted with PBS. (Bottom) Analysis of properdin bound to apoptotic T cells after their incubation with activated neutrophils. TNF-α (100 nM) was added to freshly isolated neutrophils mixed with apoptotic T cells, and cell mixtures were incubated for 15 min at 37°C in RPMI medium 1640. Cell mixtures were analyzed for deposition of properdin on T cells by FACS. The shaded histograms depict staining with the isotype control mAb. Bound properdin is depicted by the nonshaded histograms. All datasets shown are one representative result of at least three independently performed experiments. Activation of neutrophils with PMA or GM-CSF resulted in comparable amounts of properdin deposition on apoptotic T cells (data not shown).
Discussion
Several pathways ensure the rapid recognition of apoptotic cells (6). According to the current paradigm, complement-dependent recognition of apoptotic cells is initiated through the CP and/or LP, which in turn activates the AP (12, 15, 17–19). We describe here experiments that demonstrate that properdin binds apoptotic T cells, promoting AP activation and phagocytosis. Properdin recognition was cell stage-specific: it bound neither resting T cells nor nonapoptotic activated T cells. In addition, properdin did not bind to any cells within the PBMC populations freshly isolated from blood or to red blood cells (data not shown and ref. 22). Properdin binding was most marked in early apoptotic T cells and reduced upon secondary necrosis (Fig. S6). We also present evidence that recognition involved the binding of properdin to HS and/or CS chains of surface proteoglycans although additional interactions were not precluded.
Surface-bound properdin directed the opsonization of apoptotic T cells with C3 activation fragments and uptake by macrophages and DCs. Although maximum uptake depended on complement activation, we also found, unexpectedly, that properdin bound to apoptotic T cells promoted their uptake in the absence of complement activation or other serum proteins. These results, together with our observation that differentiated macrophages and DCs can bind properdin (Fig. S4), strongly suggest that properdin:target complexes can direct phagocytosis without further complement involvement. Thus, we envision two possible mechanisms by which properdin could promote phagocytosis of apoptotic T cells: In the first case, properdin binds to apoptotic T cells, initiates AP-mediated C3b deposition, and promotes cell uptake by CR3-bearing phagocytes (17, 31). In the second case, properdin binds to apoptotic T cells and mediates contact with phagocytes directly. In this latter scenario, properdin functions as a bridging protein. Properdin:T cell and properdin:DC interactions could be directly mediated by surface GAGs. Although our findings were made only with apoptotic T cells, they suggest to us that properdin could play a similar role during apoptosis of other cell types.
Apoptotic cells must be rapidly eliminated to avoid harmful inflammatory and autoimmune reactions. We have shown that properdin can identify apoptotic T cells and facilitate their clearance. Although no published studies have examined clearance of apoptotic cells in these properdin-deficient individuals, and only isolated reports have suggested a possible relationship between properdin deficiency and autoimmune disease (32, 33), other pathways can also promote the clearance of apoptotic cells (6) and so in some cases the lack of properdin may not preclude the safe clearance of apoptotic cells. We have also observed that properdin binds malignant T cell lines. This could indicate that properdin deficiency or dysfunction could potentially be a risk factor in the development of certain T cell malignancies. Interestingly, somatic mutations of the properdin gene have been linked to some breast tumors (34).
Properdin occurs in dimer, trimer, or tetramer arrangements of a 53-kDa subunit (35), which is in turn composed of six thrombospondin repeats (TSRs) (36). TSRs in thrombospondin, TRAP, and other proteins harbor heparin-binding sites (37). Structural analyses of several TSRs have revealed a possible GAG “recognition face” composed of disulfide-stabilized alternating tryptophan and arginine side chains (37, 38). The arginine side chains are ≈9 Å apart, which matches the length of the GAG:disaccharide repeat. Thus, it has been proposed that TSR:GAG interactions are facilitated by electrostatic bonds between the TSR arginine pairs and GAG sulfate pairs (38). The key amino acids in this model have been conserved in five of the six properdin TSRs, and so one or more similar GAG recognition faces could facilitate properdin:GAG interactions. CS-C (GlcA-GalNAc6S) and CS-E (GlcA-GalNAc4, 6S) inhibited properdin interactions with apoptotic and malignant T cells and with DCs. Their common sulfate moieties at the carbon 6 position of GalNAc may play an important role in recognition. CS-D (GlcA2S-GalNAc6S) also has a sulfate at the GalNAc carbon 6 position but did not affect properdin binding, possibly because of interference from its unique GlcA carbon 2 sulfate.
