Abstract
Diabetic patients continue to develop inflammation and vascular complications even after achieving glycemic control. This poorly understood “metabolic memory” phenomenon poses major challenges in treating diabetes. Recent studies demonstrate a link between epigenetic changes such as chromatin histone lysine methylation and gene expression. We hypothesized that H3 lysine-9 tri-methylation (H3K9me3), a key repressive and relatively stable epigenetic chromatin mark, may be involved in metabolic memory. This was tested in vascular smooth muscle cells (VSMC) derived from type 2 diabetic db/db mice. These cells exhibit a persistent atherogenic and inflammatory phenotype even after culture in vitro. ChIP assays showed that H3K9me3 levels were significantly decreased at the promoters of key inflammatory genes in cultured db/db VSMC relative to control db/+ cells. Immunoblotting demonstrated that protein levels of the H3K9me3 methyltransferase Suv39h1 were also reduced in db/db VSMC. Furthermore, db/db VSMC were hypersensitive to TNF-α inflammatory stimulus, which induced dramatic and sustained decreases in promoter H3K9me3 and Suv39h1 occupancy. Recruitment of corepressor HP1α was also reduced under these conditions in db/db cells. Overexpression of SUV39H1 in db/db VSMC reversed this diabetic phenotype. Conversely, gene silencing of SUV39H1 with shRNAs in normal human VSMC (HVSMC) increased inflammatory genes. HVSMC cultured in high glucose also showed increased inflammatory gene expression and decreased H3K9me3 at their promoters. These results demonstrate protective roles for H3K9me3 and Suv39h1 against the preactivated state of diabetic VSMC. Dysregulation of epigenetic histone modifications may be a major underlying mechanism for metabolic memory and sustained proinflammatory phenotype of diabetic cells.
Keywords: chromatin, diabetic inflammation, H3K9me3, Suv39h1
The Diabetes Control and Complications Trial (DCCT) and follow-up Epidemiology of Diabetic Interventions and Complications (EDIC) study have revealed that patients with diabetes who were previously on standard insulin therapy continue to exhibit low-grade inflammation and develop vascular complications despite subsequent intensive therapy (1), suggesting a “metabolic memory” phenomenon mediated by hyperglycemia (2). However, the mechanisms mediating this mysterious metabolic memory are not well understood. Chromatin remodeling events include histone modifications of the N-terminal tails of core histones. These “epigenetic” marks form the basis of the “histone code,” which postulates that the type and number of histone tail modifications specify transcriptional memory and biological outcomes (3). We hypothesized that such chromatin changes arising from dysregulated histone lysine methylation may be the underlying epigenetic mechanism for sustained vascular inflammation, diabetic complications, and metabolic memory.
Chromatin remodeling is a dynamic process in which inaccessible, compact, and repressed chromatin is converted into an open accessible form for active gene transcription or vice versa. Both the repressed and transcriptionally active states involve the recruitment of protein complexes that alter chromatin structure through covalent modifications of histone tails (3). These include histone H3 lysine acetylation, generally associated with gene activation, and histone H3 lysine methylation (H3Kme), associated with either gene activation or repression depending on the site of methylation (3, 4). As an added layer of regulation, lysine residues can be mono- (me1), di- (me2), or tri (me3) methylated (4) by histone methyl transferases (HMTs) with specificity for a particular lysine residue. The epigenetic modification H3K9me3, which is usually linked to gene repression, is catalyzed by the conserved mammalian ortholog of the Drosophila suppressor of position-effect variegation, Su(var)3–9-related HMTs, SUV39H1 in humans, and Suv39h1 in mice (4–7). H3K9me3 plays key roles in gene silencing by recruiting repressors and cofactors including histone deacetylases (HDACs) and heterochromatin protein-1α (HP1α). HP1α can then recruit or interact with other corepressor complexes and further propagate methylation and chromatin condensation (6, 7).
Although it was once regarded as stable or irreversible, current evidence suggests that histone Kme is a more dynamic process, with Kme changes detected at inflammatory gene promoters upon stimulation (8–10). This is further exemplified by the recent identification of histone demethylases such as lysine-specific demethylase 1 (LSD1) and the JMJD2 family members capable of reversing histone H3K4 and H3K9 methylation, respectively (11). Furthermore, although these reports suggest a role for H3K9me in gene repression, the regulation of inflammatory genes by histone Kme in vascular smooth muscle cells (VSMC) under diabetic conditions has not been explored.
Chronic inflammation resulting from the interactions among macrophages, endothelial cells, and VSMCs can lead to the formation of atherosclerotic lesions/plaques that can rupture and result in myocardial infarction and stroke. These complications are significantly accelerated in diabetes (12, 13). Increased inflammatory gene expression in diabetes can promote VSMC migration and proliferation, key processes in atherosclerosis. Enhanced levels of inflammatory cytokines and chemokines such as interleukin-6 (IL-6), macrophage colony stimulating factor-1 (MCSF), and monocyte chemoattractant protein-1 (MCP-1) have been found in both diabetes and atherosclerosis (14–16). Although inflammatory genes have been implicated in diabetic complications, little is known about how they are regulated at the chromatin level and promote sustained vascular inflammation.
