Abstract
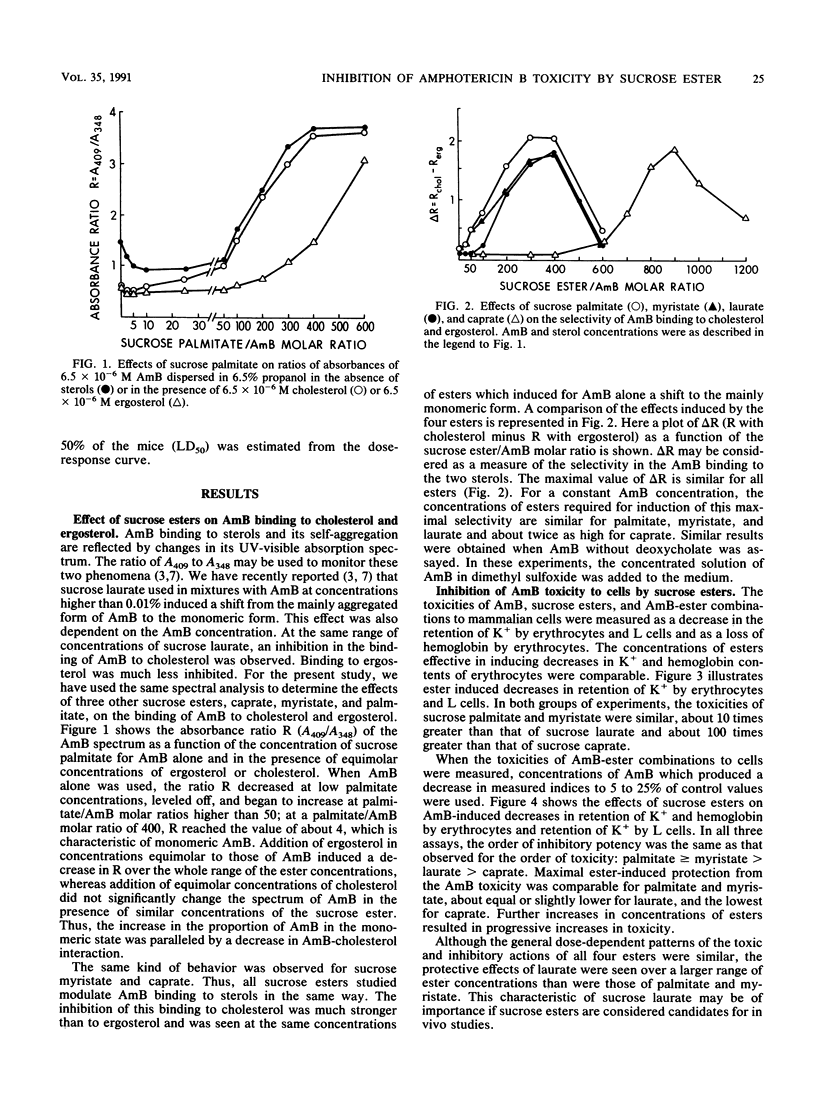
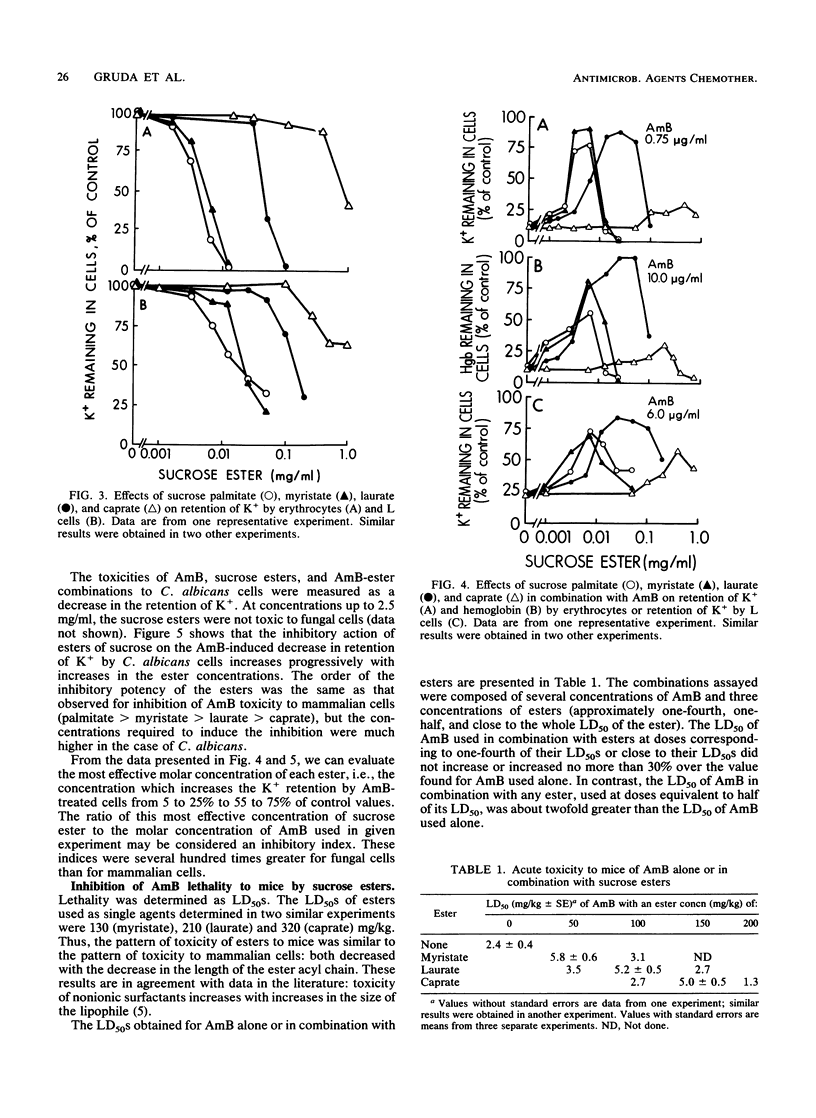
The effects of four monoesters of sucrose with different acyl chain lengths (palmitate, C16; myristate, C14; laurate, C12; and caprate, C10) on the aggregation state of amphotericin B (AmB), its binding to cholesterol and ergosterol, its toxicity to cells, and its lethality to mice were determined. In solution, all four of these esters inhibited AmB binding to cholesterol more than to ergosterol; this effect correlated with the ester-induced shift from the mainly aggregated form of AmB to the mainly monomeric form. In experiments with cells, the esters inhibited the toxicity of AmB to mouse erythrocytes and cultured mouse fibroblast L-929 cells more than its toxicity to Candida albicans cells. When injected intravenously with AmB, these esters decreased AmB lethality to mice. In all of these assays, the ester with the shortest chain length (caprate) was much less potent than the other three esters. Our results indicate a correlation between in vitro and in vivo assays and suggest that the in vitro and in vivo selectivity of AmB may be enhanced by surface-active agents which modulate the aggregation state of AmB.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagiński M., Tempczyk A., Borowski E. Comparative conformational analysis of cholesterol and ergosterol by molecular mechanics. Eur Biophys J. 1989;17(3):159–166. doi: 10.1007/BF00254770. [DOI] [PubMed] [Google Scholar]
- Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta. 1986 Dec 22;864(3-4):257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- Brajtburg J., Gruda I., Daigle I., Medoff G. Concentration dependent dual effect of the monolauryl ester of sucrose on the antifungal activity and absorption spectra of amphotericin B (Fungizone). Biochim Biophys Acta. 1989 Nov 3;985(3):307–312. doi: 10.1016/0005-2736(89)90417-3. [DOI] [PubMed] [Google Scholar]
- Davis S. S., Washington C., West P., Illum L., Liversidge G., Sternson L., Kirsh R. Lipid emulsions as drug delivery systems. Ann N Y Acad Sci. 1987;507:75–88. doi: 10.1111/j.1749-6632.1987.tb45793.x. [DOI] [PubMed] [Google Scholar]
- Ernst R., Arditti J. Biological effects of surfactants, IV. Effects of non-ionics and amphoterics on HeLa cells. Toxicology. 1980;15(3):233–242. doi: 10.1016/0300-483x(80)90056-6. [DOI] [PubMed] [Google Scholar]