Complement activation is controlled in part by serum inhibitors that function to protect self-tissue from complement-mediated damage: factor H regulates AP activation, and C4b-binding protein regulates CP and LP activation (13). We observed that apoptotic T cells strongly bind properdin purified from serum as well as properdin freshly released from neutrophils, but they bind poorly the properdin present in unfractionated serum (Fig. 5). This observation indeed suggests stringent serum-level regulation of properdin:target recognition. We have shown that soluble/fluid phase GAGs, especially certain chondroitin sulfates, can inhibit binding of properdin to apoptotic cells (Fig. 4c). It is feasible that free or protein-associated GAGs in circulation suppress systemic properdin:target interactions and thereby regulate/control properdin-induced complement activation. By this view, properdin-dependent recognition of apoptotic cells/targets is mediated largely by neutrophil-derived properdin, whereas the properdin in the plasma functions primarily to stabilize C3 convertases.
The innate immune system constantly monitors a spectrum of biological agents including those of self, altered self, and non-self. It distinguishes those that are dangerous from those that are benign and initiates appropriate immunogenic or tolerogenic responses, often mediated by adaptive immunity (39, 40). Potential targets are recognized on the basis of their characteristic surface-level molecular patterns. In this study, we show for the first time, to our knowledge, that properdin recognizes apoptotic and malignant T cells (altered self) and CHO cells (non-self) but neither resting T cells nor resting B cells, and we have previously observed that properdin does not recognize human red blood cells (22). Thus, properdin appears to distinguish certain harmful cells. We also show here that GAGs associated with surface glycoproteins facilitate properdin:cell contact. Thus, specific GAG structures could direct properdin:cell recognition.
Methods
Media, Cell Lines, Antibodies, Additional proteins, and Inhibitors.
Media, cell lines, sera, antibodies, and proteins used are listed in SI Methods.
T Cell Isolation and Activation.
Blood samples were obtained from healthy volunteers by using protocols approved by the Washington University Human Studies Committee. CD4+ T cells were purified from whole blood as previously described using the human CD4+ MicroBeads from Miltenyi Biotec (41). The purity of the obtained T cell population was consistently >97%. In vitro stimulation of isolated CD4+ T cells was performed in 96-well culture plates coated with mAbs to CD3 (1 μg/ml) and CD28 (1 μg/ml). The wells were washed, and purified CD4+ lymphocytes (1.5–2.0 × 105 cells per well) were added in 100 μl of culture medium supplemented with 25 units/ml recombinant human IL-2. Cells were cultured at 37°C in 5% CO2 for 2–3 days and used in subsequent assays with or without induction of apoptosis or necrosis.
Neutrophil Isolation and Activation/Induction of Properdin Release.
Human neutrophils were isolated as previously described (29). Briefly, freshly drawn blood was separated using a Ficoll gradient. The neutrophil-containing cell pellet was resuspended in room temperature Hanks' buffered saline solution (HBSS), mixed with an equal volume of 3% Dextran (Sigma–Aldrich) in 0.9% saline solution, and incubated for 20 min at 37°C to allow neutrophils to segregate to the surface. The top 2–3 ml of the solution was then collected, and neutrophils were pelleted through centrifugation at 250 × g for 6 min at 4°C. From here on, cells and solutions were kept on ice. Neutrophils were depleted of contaminating erythrocytes through hypotonic lysis and then resuspended in PBS containing 0.2% glucose. Activation of neutrophils and release of properdin from granules was induced through the addition of 30 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma), 100 ng/ml TNF-α (BioSource International), or 10 ng/ml GM-CSF.
Generation of DCs and Macrophages.
Monocytes were isolated from PBMCs via positive selection by using human CD14 MicroBeads (Miltenyi Biotec) and immature or mature DCs generated as previously described via culture in media supplemented with 10% FCS (HyClone), GM-CSF, and IL-4 (41). Maturation status of DCs was monitored via analyzing for the expected CD14, CD80, CD86, and HLA-DR expression profile. Cultures contained consistently >95% of DCs with the desired maturation level. Macrophages were generated by incubating freshly purified PBMCs in RPMI medium 1640 in 24- or 48-well plates for 2 h at 37°C. Nonadherent cells were washed away, and adherent enriched monocytes were differentiated into macrophages by culture in media containing 50 ng/ml recombinant human M-CSF for 5–7 days. Media was exchanged every other day. The percentage of differentiated macrophages in the culture was assessed by measuring CD220 expression and was >90% each time.
Induction and Detection of Apoptosis and Necrosis.
Apoptosis was induced in activated T cells through the addition of 10 μg/ml monoclonal anti-FAS (CD95) antibody (BD Biosciences) for 12 h or through addition of 250 nM staurosporin (Sigma) for 5 h. Necrosis was induced by incubating activated T cells for 4 h in culture medium containing 10 nM H2O2 (Sigma). Monitoring of apoptosis or necrosis was performed by staining with annexin V and PI (Sigma). Cells negative for PI (MFI ≤ 15) but positive for annexin V (MFI ≥ 25) were defined as apoptotic cells. Late apoptotic and necrotic cells were identified by strong PI staining (MFI ≥ 500).