We recently demonstrated that VSMC from db/db mice, an established model for type 2 diabetes and insulin resistance (17), retain the diabetic phenotype even after isolation from the mice and in vitro culture for 5–8 weeks. These cells exhibit persistent increased inflammatory gene expression, enhanced VSMC migration, and monocyte-VSMC binding relative to control db/+ mice (18, 19), thereby providing an excellent model in which to study the mechanistic aspects of metabolic memory. In this study, we demonstrate that metabolic memory in VSMC can arise from epigenetic mechanisms involving the down-regulation of repressive chromatin marks established by the HMT Suv39h1. This results in decreased H3K9me3 and occupancies of Suv39h1 at inflammatory gene promoters, leading to derepression of these target genes and sustained proatherogenic responses implicated in diabetic vascular complications.
Results
Inflammatory Gene Expression Is Increased and Repressive H3K9me3 Levels Are Decreased in db/db VSMC.
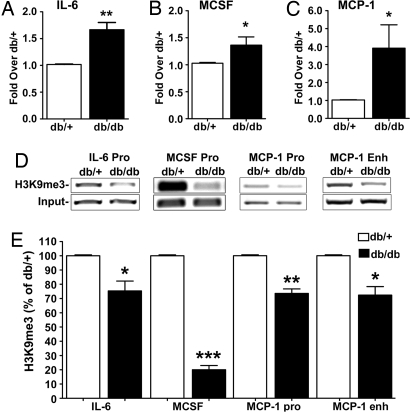
Our previous results showed that mouse VSMC (MVSMC) cultures derived from aortas of diabetic db/db mice exhibit enhanced inflammatory gene expression relative to db/+ control mice. Here, we examined whether this was associated with changes in the repressive epigenetic marker H3K9me3 on gene promoters. First, we measured the mRNA expression of key inflammatory genes by RT-quantitative (q)PCR. As shown, significant increases in IL-6 (Fig. 1A), MCSF (Fig. 1B), and MCP-1 (Fig. 1C) mRNA levels were seen in db/db MVSMC relative to control db/+ even after multiple passages in in vitro culture (5–8 weeks). These results confirmed our previous gene expression data with IL-6 and MCP-1 (18) and further showed that MCSF is also up-regulated in db/db MVSMC. We next performed ChIP assays with H3K9me3-specific antibodies. As shown in Fig. 1 D and E, H3K9me3 levels at the promoters of IL-6, MCSF, and MCP-1 as well as MCP-1 enhancer were significantly reduced in VSMC from diabetic db/db mice compared with control db/+. IL-6 promoter results were further confirmed by real-time qPCR (59 ± 2.52% decrease; P < 0.0001, n = 3). In contrast, there was no significant difference in gene expression or promoter H3K9me3 levels for cyclophilin A (CypA) housekeeping gene in db/db versus db/+ VSMC [see supporting information (SI) Fig. S1 A and B]. These results suggest that increased inflammatory gene expression seen in the diabetic VSMC may be due to a loss of repressive epigenetic histone modifications. This diabetic phenotype and reduction of repressive marks are maintained even after removal from the mice and in vitro culture, suggesting a mechanism for transcriptional memory and sustained gene expression. Loss of H3K9me3 may therefore play a key role in the metabolic memory of vascular cells.
Fig. 1.
Inflammatory gene expression is increased and repressive H3K9me3 levels are decreased in db/db VSMC. A-C Total RNA from cultured db/db and db/+ VSMC was analyzed by RT-qPCR and normalized to internal control β-actin gene. Results are expressed as fold over db/+. (A) IL-6 mRNA levels (mean ± SE; **, P < 0.01 vs. db/+, n = 4). (B) MCSF mRNA levels (mean ± SE; *, P < 0.05 vs. db/+, n = 4). (C) MCP-1 mRNA levels (mean ± SE; *, P < 0.05 vs. db/+, n = 3). (D and E) H3K9me3 levels in db/+ and db/db VSMC determined by ChIP assays with H3K9me3 antibodies. (D) Immunoprecipitated DNA and input DNA analyzed by PCR to amplify the IL-6, MCSF, and MCP-1 promoters and MCP-1 enhancer. (E) Bar graphs representing relative H3K9me3 levels at the specified promoters quantified by densitometry. Results were normalized to input and expressed as the percentage of db/+ (mean ± SE; *, P < 0.05; **, P < 0.01; ***, P < 0.0001 vs. db/+, n = 3).
VSMC from Diabetic Mice Are Hypersensitive to Inflammatory Stimuli.
