Abstract
The development of Th17 cells is a key event in the pathogenesis of experimental autoimmune encephalomyelitis (EAE), a murine model of human multiple sclerosis (MS). Previous studies have demonstrated that an IL-6-dependent pathway is involved in the differentiation of Th17 cells from naïve CD4-positive T cells in vitro. However, the role of IL-6 in vivo in the development of Th17 cells in EAE has remained unclear. In the present study, we found that IL-6 blockade by treatment with an anti-IL-6 receptor monoclonal antibody (anti-IL-6R mAb) inhibited the development of EAE and inhibited the induction of myelin oligodendrocyte glycoprotein (MOG) peptide-specific CD4-positive, CD8-positive, and Th17 T cells, in inguinal lymph nodes. Thus, the protective effect of IL-6 blockade in EAE is likely to be mediated via the inhibition of the development of MOG-peptide-specific Th17 cells and Th1 cells, which in turn leads to reduced infiltration of T cells into the CNS. These findings indicate that anti-IL-6R mAb treatment might represent a novel therapy for human MS.
Keywords: autoimmunity, multiple sclerosis, T cells
Multiple Sclerosis (MS) is an inflammatory demyelinating disease of the CNS. More than 2 million people worldwide are affected with this disease; however, an effective therapy for MS has not yet been established. Although the cause of MS is not fully understood, infiltration of CD4+ T cell and CD8+ T cell into the CNS is believed to be important in the pathogenesis of this disease (1).
Experimental autoimmune encephalomyelitis (EAE) is a murine model of human MS that shares many pathological and histological characteristics with human MS. Initially, EAE was considered to be a Th1-mediated disease; however, recent studies have revealed that the major pathogenic T cell subset in EAE are Th17 cells (2, 3), which are characterized by CD4-positive T cells producing IL-17A (IL-17) (4, 5). Th17 cells are believed to play an important role in host defense against extracellular pathogens, which are not effectively cleared by Th1 or Th2 cells. Because Th17 cells are highly proinflammatory, Th17 cells directed against self antigens cause severe autoimmune disease in mice, including EAE and collagen-induced arthritis (CIA) (3, 6).
Previous studies have suggested a pathogenic role for IL-17 in MS. Matusevicius et al. (7) reported that IL-17-secreting lymphocytes were detected in the cerebrospinal fluid of MS. Lock et al. (8) revealed that increased levels of transcripts for IL-17 and IL-6 are detected in chronic lesions of patients with MS. Furthermore, Tzartos et al. (9) reported that IL-17-producing CD4+ T cells are present within active areas of MS. Because IL-17 signaling is important for the production of various chemokines from fibroblasts and epithelial cells, which attract antigen-presenting cells to the CNS, resulting in demyelination, the suppression of the development or the proliferation of Th17 cells may represent a promising therapy for MS.
Recently, three independent groups demonstrated that the cytokines IL-6 and TGF-β synergistically induce the differentiation of Th17 cells in mice in vitro (10–12). More recently, IL-21 produced by Th17 cells themselves contributes to the amplification of differentiated Th17 cells (13–15). Moreover, IL-23 has been shown to contribute to the proliferation and stabilization of Th17 cells (12). Thus, because IL-6 is a regulator of Th17 differentiation in vitro, it represents a potential target for the inhibition of Th17 development in vivo. IL-6-deficient mice have been shown to be highly resistant to the induction of EAE (16, 17). However, data obtained from IL-6-deficient mice may not equate to data obtained by IL-6 blockade using neutralizing antibody, because the complete absence of IL-6 in knockout mice has been shown to display hematopoietic defects (18). Two independent groups have performed anti-IL-6 therapy against EAE, but the results were conflicting. Gijbels et al. (19) reported that treatment of anti-IL-6 antibody was protective against the development of EAE. By contrast, Willenborg et al. (20) reported that anti-IL-6 therapy has no significant protective effect in EAE. Therefore, the role of IL-6 in EAE remains unclear. Furthermore, because these studies were conducted before the discovery of the Th17 T cell subset, the effect of IL-6 blockade on T cell development in EAE, particularly highly proinflammatory Th17 cells, also remains unclear.
In the present study, we investigated the in vivo role of IL-6 in the development of T cells, particularly Th17 cells, in EAE, using an anti-IL-6R monoclonal antibody (anti-IL-6R mAb), which shows significant protective effect in CIA (21).
Results
Anti-IL-6R mAb Treatment Inhibited the Development of EAE.