- Gruda I., Dussault N. Effect of the aggregation state of amphotericin B on its interaction with ergosterol. Biochem Cell Biol. 1988 Mar;66(3):177–183. doi: 10.1139/o88-024. [DOI] [PubMed] [Google Scholar]
- Gruda I., Gauthier E., Elberg S., Brajtburg J., Medoff G. Effects of the detergent sucrose monolaurate on binding of amphotericin B to sterols and its toxicity for cells. Biochem Biophys Res Commun. 1988 Aug 15;154(3):954–958. doi: 10.1016/0006-291x(88)90232-x. [DOI] [PubMed] [Google Scholar]
- Janoff A. S., Boni L. T., Popescu M. C., Minchey S. R., Cullis P. R., Madden T. D., Taraschi T., Gruner S. M., Shyamsunder E., Tate M. W. Unusual lipid structures selectively reduce the toxicity of amphotericin B. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6122–6126. doi: 10.1073/pnas.85.16.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien S., Brajtburg J., Bolard J. Affinity of amphotericin B for phosphatidylcholine vesicles as a determinant of the in vitro cellular toxicity of liposomal preparations. Biochim Biophys Acta. 1990 Jan 15;1021(1):39–45. doi: 10.1016/0005-2736(90)90381-w. [DOI] [PubMed] [Google Scholar]
- Lamy-Freund M. T., Ferreira V. F., Schreier S. Polydispersity of aggregates formed by the polyene antibiotic amphotericin B and deoxycholate. A spin label study. Biochim Biophys Acta. 1989 Jun 6;981(2):207–212. doi: 10.1016/0005-2736(89)90030-8. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G. Liposomes in infectious diseases: present and future. Curr Clin Top Infect Dis. 1989;10:241–253. [PubMed] [Google Scholar]
- Mehta R., Lopez-Berestein G., Hopfer R., Mills K., Juliano R. L. Liposomal amphotericin B is toxic to fungal cells but not to mammalian cells. Biochim Biophys Acta. 1984 Mar 14;770(2):230–234. doi: 10.1016/0005-2736(84)90135-4. [DOI] [PubMed] [Google Scholar]
- Patterson T. F., Miniter P., Dijkstra J., Szoka F. C., Jr, Ryan J. L., Andriole V. T. Treatment of experimental invasive aspergillosis with novel amphotericin B/cholesterol-sulfate complexes. J Infect Dis. 1989 Apr;159(4):717–724. doi: 10.1093/infdis/159.4.717. [DOI] [PubMed] [Google Scholar]
- Szoka F. C., Jr, Milholland D., Barza M. Effect of lipid composition and liposome size on toxicity and in vitro fungicidal activity of liposome-intercalated amphotericin B. Antimicrob Agents Chemother. 1987 Mar;31(3):421–429. doi: 10.1128/aac.31.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]