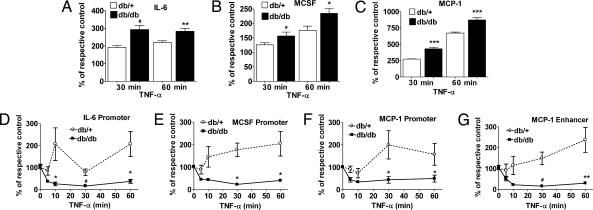
Next, we examined whether reduced repressive H3K9me3 sensitizes the diabetic cells to inflammatory stimuli. We used TNF-α as an inflammatory stimulus because it is up-regulated in diabetes, activates the proinflammatory transcription factor NF-κB, and subsequently enhances expression of NF-κB-regulated inflammatory genes (20, 21). Serum-depleted MVSMC were stimulated with TNF-α (10 ng/ml) for 30–60 min and inflammatory genes quantified by RT-qPCR. Results normalized to β-actin show that TNF-α-stimulated IL-6, MCSF, and MCP-1 levels were all significantly greater in db/db MVSMC relative to db/+ at both 30 and 60 min (Fig. 2A, B, and C, respectively). In contrast, CypA expression was not altered under these conditions (Fig. S2A). Thus db/db VSMC are preactivated relative to db/+ and maintain a metabolic memory even when cultured ex vivo, rendering them more sensitive to inflammatory stimuli.
Fig. 2.
Enhanced TNF-α-induced gene expression and a reciprocal decrease in H3K9me3 at inflammatory gene promoters in diabetic VSMC. (A–C) Serum-depleted VSMC were treated with or without TNF-α (10 ng/ml) for 30 and 60 min. Total RNA was used to measure IL-6 (A), MCSF (B), and MCP-1 (C) gene expression by RT-qPCR, and results are expressed as the percentage of respective untreated control after normalization with internal control β-actin. Data represent mean ± SE of triplicate qPCRs from two independent experiments (*, P < 0.05; **, P < 0.01; #, P < 0.001; ***, P < 0.0001 vs. TNF-α-treated db/+). (D–G) Time course of TNF-α-induced H3K9me3. Serum-depleted VSMC were treated with or without 10 ng/ml TNF-α for the indicated time points, and cell lysates were subjected to ChIP assays using H3K9me3 antibody. ChIP-enriched DNA samples were analyzed by qPCR using primers specific for promoters of IL-6 (D), MCSF (E), MCP-1 (F), and MCP-1 enhancer (G). The line graph represents relative H3K9me3 levels normalized to input and expressed as the percentage of untreated control (mean ± SE; *, P < 0.05; **, P < 0.01; #, P < 0.001 vs. TNF-α-treated db/+, n = 3).
TNF-α Stimulation Results in a Sustained Decrease in the Repressive H3K9me3 at Key Inflammatory Gene Promoters in db/db VSMC.
Because TNF-α led to enhanced inflammatory gene expression in db/db VSMC, we next tested whether this was via modulation of H3K9me3. Serum-depleted MVSMC were stimulated with TNF-α for 5–60 min, followed by ChIP assays with H3K9me3 antibodies. ChIP-enriched DNA was analyzed by real-time qPCR. As shown in Fig. 2 D–G, TNF-α induced a slight decrease in H3K9me3 in nondiabetic db/+ cells at early time points (5–10 min) at the IL-6, MCSF, and MCP-1 promoters and MCP-1 enhancer, which reverted to an increase in H3K9me3 levels by 30–60 min. On the other hand, TNF-α-induced a much more dramatic and sustained decrease in promoter H3K9me3 in the diabetic db/db cells that inversely correlated with the enhanced gene expression. CypA promoter showed no significant changes in H3K9me3 levels, confirming the specificity of these findings (Fig. S2B). Conventional PCR of ChIP samples (30- and 60-min time points) showed similar results (Fig. S3). These results demonstrate that sustained low levels of the repressive H3K9me3 marker may hypersensitize diabetic cells to inflammatory stimuli.
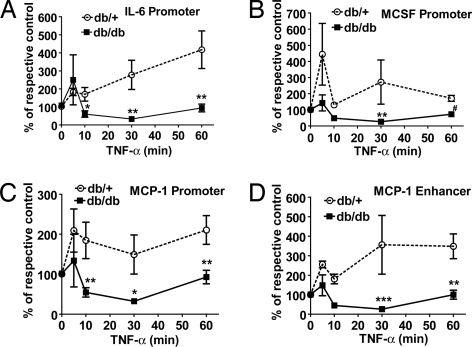
Sustained Inhibition of Suv39h1 Recruitment in db/db VSMC Stimulated with TNF-α.
Evidence shows that HMT SUV39H1 responsible for H3K9me3 in heterochromatin also plays a role in euchromatic H3K9me3 (22, 23). Hence, we used ChIP assays in TNF-α-treated MVSMC to examine the recruitment of Suv39h1 (mouse homolog of human SUV39H1) using SUV39H1 antibody that also recognizes Suv39h1. ChIP-qPCR data showed significantly sustained decreases in Suv39h1 recruitment at promoters of IL-6, MCSF, MCP-1, and MCP-1 enhancer in db/db VSMC relative to db/+ (Fig. 3 A–D). These results are strikingly similar to the decreased H3K9me3 (Fig. 2 D–G) and correlate well with the increased inflammatory gene expression (Fig. 2 A–C). There was no significant difference in Suv39h1 recruitment to CypA promoter (Fig. S2C). This suggests a key role for SUV39H1-mediated H3K9me3 in repression of inflammatory genes and that its dysfunction can mediate the memory of the diabetic cells.