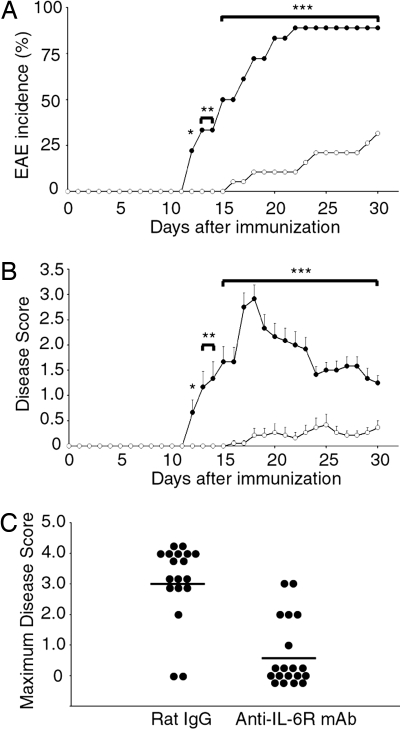
To investigate a protective effect of anti-IL-6R mAb treatment against the development of EAE, we immunized C57BL/6J mice with MOG35–55 peptide emulsified with CFA, followed by i.p. treatment of 8 mg of anti-IL-6R mAb or control rat IgG at the same day of immunization. Compared with a control rat-IgG-treated group, the incidence of EAE was reduced and the onset of EAE was delayed in the anti-IL-6R-mAb-administered group (Fig. 1A). In anti-IL-6R-mAb-treated mice, the clinical scores were also significantly lower than those of control rat-IgG-treated mice (Fig. 1 B and C).
Fig. 1.
Anti-IL-6R mAb suppressed the onset of actively induced EAE. EAE was induced in C57BL/6J mice and treated with 8 mg of anti-IL-6R mAb or rat IgG injected at the same day of EAE induction. (A) Incidence of EAE. (B) Average clinical scores of diseased mice. Filled circles indicate the rat-IgG-treated group (n = 18), and open circles indicate the anti-IL-6R-mAb-treated group (n = 19). Clinical scores (averages ± SEM) combining three independent experiments are shown. Statistics were calculated with the χ2 test (A) and Mann–Whitney U test (B). *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) Maximum severity of clinical disease for each mouse. Indicated antibodies were treated per group.
Anti-IL-6R-mAb-Treated Mice Are Devoid of CNS-Infiltrated T Cells.
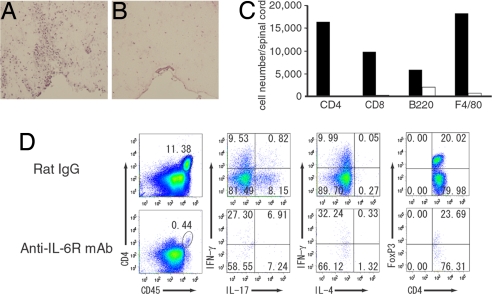
At the peak stage (19 days after antigen immunization) of EAE, histopathology of CNS in the control rat-IgG-treated mice showed intense infiltration of mononuclear cells into the white matter of spinal cords (Fig. 2A). In contrast, cellular infiltration was markedly reduced in anti-IL-6R-mAb-treated mice, which was consistent with their decreased clinical scores (Fig. 2B). Similarly, demyelination was also detected in control rat-IgG-received mice and not found in anti-IL-6R-mAb-treated mice (data not shown). To investigate the population of lymphocytes infiltrated into the CNS, we recovered mononuclear cells from the spinal cords and surface stained with CD4, CD8, B220, and F4/80 antibody. At peak stage of EAE, CD4+ T cells, CD8+ T cells, B cells, and macrophages were detected in control rat-IgG-treated mice (Fig. 2C). In anti-IL-6R-mAb-treated mice, CD4+ T cells, CD8+ T cells, and macrophages were hardly detected, whereas approximately one-third the number of B cells were found compared with control (Fig. 2C). Furthermore, intracellular cytokine staining of CNS-infiltrating lymphocytes demonstrated that intense infiltration of Th17, Th1, and FoxP3 positive regulatory T (RegT) cells were detected, whereas anti-IL-6R-mAb-treated mice had a paucity of cells in the CNS (Fig. 2D). Thus, anti-IL-6R mAb treatment on day 0 suppressed the presence of lymphocytes into the spinal cord.
Fig. 2.
Anti-IL-6R-mAb-treated mice are devoid of CNS-infiltrated T cells. (A and B) H&E histology of spinal cords from antibody-treated mice taken at peak disease (19 days after antigen immunization). (Original magnifications: ×20.) (C) Analysis of mononuclear cells infiltrating into the spinal cords. Mononuclear cells were recovered from spinal cord at 19 days after antigen immunization. Isolated cells were surface-stained with antibodies against CD45, CD4, CD8, B220, and F4/80. CD45 high populations were gated. (D) Intracellular cytokine staining and FoxP3 staining of spinal-cord-infiltrating cells. Mononuclear cells were recovered from spinal cord at 19 days after immunization, and cells were stimulated with PMA and ionomycin in the presence of Brefeldin A; all plots are gated on CD4+ CD45+ T cells. Flow-cytometric analysis was done on pooled spinal cord from four mice per group, and results are representative of two independent experiments.