Detection of Purified Properdin on Apoptotic T Cells and CHO Cells.
Cells were washed and resuspended to 5 × 105 cells in 100 μl of PBS containing 1% FCS. Purified properdin was added to the cells to a final concentration of 5 μg/ml, and the mixture was incubated for 15 min at 37°C. Cells were then washed twice with PBS plus 1% heat-inactivated FCS, and bound properdin was detected by incubating the cells first with the biotinylated anti-properdin mAb followed by an incubation with fluorochrome-labeled streptavidin and FACS analysis.
Detection of Neutrophil-Derived Properdin on Apoptotic T Cells.
Apoptotic T cells were mixed with freshly purified autologous neutrophils at a 1:1 cell ratio (5 × 105 cells of each cell population) in 48-well plates in RPMI medium 1640. Activation of neutrophils was induced through the addition of PMA, TNF-α, or GM-CSF, and cell mixtures were incubated at 37°C. At 0, 1, 5, 10, and 30 min, reaction samples were transferred to ice and washed twice with ice-cold PBS containing 1% heat-inactivated FCS. Cell mixtures were then stained for cell surface-bound properdin and analyzed via FACS. Apoptotic T cells and neutrophils were identified by their distinct laser scatter profiles.
Phagocytosis Assay.
DCs or macrophages were grown to confluence in 24-well plates and labeled with CFSE. Apoptotic T cells, preincubated with purified properdin and then washed or left untreated, were labeled with an APC-conjugated anti-CD4 mAb and added to the phagocytes at 0.5 to 1 × 106 per well in prewarmed RPMI medium 1640 containing 15% NHS, 15% heat-inactivated human serum (ΔHS), or 15% properdin-depleted serum (ΔP). Cell mixtures were incubated at 37°C for up to 1 h. Wells containing cell mixtures were then washed several times with cold PBS to aspirate noningested T cells. To detach DCs or macrophages, cells were incubated for 20 min at 37°C in a 0.5% trypsin/5 mM EDTA solution, collected by forceful pipetting, and immediately analyzed by FACS. DCs or macrophages that had ingested T cells were identified by their positive signal for both CSFE and APC.
Statistical Analysis.
Data represent mean ± SD of at least three independent experiments using cell populations from different donors with each condition conducted in triplicate. Results were compared and analyzed for statistical significance by using the paired Student t test. Significant differences were defined as P < 0.05.
Note Added in Proof.
After this article had been accepted for publication, a report was published that describes properdin binding to late apoptotic and necrotic Jurkat cells (42). Although we conclude in the current investigation that properdin binds primarily to early apoptotic cells, our conclusion is based on studies of apoptotic and necrotic primary T cells. The apparent disparity between the two studies is, therefore, likely due to methodological differences and should not detract from the potential significance of properdin in the recognition and clearance of apoptotic cells.
Supplementary Material
Acknowledgments.
We thank Dr. Peter Densen (University of Iowa, Iowa City) for his generous gift of the properdin-deficient human serum and Dr. Wilhelm Schwaeble (University of Leicester, Leicester, UK) for purified mannose-binding lectin. We also thank Dr. Panisadee Avirutnan for helpful advice, Drs. Anja Fuchs and Christine Pham for valuable discussions of the results and their critical readings of the manuscript, and Madonna Bogacki for help with the manuscript. This work was supported by National Institutes of Health Grant AI05143 (to D.E.H.), the Sandler Program for Asthma Research, and National Institutes of Health Grant U19 AI070489.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801015105/DCSupplemental.