Fig. 3.
TNF-α stimulation leads to a sustained decrease in Suv39h1 recruitment in db/db VSMC but not db/+. Suv39h1 occupancy at promoters of IL-6 (A), MCSF (B), MCP-1 (C), and MCP-1 enhancer (D) analyzed by ChIP assays using SUV39H1 antibody in MVSMC stimulated with 10 ng/ml TNF-α for indicated time points. Results were expressed as the percentage of untreated control (mean ± SE; *, P < 0.05; **, P < 0.01; #, P < 0.001; ***, P < 0.0001 vs. TNF-α-treated db/+, n = 3). Stimulation with TNF-α, ChIP assays, and qPCRs were performed as described in Fig. 2.
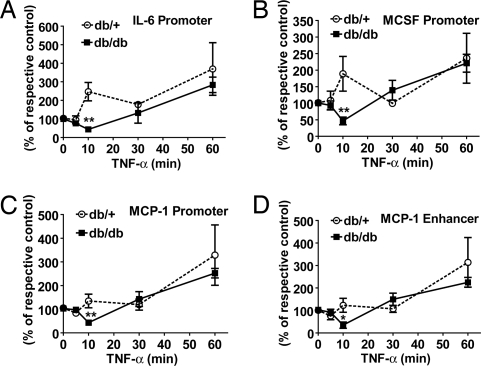
TNF-α also Decreases HP1α Recruitment Transiently in db/db VSMC.
To further elucidate the mechanism for apparent loss of repression underlying the increased inflammatory gene expression, ChIP assays were performed with antibodies against HP1α, a corepressor recruited by Suv39h1 and H3K9me3 (24). As shown in Fig. 4 A–D, HP1α recruitment was also reduced at inflammatory gene promoters within 10 min of TNF-α stimulation in db/db VSMC. However, its recruitment rebounded to similar higher levels in both cells types at later time points. Again, there was no significant difference in HP1α recruitment to the CypA promoter (Fig. S2D). Thus, the coordinated removal of repressive proteins that regulate H3K9me3, as noted by decreased occupancies of HP1α and Suv39h1, at inflammatory gene promoters could be a key underlying mechanism for formation of a more open chromatin structure that is sustained in db/db VSMC even after short-term culture.
Fig. 4.
TNF-α stimulation leads to decreased HP1α recruitment in db/db VSMC. Levels of HP1α on promoters of IL-6 (A), MCSF (B), MCP-1 (C), and enhancer of MCP-1 (D) genes analyzed by ChIP assays using HP1α antibodies in VSMC stimulated with 10 ng/ml TNF-α for indicated time points. The line graph represents relative HP1α recruitment normalized to input expressed as the percentage of untreated control (mean ± SE,*, P < 0.05; **, P < 0.01 vs. TNF-α-treated db/+, n = 3).
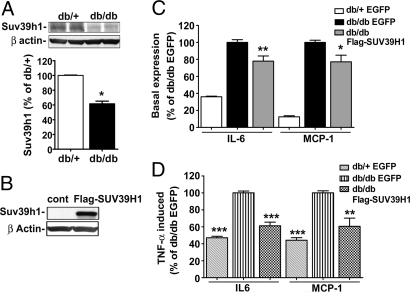
Suv39h1 Protein Levels Are Decreased in Diabetic MVSMC.
We next examined whether Suv39h1 and HP1α levels were themselves altered under diabetic conditions. Immunoblotting with specific antibodies showed that Suv39h1 protein levels were significantly decreased in the db/db VSMC compared with db/+, whereas the internal control β-actin levels were unaffected (Fig. 5A). However, HP1α protein levels did not change (data not shown). Thus, reduced Suv39h1 protein levels may be one of the mechanisms involved in the decreased H3K9me3 and Suv39h1 occupancy, leading to increased inflammatory gene expression under diabetic conditions.
Fig. 5.
Suv39h1 levels in VSMC regulate inflammatory gene expression. (A) Western blot analysis of cell lysates from db/+ and db/db VSMC using SUV39H1 and β-actin antibodies. The bar graph represents mouse Suv39h1 protein levels by densitometry and are expressed as the percentage of db/+ VSMC (mean ± SE; *, P < 0.023 vs. db/+, n = 3). (B) Western blot analysis of cell lysates from db/db VSMC transfected with EGFP or FLAG-SUV39H1 vectors by using SUV39H1 and β-actin antibodies. (C and D) VSMC were transfected with EGFP or FLAG-SUV39H1 vectors and inflammatory gene levels measured in basal or TNF-α-treated cells (10 ng/ml for 1 h). RT-qPCR results shown as the percentage of db/db-EGFP. (C) Basal gene expression. Data represent mean ± SE of triplicate qPCRs from two independent experiments (*, P < 0.05; **, P < 0.01 vs. db/db-EGFP). (D) TNF-α-induced gene expression. Results represent mean ± SE of triplicate qPCRs, n = 2 for IL-6 and n = 3 for MCP-1 (**, P < 0.01; ***, P < 0.0001 vs. db/db-EGFP).