Anti-IL-6R mAb Treatment Suppressed the Induction of MOG35–55-Peptide-Specific T Cells in Peripheral Lymphoid Tissue.
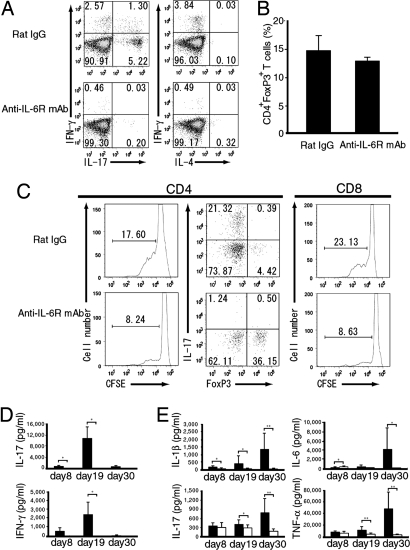
To investigate whether the deficiency of CNS-infiltrating CD4+ T cells in anti-IL-6R-mAb-treated mice was due to a T cell priming defect or not, we analyzed MOG35–55-peptide-specific CD4+ T cells from draining lymph nodes. In the lymphocytes prepared from inguinal lymph nodes at priming stage (8 days after antigen immunization), the development of Th17 cells as well as Th1 cells was highly suppressed in anti-IL-6R-mAb-treated mice compared with rat-IgG-treated mice (Fig. 3A). In contrast, Th2 cells were not induced in mice treated with rat IgG or anti-IL-6R mAb in EAE (Fig. 3A). In addition, the population of FoxP3-positive RegT cells was also not changed significantly in anti-IL-6R-mAb-treated mice (Fig. 3B). To examine the effect of IL-6 blockade against the proliferation of MOG35–55-peptide-specific T cells, we performed CFSE dilution assay and found that anti-IL-6R mAb treatment at the same day of antigen immunization suppressed the proliferation of MOG35–55-peptide-specific CD4+ T cells in vitro (Fig. 3C Left). To further elucidate the subsets of MOG35–55-peptide-specific CD4+ T cells, CFSElow CD4+ T cells were gated and MOG35–55-peptide-specific Th17 and FoxP3+ RegT cells were analyzed. An increased population of Th17 cells was detected in rat-IgG-treated mice. In contrast, although the absolute number of MOG35–55-peptide-responsive CD4+ T cells was lower than in the rat-IgG-treated group, a remarkably higher population of FoxP3+ RegT cells and a lower population of Th17 cells were found in anti-IL-6R-mAb-treated group (Fig. 3C Center). In addition to the suppression of MOG35–55-peptide-specific CD4+ T cells, anti-IL-6R mAb also suppressed the proliferation of MOG35–55-peptide-specific CD8+ T cells (Fig. 3C Right).
Fig. 3.
IL-6 blockade suppressed the induction of Th17 cells in the lymph nodes. (A) Intracellular cytokine staining of lymphocytes stimulated with MOG35–55 peptide. Inguinal lymph node cells were recovered at 8 days after antigen immunization. All plots were gated on CD4+ T cells. (B) FoxP3 staining of CD4+ T cells recovered from inguinal lymph node at 8 days after immunization, and FoxP3-positive CD4+ T cells analyzed by FACS. (C) Analysis of MOG35–55-peptide-specific T cells by CFSE dilution assay. All plots were gated on CD4+ T or CD8+ T and CFSElow populations, and a population of IL-17- or FoxP3-positive CD4+ T cells was analyzed by FACS. Data are representative of three independent results. (D) IL-6 blockade suppressed the antigen-specific cytokine production of IL-17 and IFN-γ from lymphocytes. Inguinal lymphoid node cells were recovered at the indicated days after immunization, and lymph node cells were restimulated with 50 μg/ml MOG35–55 peptide for 72 h. IL-17 and IFN-γ concentrations in the supernatant were determined by using BioPlex. (E) IL-6 blockade suppressed the serum proinflammatory cytokine levels. Mice were treated with anti-IL-6R mAb or rat IgG on day 0, and serum samples were prepared at the indicated days after antigen immunization. IL-1β, IL-6, IL-17, and TNF-α cytokine concentrations in the serum were analyzed by using BioPlex. All p values were determined by using the Student t test. *, P < 0.05; **, P < 0.005.