References
- 1.Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krammer PH. CD95's deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 3.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 4.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 6.Fadok VA, Bratton DL, Henson PM. Phagocyte receptors for apoptotic cells: Recognition, uptake, and consequences. J Clin Invest. 2001;108:957–962. doi: 10.1172/JCI14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fadok VA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 8.Fadok VA, et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 9.Ren Y, Silverstein RL, Allen J, Savill J. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J Exp Med. 1995;181:1857–1862. doi: 10.1084/jem.181.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;182:1597–1601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schagat TL, Wofford JA, Wright JR. Surfactant protein A enhances alveolar macrophage phagocytosis of apoptotic neutrophils. J Immunol. 2001;166:2727–2733. doi: 10.4049/jimmunol.166.4.2727. [DOI] [PubMed] [Google Scholar]
- 12.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volanakis JE. In: The human Complement System in Health and Disease. Volanakis JE, Frank MM, editors. New York: Marcel Dekker; 1998. pp. 9–32. [Google Scholar]
- 14.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol. 2008;6:132–142. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogden CA, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor PR, et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;7:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roos A, et al. Mini-review: A pivotal role for innate immunity in the clearance of apoptotic cells. Eur J Immunol. 2004;34:921–929. doi: 10.1002/eji.200424904. [DOI] [PubMed] [Google Scholar]
- 18.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes. J Immunol. 1997;158:4525–4528. [PubMed] [Google Scholar]
- 19.Botto M, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 20.Fearon DT, Austen KF. Properdin: Binding to C3b and stabilization of the C3b-dependent C3 convertase. J Exp Med. 1975;142:856–863. doi: 10.1084/jem.142.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hourcade D. The role of properdin in the assembly of the alternative pathway C3 convertases of complement. J Biol Chem. 2006;281:2128–2132. doi: 10.1074/jbc.M508928200. [DOI] [PubMed] [Google Scholar]
- 22.Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J Immunol. 2007;179:2600–2608. doi: 10.4049/jimmunol.179.4.2600. [DOI] [PubMed] [Google Scholar]
- 23.Nolan KF, Reid KB. Properdin. Methods Enzymol. 1993;223:35–46. doi: 10.1016/0076-6879(93)23036-m. [DOI] [PubMed] [Google Scholar]
- 24.Yu H, Munoz EM, Edens RE, Linhardt RJ. Kinetic studies on the interactions of heparin and complement proteins using surface plasmon resonance. Biochim Biophys Acta. 2005;1726:168–176. doi: 10.1016/j.bbagen.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esko JD, Selleck SB. Order out of chaos: Assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Lawrence R, Frazier BA, Esko JD. CHO glycosylation mutants: Proteoglycans. Methods Enzymol. 2006;416:205–221. doi: 10.1016/S0076-6879(06)16013-9. [DOI] [PubMed] [Google Scholar]
- 27.Esko JD, Stewart TE, Taylor WH. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci USA. 1985;82:3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baeuerle PA, Huttner WB. Chlorate—a potent inhibitor of protein sulfation in intact cells. Biochem Biophys Res Commun. 1986;141:870–877. doi: 10.1016/s0006-291x(86)80253-4. [DOI] [PubMed] [Google Scholar]
- 29.Wirthmueller U, et al. Properdin, a positive regulator of complement activation, is released from secondary granules of stimulated peripheral blood neutrophils. J Immunol. 1997;158:4444–4451. [PubMed] [Google Scholar]
- 30.Schwaeble WJ, Reid KB. Does properdin crosslink the cellular and the humoral immune response? Immunol Today. 1999;20:17–21. doi: 10.1016/s0167-5699(98)01376-0. [DOI] [PubMed] [Google Scholar]
- 31.Wright SD, Silverstein SC. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983;158:2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sjoholm AG, et al. Dysfunctional properdin in a Dutch family with meningococcal disease. N Engl J Med. 1988;319:33–37. doi: 10.1056/NEJM198807073190106. [DOI] [PubMed] [Google Scholar]
- 33.Holme ER, et al. Familial properdin deficiency associated with chronic discoid lupus erythematosus. Clin Exp Immunol. 1989;76:76–81. [PMC free article] [PubMed] [Google Scholar]
- 34.Sjoblom T, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 35.Smith CA, Pangburn MK, Vogel CW, Muller-Eberhard HJ. Molecular architecture of human properdin, a positive regulator of the alternative pathway of complement. J Biol Chem. 1984;259:4582–4588. [PubMed] [Google Scholar]
- 36.Nolan KF, et al. Molecular cloning of the cDNA coding for properdin, a positive regulator of the alternative pathway of human complement. Eur J Immunol. 1991;21:771–776. doi: 10.1002/eji.1830210333. [DOI] [PubMed] [Google Scholar]
- 37.Tossavainen H, et al. The layered fold of the TSR domain of P. falciparum TRAP contains a heparin binding site. Protein Sci. 2006;15:1760–1768. doi: 10.1110/ps.052068506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan K, et al. Crystal structure of the TSP-1 type 1 repeats: A novel layered fold and its biological implication. J Cell Biol. 2002;159:373–382. doi: 10.1083/jcb.200206062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medhzitov R, Janeway CJ. Decoding the patterns of self and non-self by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 40.Kabelitz D, Medzhitov R. Innate immunity—cross-talk with adaptive immunity through pattern recognition receptors and cytokines. Curr Opin Immunol. 2007;19:1–3. doi: 10.1016/j.coi.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 41.Barchet W, et al. Complement-induced regulatory T cells suppress T cell responses but allow for dendritic cell maturation. Blood. 2006;107:1497–1504. doi: 10.1182/blood-2005-07-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu W, et al. Properdin binds to late apoptotic and necrotic cells independently of C3b and regulates alternative pathway complement activation. J Immunol. 2008;180:7613–7621. doi: 10.4049/jimmunol.180.11.7613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.