Reversal of the Diabetic Phenotype by Overexpression of FLAG-SUV39H1 in db/db VSMC.
To further explore the functional role of Suv39h1, we tested the hypothesis that overexpression of this protein would reverse the proinflammatory diabetic phenotype. We transfected db/db cells with FLAG-tagged human SUV39H1 or EGFP (control) vectors. Expression was confirmed by immunoblotting cell lysates of transfected db/db VSMC with SUV39H1 antibody (Fig. 5B). Next, we examined inflammatory gene expression by RT-qPCR in db/db VSMC transfected with FLAG-SUV39H1 or EGFP. We also transfected db/+ VSMC with EGFP as a control for db/db cells. As expected, db/db VSMC transfected with EGFP exhibited increased IL-6 and MCP-1 levels compared with db/+ mice transfected with EGFP (Fig. 5C). However, db/db VSMC transfected with FLAG-SUV39H1 showed significantly decreased inflammatory gene levels relative to EGFP vector (Fig. 5C), supporting our hypothesis.
In addition, we also examined whether FLAG-SUV39H1 overexpression could also reverse the enhanced sensitivity of db/db cells to TNF-α. Transfected cells were serum-depleted for 24 h and stimulated with TNF-α for 1 hr, and gene expression was analyzed by RT-qPCR. As shown in Fig. 5D, TNF-α-induced IL-6 and MCP-1 expression was greater in db/db cells transfected with EGFP relative to db/+, but this was significantly attenuated in db/db cells transfected with FLAG-SUV39H1. These results demonstrate that increasing SUV39H1 protein levels in diabetic VSMC can reverse their metabolic memory and decrease their hypersensitivity to inflammatory stimuli.
High Glucose (HG) Leads to Increased Inflammatory Gene Expression and Dysregulation of H3K9me3 in VSMC.
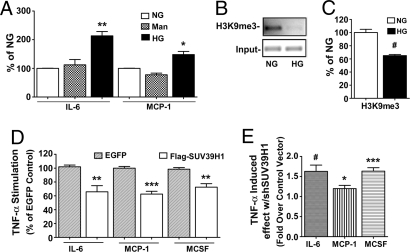
HG is a major risk factor for diabetic complications as also exemplified by DCCT/EDIC trials. Therefore, we examined whether the sustained inflammatory phenotype in the diabetic VSMC is due to prior exposure to hyperglycemia. In these experiments, human VSMC (HVSMC) were cultured for 2 weeks in normal glucose (NG) (5.5 mM), HG (25 mM), or mannitol (20 mM, osmotic control), and total RNA was analyzed by RT-qPCR. We observed a significant increase in IL-6 and MCP-1 mRNA levels in HG relative to NG-treated HVSMC (Fig. 6A), whereas mannitol had no significant effect. Next, ChIP assays showed that H3K9me3 levels were significantly decreased at the IL-6 promoter in HVSMC cultured in HG relative to NG (Fig. 6 B and C), similar to results seen in diabetic db/db VSMC.
Fig. 6.
HG effect in HVSMC and the role of SUV39H1. (A) IL-6 and MCP-1 mRNA levels in HVSMC grown in NG, mannitol (Man), or HG. RT-qPCR results shown as the percentage of NG. (mean ± SE; *, P < 0.05; **, P < 0.01 vs. NG, n = 3). (B and C) ChIP assay with NG- and HG-treated VSMC using H3K9me3 antibodies. Immunoprecipitated DNA and input DNA was analyzed by PCR with IL-6 promoter specific primers. The bar graph represents relative H3K9me3 levels normalized to input (quantified by densitometry) and shown as the percentage of NG (mean ± SE; #, P < 0.001 vs. NG, n = 3). (D) HVSMC were transfected with EGFP or FLAG-SUV39H1 vectors and inflammatory gene levels measured in total RNA extracted from TNF-α-treated cells (10 ng/ml, 1 h). RT-qPCR results are shown as the percentage of EGFP-transfected cells (mean ± SE of triplicate qPCRs; **, P < 0.01; ***, P < 0.0001 vs. TNF-α-treated EGFP cells; IL-6 n = 3, MCP-1 and MCSF n = 2). (E) HVSMC were transfected with expression plasmids for scrambled control or shSUV39H1 and inflammatory genes quantified from TNF-α-treated cells. RT-qPCR results are shown as TNF-α-induced effect with shSUV39H1, fold over that with control vector (mean ± SE of triplicate qPCRs, *, P < 0.05; #, P < 0.001; ***, P < 0.0001 vs. TNF-α effect in control; IL-6, and MCSF n = 3, MCP-1 n = 2).