To investigate the effect of IL-6 blockade against MOG35–55-peptide-specific cytokine production, we quantified the concentrations of cytokine levels secreted into the culture supernatant of lymphocytes from inguinal lymph nodes, which were restimulated with MOG35–55 peptide. We found that the production of IL-17 and IFN-γ was significantly suppressed during all stages in anti-IL-6R-mAb-treated mice, although these cytokine levels were higher at peak stage in control mice, which correlated with clinical scores (Fig. 3D). We could not detect IL-4, IL-6, or TNF-α in the culture supernatant. In contrast, the concentrations of serum cytokines including IL-1β, IL-6, IL-17, and TNF-α were significantly higher at the recovery stage than the peak stage of rat-IgG-treated mice, and these serum cytokine levels were also suppressed in anti-IL-6R-mAb-treated mice during the peak and recovery stages (Fig. 3E). Serum cytokine levels of IFN-γ and IL-4 were below the limit of detection in both antibody-treated groups. At the recovery stage, increased serum cytokine levels, such as IL-17 and IL-6, in rat-IgG-treated mice might be mediated by activated lymphocytes that were migrated from the CNS to peripheral lymphoid tissues. These results suggest that suppression of the differentiation of MOG35–55-peptide-specific Th17 and Th1 cells by IL-6 blockade contributes to the protective effect against the development of EAE.
Delayed Treatment of Anti-IL-6R mAb Failed to Suppress EAE.
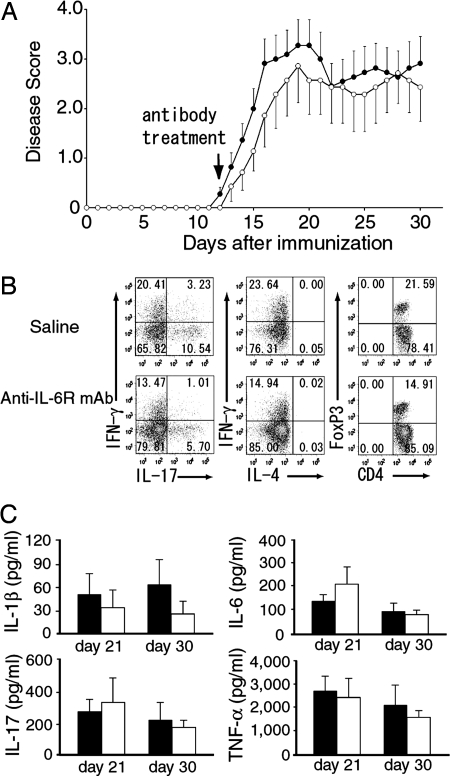
To determine whether administration of anti-IL-6R mAb inhibits the proliferation of already committed Th17 cells in vivo or not, we treated antibody during onset stage (12 days after antigen immunization) and investigated the effect of anti-IL-6R mAb against EAE. There were no significant differences in the clinical scores between anti-IL-6R mAb and saline treated groups (Fig. 4A). Although the population of Th17, Th1 and FoxP3+ RegT cells infiltrated into the spinal cord was lower in anti-IL-6R-mAb-treated mice than saline treated mice (Fig. 4B), it was not enough to reduce disease severity. In addition, there were no differences in serum cytokine levels (Fig. 4C). These results indicate that IL-6 blockade is not efficacious in inhibiting an established EAE.
Fig. 4.
Delayed treatment of anti-IL-6R mAb failed to suppress EAE. (A) Average clinical scores of mice treated with 8 mg of anti IL-6R mAb (n = 7) or saline (n = 11) injected at 12 days after antigen immunization. Clinical scores (averages ± SEM) combining two independent experiments are shown. (B) Intracellular cytokine staining and FoxP3 staining of spinal cord infiltrating cells recovered from peak stage (21 days after antigen immunization) of EAE. All plots were gated on CD4+ CD45+ T cells. Flow cytometric analysis was done on pooled spinal cord from 4 mice/group and results are representative of two independent experiments. (C) IL-6 blockade has no effect on the serum pro-inflammatory cytokine levels. Mice were treated with anti-IL-6R mAb or saline on day 12, and serum were prepared at indicated days after antigen immunization. IL-1β, IL-6, IL-17 and TNF-α cytokine concentrations in the serum were analyzed by BioPlex.
IL-6 Blockade Cannot Prevent the Infiltration of Activated Lymphocytes into the CNS.