To verify the role of SUV39H1, we evaluated gain- and loss-of-function approaches. HVSMC were transfected with either FLAG-SUV39H1 or EGFP vectors, followed by TNF-α stimulation. RT-qPCRs showed that although TNF-α could induce IL-6, MCP-1 and MCSF gene expression, this induction could be reversed by ∼40% in HVSMC overexpressing FLAG-SUV39H1 compared with EGFP control (Fig. 6D). We next examined whether loss of function using shRNAs to down-regulate human SUV39H1 in HVSMC can, conversely, mimic the diabetic phenotype of increased inflammatory gene expression. HVSMC were transfected with shSUV39H1 or scrambled control vectors. Immunoblotting showed a 30% reduction in SUV39H1 protein levels by shSUV39H1 compared with control vector (data not shown). RT-qPCR data showed that TNF-α-induced IL-6, MCP-1, and MCSF mRNA levels were significantly greater in cells transfected with shSUV39H1 relative to control vector (Fig. 6E). These results further support the notion that SUV39H1-mediated H3K9me3 plays an important role in the negative regulation of inflammatory genes in VSMC and that down-regulation of SUV39H1 under diabetic conditions leads to a sustained increase in inflammatory gene expression.
Discussion
Our results demonstrate that H3K9me3 and SUV39H1 play important roles in the repression of inflammatory genes in VSMC. Furthermore, they also suggest that epigenetic mechanisms involving the reduction of promoter H3K9me3 repressive mark may lead to metabolic memory.
H3K9me3 and SUV39H1 play important roles in gene repression and silencing (4, 5). Mechanisms include assembly of repressive complexes at H3K9me3 sites including HP1α and HDACs. HDACs remove H3K9acetylation, a chromatin marker normally associated with transcriptional activation (6, 7), and this promotes H3K9me formation to further amplify repression. Therefore, reduced SUV39H1/Suv39h1-mediated H3K9me3 on promoters of inflammatory genes in pathological conditions may lead to loss of repressive proteins and formation of open chromatin poised for transcriptional activation as noted in the diabetic cells in the current study. Our results show that VSMC from db/db mice have reduced Suv39h1 protein levels in parallel with decreased H3K9me3 on the IL-6, MCSF, and MCP-1 promoters and MCP-1 enhancer. A correlated increased expression of these genes supports our hypothesis that loss of repression via altered histone modifications is a key mechanism underlying the increased inflammatory gene expression observed under diabetic conditions. These phenomena were retained in db/db relative to db/+ VSMC even after short-term culture (up to 8 weeks) outside the animal, indicating the existence of a metabolic memory.
Cultured db/db VSMC were hypersensitive to TNF-α-induced inflammatory gene expression, which inversely correlated with a more dramatic decrease in promoter H3K9me3 in db/db cells relative to db/+. Although H3K9me3 levels in db/+ VSMC rebounded by 30–60 min, db/db H3K9me3 levels remained persistently low. Dynamic removal of H3K9me3 upon stimulation, followed by remethylation at later time points at the promoters of inducible inflammatory genes has been reported in LPS-stimulated macrophages (8), suggesting a negative feedback mechanism to shut off prolonged expression of such genes. Our results suggest that this protective mechanism is dysregulated in db/db VSMC, thus making them more sensitive to inflammatory stimuli such as TNF-α. Because diabetic cells express higher levels of several inflammatory genes, this can create amplifying loops that further propagate vascular inflammation. Thus, the aberrant behavior of the db/db VSMC demonstrates a “memory” of prior exposure to diabetic conditions leading to an open chromatin structure at the inflammatory gene promoters.
Occupancies of Suv39h1 and HP1α were also significantly decreased in parallel at inflammatory gene promoters. Interestingly, although Suv39h1 recruitment and H3K9me3 were maintained at low levels in db/db VSMC, lower HP1α occupancy in db/db rebounded relatively much more quickly. The mechanisms involved in the early return of HP1α need further investigation. Despite normal levels of HP1α at later time points (30 and 60 min), TNF-α-induced gene expression was still elevated in db/db VSMC. This may be due to the significantly reduced levels of Suv39h1 and H3K9me3 at the gene promoters, further supporting the key role of H3K9me3. To confirm specificity, we also tested an inflammatory gene that was not enhanced in the db/db cells. We noted that, under basal as well as TNF-α-stimulated conditions, cycloxygenase-1 (Cox-1) mRNA levels were not significantly different in db/db MVSMC versus db/+. In parallel, there were also no differences in Cox-1 promoter basal or TNF-α-induced H3K9me3 (Fig. S4 A–D).
H3K9me3 formation by SUV39H1 has been well characterized in heterochromatic gene silencing (5). However, recent reports indicate that SUV39H1-mediated H3K9me3 also occurs at euchromatic gene promoters involved in muscle differentiation and cell cycle regulation (22–24). Suv39h1 has wide ranging expression patterns in mouse tissues (25). However, the expression pattern of Suv39h1 specifically in db/db mice has not yet been explored. Our article demonstrates a role for Suv39h1 in db/db mice VSMC under diabetic conditions. Our data with SUV39H1 gene silencing and overexpression suggest a role in the negative regulation of inflammatory genes IL-6, MCSF, and MCP-1. This repression is lost under diabetic conditions because of reduced protein levels and promoter recruitment. Importantly, this diabetic phenotype is retained even in cells passaged and cultured in vitro and can be reversed partially by overexpressing SUV39H1 in db/db VSMC, demonstrating a key role for SUV39H1 in metabolic memory.