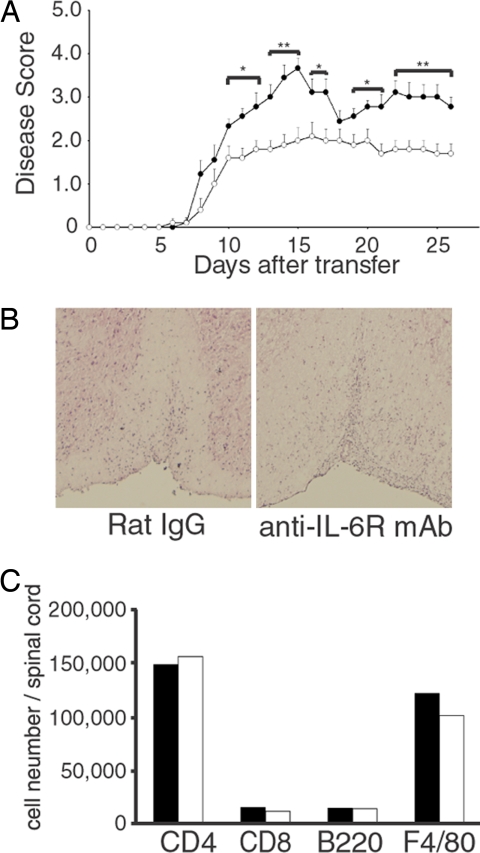
Next, we investigated the effect of IL-6 blockade against passive induction of EAE to elucidate whether administration of anti-IL-6R mAb prevents the infiltration of activated lymphocytes into the spinal cord. IL-6 has been reported to induce cell-adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1) in endothelial cells (22); therefore, IL-6 blockade might inhibit the infiltration of lymphocytes into the spinal cord. For the passive induction of EAE, lymphocytes from MOG35–55 peptide/CFA-immunized C57BL/6J mice were stimulated with MOG35–55 peptide for 4 days in vitro, and viable lymphocytes were transferred into naïve wild-type mice. When mice were i.p. treated with 8 mg of anti-IL-6R mAb 1 day before transfer, there was no difference in the disease onset between anti-IL-6R-mAb- and rat-IgG-treated mice; however, clinical scores were partially inhibited in anti-IL-6R-mAb-treated mice (Fig. 5A). Histopathology of CNS in the anti-IL-6R-mAb-treated mice showed massive infiltration of mononuclear cells into the white matter of spinal cord, and it was comparable with those of control rat-IgG-treated mice (Fig. 5B). Moreover, there were also no differences in the absolute numbers of CD4+ T cells, CD8+ T cells, B cells, and macrophages infiltrating into the CNS (Fig. 5C). These results indicate that anti-IL-6R mAb treatment cannot prevent the infiltration of activated lymphocytes into the spinal cord.
Fig. 5.
IL-6 blockade cannot prevent the infiltration of activated lymphocytes into the CNS. (A) IL-6 blockade failed to suppress the disease onset but partially inhibits the disease severity of passive transfer EAE. Eight mg of anti-IL-6R mAb (n = 10) or rat IgG (n = 9) was treated at one day before transfer. EAE was passively induced and clinical scores (averages ± SEM) are shown. Statistics were calculated with the Mann–Whitney U test. *, P < 0.05; **, P < 0.005. (B) H&E histology of spinal cords from antibody-treated mice taken at peak disease. (Original magnification: ×20.) Anti-IL-6R-mAb and rat-IgG-treated mice show inflammation in the white matter of the CNS. (C) Analysis of mononuclear cells infiltrating into the spinal cord. Mononuclear cells were recovered from pooled spinal cord from three mice per group at 14 days after transfer. Isolated cells were surface-stained with antibodies against CD45, CD4, CD8, B220, and F4/80, and analyzed by FACS. CD45 high populations were gated.
Discussion
The recent identification of the highly proinflammatory Th17 effector T cell subset has focused attention to the role of Th17 cells in the pathogenesis of autoimmune disease. In mice, autoantigen-specific Th17 cells have been shown to be the dominant pathogenic T cell subset in EAE (3). Moreover, IL-17-deficient mice have been reported to develop EAE with delayed onset and reduced severity (23). In humans, Th17 cells have been identified in the CNS of patients with MS (9). These studies highlight the importance of understanding the regulation of Th17 cell development in autoimmune disease.
It has been demonstrated that IL-6 and TGF-β synergistically induce the differentiation of naïve CD4-positive T cells into Th17 cells in mice (10–12). However, our previous studies (24) and those of other groups (13–15) have highlighted both IL-6-dependent and -independent pathways in the differentiation of Th17 cells in vitro. IL-6 knockout mice have been shown to be highly resistant to the development of EAE (16, 17). However, treatment with anti-IL-6 mAb has been reported to be protective in EAE in one study (19) and not protective in another study (20). Therefore, the role of IL-6 in the induction of T cells (particularly Th17 cells) in EAE remains unclear. In this study, we investigated the in vivo role of IL-6 in T cell development in EAE, using an anti-IL-6R mAb.
In this article, we showed that treatment with an anti-IL-6R mAb at the same day of antigen immunization effectively suppressed the disease incidence and severity of EAE (Fig. 1 A and B). Willenborg et al. (20), however, failed to suppress EAE disease using anti-IL-6 antibody; this may be due to the differences in the dose and/or timing of antibody treatment. Anti-IL-6R-mAb-treated mice were devoid of mononuclear cells in the spinal cord (Fig. 2 B and C). These results are consistent with previous studies of EAE in IL-6-deficient mice (16, 17). Because a subset of CD8+ T cells has been demonstrated to be suppressive against EAE (25), it is uncertain whether the decrease in the number of CD8+ T cells infiltrated into the CNS contributes to the amelioration of the clinical score.
Importantly, anti-IL-6R mAb inhibited the induction of MOG35–55-peptide-specific Th17 cells (Fig. 3A) in vivo. As previously reported, the combination of IL-6 and TGF-β is important for the differentiation of naïve CD4-positive T cells into Th17 cells in vitro (10–12). Our data support the importance of IL-6 in the differentiation of Th17 cells in vivo.
We observed that anti-IL-6R mAb treatment suppressed the induction of Th1 cells during the priming stage of lymph nodes (Fig. 3A), whereas Th2 cells were not induced in EAE (Fig. 3A). It is not clear whether IL-6 directly regulates the differentiation of Th1 cells. Whereas IL-6 has been shown to suppress the induction of TGF-β-inducible RegT cells from naïve CD4+ T cells (10), the suppression of MOG35–55-peptide-specific Th1 cells might be mediated by MOG35–55-peptide-specific TGF-β-inducible RegT cells, which were increased by IL-6 blockade in EAE (Fig. 3C). In agreement with this, it has been reported that IL-6 deficiency promotes the generation or expansion of MOG35–55-peptide-specific RegT cells and inhibits the induction of effector T cell responses, including Th17 and Th1 cells, although MOG35–55-peptide-specific CD8+ T cells were not studied (13). Recently, Selvaraj et al. (26) reported that development of EAE was suppressed after passive transfer of inducible RegT cells and that the induction of both Th1 and Th17 cells was also suppressed, thus supporting a possible protective role for inducible RegT cells in anti-IL-6R-mAb-treated mice. When RegT cells were depleted from lymph node cells before CFSE dilution assay by using antibody against folate receptor 4 (a surface marker of RegT cells), we did not observe changes in the population of MOG35–55-peptide-specific Th1 cells (data not shown), suggesting that the differentiation of MOG35–55-peptide-specific Th1 cells is suppressed by RegT in vivo before the in vitro analysis, in anti-IL-6R-mAb-treated mice. In addition to the MOG35–55-peptide-specific CD4+ T cells, anti-IL-6R-mAb-treated mice showed impaired proliferation of MOG35–55-peptide-specific CD8+ T cells (Fig. 3C). It has been reported that the proliferation of antigen-specific CD8+ T cells was suppressed by RegT cells (27). Therefore, the decreased population of MOG35–55-peptide-specific CD8+ T cells might be mediated by the MOG35–55-peptide-specific TGF-β-inducible RegT cells.
We also investigated the effect of anti-IL-6R mAb during disease onset stage, and no differences were found in the clinical score of EAE (Fig. 4A). Although the populations of Th17 and Th1 cells were partially decreased (Fig. 4B), this might be due to the prevention of newly differentiating Th17 cells from naïve CD4+ T cells by IL-6 blockade. However, a partial decrease in the populations of Th17 and Th1 cells was not sufficient to reduce disease severity significantly. These results indicate that IL-6 blockade is not effective in inhibiting the proliferation of already committed Th17 cells in vivo.
Infiltration of MOG35–55-peptide-specific CD4+ T cells into the CNS is an important step in EAE disease onset (28), and IL-6 has been reported to induce ICAM-1 in endothelial cells (22), a protein possibly involved in the infiltration of lymphocytes through the blood–brain barrier (BBB) (28). Therefore, we investigated the effect of anti-IL-6R mAb treatment against passive induction of EAE to elucidate the effect of IL-6 blockade against infiltration of activated lymphocytes into the spinal cord. We did not observe any difference in the disease onset between anti-IL-6R mAb and rat-IgG-treated mice (Fig. 5A). Furthermore, there were no differences in the absolute number of CD4+ T cells, CD8+ T cells, B cells, and macrophages infiltrating into the CNS (Fig. 5C). These results indicate that IL-6 is dispensable for the infiltration of activated lymphocytes through the BBB and argues against a role for IL-6 in the induction of cell-adhesion molecules on the endothelial cells in EAE. Therefore, the absence of activated lymphocytes in the spinal cord of anti-IL-6R-mAb-treated mice (Fig. 2B) is likely to be mediated via the inhibition of the induction of MOG35–55-peptide-specific CD4+ T cells, including Th17 cells, and CD8+ T cells in the peripheral lymphoid tissue rather than via the inhibition of infiltration of activated lymphocytes into the spinal cord. Interestingly, the clinical score of passively induced EAE was partially inhibited in anti-IL-6R-mAb-treated mice compared with rat-IgG-treated mice (Fig. 5A). This indicates that IL-6 not only regulates Th17 differentiation but also affects T cells or other cells such as endothelial cells or CNS glial cells including astrocytes at effector phase in EAE. However, we could not find significant amelioration of the EAE clinical scores when anti-IL-6R mAb was administered after 12 days of antigen immunization (Fig. 4A). These differences in the protective efficacy of IL-6 blockade at effector phase may be due to differences in serum cytokine levels between passively induced EAE and treatment of anti-IL-6R mAb at 12 days after antigen immunization against actively induced EAE. Thus, for example, because of prior immune activation, IL-17 (an inducer of IL-6 expression) levels are likely to be higher in the actively induced EAE model at day 12 (beginning of antibody administration) compared with IL-17 levels in the passively induced EAE model at the time of anti-IL-6R mAb administration.