Hyperglycemia is implicated in diabetic complications and linked to metabolic memory, suggesting the need for early tight metabolic control (2, 26). We also examined whether HG itself affects H3K9me3. HG increased inflammatory genes and decreased H3K9me3 levels at such promoters in HVSMC, suggesting that loss of H3K9me3 and gene repression most likely result from hyperglycemic exposure, which is then maintained and propagated in an epigenetic fashion in diabetic VSMC even after removal from the mice. Thus hyperglycemia may be the primary stimulus, although some contribution from dyslipidemia cannot be fully ruled out.
Taken together, our studies demonstrate aberrant regulation of Suv39h1 and H3K9me3 in diabetic mice. HG also similarly affected HVSMC, suggesting mechanisms for the mysterious metabolic memory and sustained inflammatory phenotype of vascular cells associated with the accelerated vascular complications in diabetes.
Materials and Methods
Materials.
For information about materials used in this study, see SI Text.
Cell Culture.
All animal protocols were approved by an Institutional Research Animal Care Committee. Mouse aortic VSMC (MVSMC) from 9-to 11-week-old male diabetic db/db mice (blood glucose levels >450 mg/dl) and heterozygous nondiabetic db/+ control littermates (blood glucose <140 mg/dl) were isolated as described (18). MVSMC were cultured in vitro for 5–8 weeks in DMEM/F12 medium supplemented with 10% FCS and used between passages 5 and 8 to obtain sufficient numbers of cells and maintain the diabetic phenotype. HVSMC were cultured in SmBM medium as described by the manufacturer. When indicated, MVSMC and HVSMC were serum-depleted for 24–48 h by using DMEM/F12 supplemented with 0.2% BSA or SmBM supplemented with 0.5% FBS, respectively, before stimulation with 10 ng/ml TNF-α for the indicated time periods.
qRT-PCR.
Total RNA was extracted and RT-PCRs performed as described (18). β-Actin was used as internal control, and levels showed no difference in db/db vs. db/+ (Fig. S5). Primer sequences and annealing temperatures can be found in the Table S1. Real-time qPCRs were performed in triplicate and mRNA levels calculated after normalization to β-actin levels, by using the formula Egene(Ctcontrol−Cttreated)/Eactin(Ctactin control−Ctactin treated), where Ct is the threshold value, and E is the amplification efficiency calculated from the slope of the standard curve generated by Applied Biosystems software using the formula 10(−1/slope) (27). Amplification efficiency was 100 ± 10% for all primers. Results were expressed as fold over control or percentage of control.
ChIP Assays.
ChIP assays were performed as described (28). ChIP-enriched samples were analyzed in triplicate by qPCR using primer sequences near the NF-κB-binding sites on the IL-6, MCSF, and MCP-1 promoters and MCP-1 enhancer. Primer sequences are listed in Table S1. Data were analyzed by using the 2−ΔΔCt method and normalized with input samples as described (28). Results were expressed as fold over control or percentage of control.
Immunoblotting.
Preparation of cell lysates and Western blot analysis were performed as described (19). Intensity of protein bands was quantified by GS-800 densitometer and Quantity One software (Bio-Rad).
Cloning of SUV39H1 Expression and shRNA Vectors.
Human SUV39H1 cDNA with FLAG tag was PCR-amplified from MS2 vector (gift from Jiemin Wong, Baylor College of Medicine, Houston, TX). PCR product digested with XhoI and XbaI was inserted into the pCR3.1 expression vector downstream of the CMV promoter (Promega). The SUV39H1 shRNA vector was constructed by using our reported methods (29). Briefly, SUV39H1 shRNA targeting the SET domain (30) was cloned under the control of U6 promoter into a vector that also expresses EGFP from a CMV promoter to monitor transfection efficiency. Plasmid vector GT expressing a scrambled sequence (gift from D. Reinberg, New York University School of Medicine, New York) was used as control for shRNA transfection.
Nucleofection of VSMC.
VSMC were transfected with indicated plasmids by using Amaxa nucleofection reagent as described (19). This yielded transfection efficiencies of 50–60%.
Statistical Analysis.
PRISM software (GraphPad) was used for data analysis with Student's t tests to compare two groups for mean comparison. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments.
We are deeply grateful to all those who generously provided plasmid vectors and reagents. This work was supported by National Institutes of Health Grants R01 HL87864 and R01 DK065073 (to R.N.), a predoctoral fellowship from the Myrtle Carr Foundation (to L.M.V.), and a Junior Faculty Award from the American Diabetes Association (to M.A.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803623105/DCSupplemental.