Recent reports demonstrated differences in the regulation of Th17 cell development in vitro in humans compared with mice (29, 30). For the induction of human Th17 cells, TGF-β is not required but inhibits their differentiation (29, 30). These groups concluded that IL-1 is an effective inducer of human Th17 differentiation. Furthermore, combination of IL-1 and IL-6 synergistically induces the differentiation of human Th17 cells in vitro (29), indicating that IL-6 is important for the induction of Th17 cells in both mice and humans in vivo. In mice, it has been reported that IL-1 augments Th17 differentiation induced by IL-6 and TGF-β in vitro (12) and that IL-1 signaling is required for the induction of Th17 cells in EAE (31). Thus, our study indicates that the observed inhibition of Th17 induction in EAE after IL-6 blockade, may be mediated in part via the inhibition of the synergistic effect of IL-6 and IL-1 in the presence of TGF-β.
In conclusion, we have demonstrated a key role for IL-6 in the differentiation of Th17 cells in EAE. Anti-IL-6R mAb treatment is most effective at the same day of antigen immunization, rather than after the commitment of Th17 cells in EAE. Furthermore, anti-IL-6R mAb therapy might be also effective in the ongoing or relapse of MS, because humanized anti-IL-6R mAb can be treated repeatedly without antigenicity. Moreover, not only effector cells but also naïve T cells are thought to contribute to antigenic spread in a relapsing EAE model (32), and anti-IL-6R mAb might suppress the differentiation of Th17 cells from naïve T cells at the relapsing phase.
Our studies suggest that the protective effect of anti-IL-6R mAb treatment in EAE is mediated not only via the suppression of IL6-induced inflammatory reactions but also via the inhibition of the induction of MOG35–55-peptide-specific Th17 and Th1 cells, which in turn leads to the reduced infiltration of T cells into the CNS. These findings indicate that anti-IL-6R mAb treatment might represent a promising therapy for human MS and other Th17-mediated chronic autoimmune diseases.
Materials and Methods
Mice.
C57BL/6J mice were purchased from Charles River Laboratories. All experiments were conducted according to the institutional ethical guidelines for animal experimentation of the National Institute of Biomedical Innovation (Osaka).
Active Induction of EAE.
Mice (9 weeks of age) were immunized s.c. with 300 μg of MOG35–55 peptide emulsified in CFA and injected with pertussis toxin twice. The severity of EAE was monitored and graded on a scale of 0–5: 0 = no disease; 1 = limb tail; 2 = hind limb weakness; 3 = hind limb paralysis; 4 = hind and fore limb paralysis; 5 = moribundity and death.
Passive Induction of EAE.
Mice were immunized s.c. with MOG35–55 peptide/CFA. Spleen cells and inguinal lymph node cells were harvested on day 15 after immunization and cultured for 4 days in the presence of 25 μg/ml MOG35–55 peptide, 10 ng/ml rmIL-23, and 5 μg/ml anti-mIFN-γ antibody. Viable lymphocytes (1.35 × 107) were transferred i.p. into naïve C57BL/6J mice.
Anti-IL-6R mAb Treatment.
For IL-6 blockade, mice were i.p. treated with 8 mg of anti-IL-6R mAb (clone MR16–1, rat IgG1) on days 0 or 12 postimmunization. Purified rat IgG (Cappel) or saline were administered as control. In the case of passive induction of EAE, 8 mg of anti-IL-6R mAb or rat IgG was administered i.p. into naïve C57BL/6J mice one day before transfer.
Histology.
Mice were perfused with PBS, and spinal cords were dissected and frozen in OCT compound. Sections 5 μm in thickness from the spinal cord were stained with H&E.
Intracellular Cytokine Staining.
Draining lymph node cells were stimulated with 50 μg/ml MOG35–55 peptide for 72 h and restimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) and 750 ng/ml ionomycin for the last 4 h in the presence of 10 μg/ml Brefeldin A. To analyze lymphocytes infiltrated into the CNS, spinal cords were removed as described above, followed by digestion with collagenase D (5.0 mg/ml; Roche Diagnostics). Cells were isolated by Percoll centrifugation as described in ref. 33 and surface-stained with antibodies against CD4 (Becton Dickinson; RM4-5), CD45 (Biolegend; 30-F11), CD8 (eBioscience, 53-6.7), B220 (eBioscience; RA3-6B2), and F4/80 (CALTAG Laboratories; Cl:A3-1). Intracellular cytokine staining and FoxP3 staining were performed as described in ref. 24. Cells were analyzed by using the FACSCanto flow cytometer (Becton Dickinson), and obtained data were analyzed by using FlowJo software (Tree Star).
CFSE Assays.
Lymph node cells were incubated in 3 μM CFSE (Molecular Probes). Stained cells (2 × 105) were cultured with 50 μg/ml MOG35–55 peptide for 72 h. After stimulation, cells were stained with antibodies against CD4, CD8, IL-17, and FoxP3 and analyzed by using the FACSCanto flow cytometer.
Cytokine Quantification.
For cytokine quantification, culture supernatants and serum were analyzed by using BioPlex (Bio-Rad) according to the manufacturer's instructions.
Statistics.
The two-tailed Student t test, χ2 test, or Mann–Whitney U test was used for the statistical analyses. Differences were considered significant when p values were <0.05.
Acknowledgments.
We thank Ms. N. Ashida and Y. Ito for their secretarial assistance. This work was supported by a Grant-in-Aid from the Ministry of Education, Science, and Culture (Japan) and the Osaka Foundation for Promotion of Clinical Immunology, and also by a Grant-in-Aid for Japan Society for the Promotion of Science Fellows, the Programme for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation and Chugai-Roche Pharmaceutical Co. Ltd., Tokyo.
Footnotes
Conflict of interest statement: T.K. is a patent holder for anti-IL-6 receptor antibody (Tocilizumab), and his laboratory is supported by a donation from Chugai-Roche Co. Ltd.
References
- 1.McFarland HF, Martin R. Multiple sclerosis: A complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 2.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 3.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 6.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matusevicius D, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 8.Lock C, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 9.Tzartos JS, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2007;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 11.Mangan PR, et al. Transforming growth factor-β induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 12.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 16.Okuda Y, et al. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int Immunol. 1998;10:703–708. doi: 10.1093/intimm/10.5.703. [DOI] [PubMed] [Google Scholar]
- 17.Samoilova EB, Horton JL, Hilliard B, Liu TS, Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: Roles of IL-6 in the activation and differentiation of autoreactive T cells. J Immunol. 1998;161:6480–6486. [PubMed] [Google Scholar]
- 18.Bernad A, et al. Interleukin-6 is required in vivo for the regulation of stem cells and committed progenitors of the hematopoietic system. Immunity. 1994;1:725–731. doi: 10.1016/s1074-7613(94)80014-6. [DOI] [PubMed] [Google Scholar]
- 19.Gijbels K, Brocke S, Abrams JS, Steinman L. Administration of neutralizing antibodies to interleukin-6 (IL-6) reduces experimental autoimmune encephalomyelitis and is associated with elevated levels of IL-6 bioactivity in central nervous system and circulation. Mol Med. 1995;1:795–805. [PMC free article] [PubMed] [Google Scholar]
- 20.Willenborg DO, Fordham SA, Cowden WB, Ramshaw IA. Cytokines and murine autoimmune encephalomyelitis: Inhibition or enhancement of disease with antibodies to select cytokines, or by delivery of exogenous cytokines using a recombinant vaccinia virus system. Scand J Immunol. 1995;41:31–41. doi: 10.1111/j.1365-3083.1995.tb03530.x. [DOI] [PubMed] [Google Scholar]
- 21.Takagi N, et al. Blockage of interleukin-6 receptor ameliorates joint disease in murine collagen-induced arthritis. Arthritis Rheum. 1998;41:2117–2121. doi: 10.1002/1529-0131(199812)41:12<2117::AID-ART6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 22.Romano M, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–325. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 23.Komiyama Y, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 24.Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci USA. 2007;104:12099–12104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YH, et al. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol. 2008;180:825–832. doi: 10.4049/jimmunol.180.2.825. [DOI] [PubMed] [Google Scholar]
- 26.Selvaraj RK, Geiger TL. Mitigation of experimental allergic encephalomyelitis by TGF-β induced Foxp3+ regulatory T lymphocytes through the induction of anergy and infectious tolerance. J Immunol. 2008;180:2830–2838. doi: 10.4049/jimmunol.180.5.2830. [DOI] [PubMed] [Google Scholar]
- 27.Piccirillo CA, Shevach EM. Cutting edge: Control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–1140. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 28.Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: Anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 30.Wilson NJ, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 31.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 33.Shin T, Matsumoto Y. A quantitative analysis of CD45Rlow CD4+ T cells in the subarachnoid space of Lewis rats with autoimmune encephalomyelitis. Immunol Invest. 2001;30:57–64. doi: 10.1081/imm-100103691. [DOI] [PubMed] [Google Scholar]