References
- 1.Nathan DM, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ihnat MA, Thorpe JE, Ceriello A. Hypothesis: The “metabolic memory”, the new challenge of diabetes. Diabetic Med. 2007;24:582–586. doi: 10.1111/j.1464-5491.2007.02138.x. [DOI] [PubMed] [Google Scholar]
- 3.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1079. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 4.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nature. 2005;6:838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 5.Peters AH, et al. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol Cell. 2003;12:1577–1589. doi: 10.1016/s1097-2765(03)00477-5. [DOI] [PubMed] [Google Scholar]
- 6.Shilatifard A. Chromatin modifications by methylation and ubiquitination: Implications in the regulation of gene expression. Annu Rev Biochem. 2006;75:243–69. doi: 10.1146/annurev.biochem.75.103004.142422. [DOI] [PubMed] [Google Scholar]
- 7.Lachner M, Jenuwein T. The many faces of histone lysine methylation. Curr Opin Cell Biol. 2002;14:286–98. doi: 10.1016/s0955-0674(02)00335-6. [DOI] [PubMed] [Google Scholar]
- 8.Saccani S, Natoli G. Dynamic changes in histone H3 Lys 9 methylation occurring at tightly regulated inducible inflammatory genes. Genes Dev. 2002;16:2219–2224. doi: 10.1101/gad.232502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–980. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 10.Gazzar ME, Yoza BK, Hu TY, Cousart SL, McCall CE. Epigenetic silencing of TNFα during endotoxin tolerance. J Biol Chem. 2007;282:26857–26864. doi: 10.1074/jbc.M704584200. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, Whetstine JR. Dynamic regulation of histone lysine methylation by demethylases. Mol Cell. 2007;25:1–14. doi: 10.1016/j.molcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Libby P, Plutzky J. Diabetic macrovascular disease. The glucose paradox? Circulation. 2002;106:2760–2763. doi: 10.1161/01.cir.0000037282.92395.ae. [DOI] [PubMed] [Google Scholar]
- 13.Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 14.Devaraj S, et al. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes. 2006;55:774–779. doi: 10.2337/diabetes.55.03.06.db05-1417. [DOI] [PubMed] [Google Scholar]
- 15.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2005;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Wautier MP, Boulanger E, Guillausseau PJ, Massin P, Wautier JL. AGEs, macrophage colony stimulating factor and vascular adhesion molecule blood levels are increased in patients with diabetic microangiopathy. Thromb Haemost. 2004;91:879–885. doi: 10.1160/TH03-07-0486. [DOI] [PubMed] [Google Scholar]
- 17.Lee GH, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 18.Li S-L, et al. Enhanced pro-atherogenic responses in macrophages and vascular smooth muscle cells derived from diabetic db/db mice. Diabetes. 2006;55:2611–2619. doi: 10.2337/db06-0164. [DOI] [PubMed] [Google Scholar]
- 19.Reddy MA, et al. Key role of Src kinase in S100b-induced activation of the receptor for advanced glycation end products in vascular smooth muscle cells. J Biol Chem. 2006;281:13685–13693. doi: 10.1074/jbc.M511425200. [DOI] [PubMed] [Google Scholar]
- 20.Yerneni KK, Bai W, Khan BV, Medford RM, Natarajan R. Hyperglycemia-induced activation of nuclear transcription factor κB in vascular smooth muscle cells. Diabetes. 1999;48:855–864. doi: 10.2337/diabetes.48.4.855. [DOI] [PubMed] [Google Scholar]
- 21.Bierhaus A, et al. Diabetes-associated sustained activation of the transcription factor, nuclear factor-κB. Diabetes. 2001;50:2792–2808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 22.Zhang CL, McKinsey TA, Olson EN. Association of class II histone deacetylases with heterochromatin protein 1: Potential role for histone methylation in control of muscle differentiation. Mol Cell Biol. 2002;22:7302–7312. doi: 10.1128/MCB.22.20.7302-7312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mal AK. Histone methyltransferase Suv39h1 represses MyoD-stimulated myogenic differentiation. EMBO J. 2006;25:3323–3334. doi: 10.1038/sj.emboj.7601229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart DM, Li J, Wong J. Relationship between histone H3 lysine 9 methylation, transcription repression and heterochromatin protein 1 recruitment. Mol Cell Biol. 2005;25:2525–2538. doi: 10.1128/MCB.25.7.2525-2538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czvitkovich S, et al. Over-expression of the SUV39H1 histone methyltransferase induces altered proliferation and differentiation in transgenic mice. Mech Dev. 2001;107:141–153. doi: 10.1016/s0925-4773(01)00464-6. [DOI] [PubMed] [Google Scholar]
- 26.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 27.Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahar S, et al. Cooperation of SRC-1 and p300 with NF-kB and CREB in angiotensin II-induced IL-6 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1528–1534. doi: 10.1161/ATVBAHA.107.145862. [DOI] [PubMed] [Google Scholar]
- 29.Li S-L, et al. Effects of silencing leukocyte-type 12/15-lipoxygenase using short interfering RNAs. J Lipid Res. 2005;46:220–229. doi: 10.1194/jlr.M400328-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Ait-Si-Ali S, et al. A Suv39h-dependent mechanism for silencing S-phase genes in differentiation but not in cycling cells. EMBO J. 2004;23:605–615. doi: 10.1038/sj.emboj.7600074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.