Abstract
Voltage-gated potassium channels are comprised of four subunits, and each subunit has a pore domain and a voltage-sensing domain (VSD). The four pore domains assemble to form one single central pore, and the four individual VSDs control the gate of the pore. Recently, a family of voltage-gated proton channels, such as HV or voltage sensor only protein (VSOP), was discovered that contain a single VSD but no pore domain. It has been assumed that VSOP channels are monomeric and contain a single VSD that functions as both the VSD and the pore domain. It remains unclear, however, how a protein that contains only a VSD and no pore domain can conduct ions. Using fluorescence measurements and immunoprecipitation techniques, we show here that VSOP channels are expressed as multimeric channels. Further, FRET experiments on constructs with covalently linked subunits show that VSOP channels are dimers. Truncation of the cytoplasmic regions of VSOP reduced the dimerization, suggesting that the dimerization is caused mainly by cytoplasmic protein–protein interactions. However, these N terminus- and C terminus-deleted channels displayed large proton currents. Therefore, we conclude that even though VSOP channels are expressed mainly as dimers in the cell membrane, single VSOP subunits could function independently as proton channels.
Keywords: dimer, FRET, Hv, voltage sensor, voltage sensor only protein
Voltage-gated proton channels (HV channels) have been found in many mammalian cells, including skeletal muscle, lungs, microglia, and blood (1). HV channels have also been shown to play a crucial role in the immune system: HV channels in macrophages are involved in the pathway for the generation of reactive oxygen species, which is critical to the process of phagocytosis and the destruction of foreign pathogens (1). HV channels are activated at depolarized voltages, and their activation can be blocked by Zn2+ (2, 3).
Recently, a family of voltage-gated proton channels, called HV or voltage sensor only protein (VSOP) channels, was cloned. VSOP channels were found to have four transmembrane domains, and these domains are homologous to the four transmembrane domains of the voltage-sensing domain (VSD) of voltage-gated potassium channels (2, 3). Voltage-gated potassium channels are comprised of four subunits, each of which has a pore domain and a VSD domain. The four pore domains come together to form one single central pore, and the four individual VSDs control the gate of the pore (4). It is not clear, however, how VSOP channels containing only a VSD and no pore domain can conduct ions. Recently, it was shown that the VSD in Na+ and K+ channels could function as a proton or cation pore independently of the centrally located pore (5–8). It was therefore suggested that a VSOP channel functions as a single, independent VSD and that this VSD makes up the entire proton channel (1, 9, 10). In the present work, we tested the hypothesis that Hv channels are expressed as monomers. We did this by examining whether HA-tagged mouse VSOP (mVSOP) channel subunits can immunoprecipitate Myc-tagged mVSOP channels and whether fluorophores attached to VSOP subunits are close enough to undergo FRET.
Results
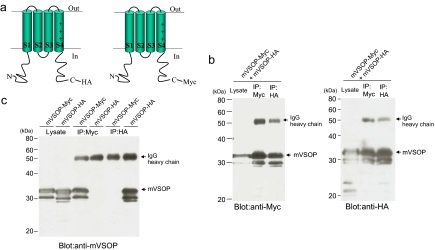
To test whether mVSOP channels are composed of multiple subunits, we made two constructs of mVSOP, one with an HA tag in the C terminus and one with a Myc tag in the C terminus (Fig. 1a). We coexpressed these two constructs in tsA201 cells. Cell extracts were precipitated with either anti-HA or anti-Myc antibodies (Fig. 1b). Myc-tagged VSOP subunits were immunoprecipitated from these extracts when either HA or Myc antibodies were used. Conversely, HA-tagged VSOP subunits were also immunoprecipitated from these extracts when either HA or Myc antibodies were used. In control experiments, HA-tagged VSOP subunits were not immunoprecipitated with Myc antibodies from extracts of cells expressing only HA-tagged VSOP subunits, nor were Myc-tagged VSOP subunits immunoprecipitated with HA antibodies from extracts of cells expressing only Myc-tagged VSOP subunits (Fig. 1c). These data show that VSOP subunits form multimers containing both Myc- and HA-tagged subunits.
Fig. 1.
Coimmunoprecipitation of VSOP channel subunits. (a and b) Mammalian expression vectors encoding the Myc-tagged and HA-tagged mVSOP were cotransfected in tsA201 cells. After the cell extracts were precipitated with anti-Myc (IP:Myc) or anti- HA (IP:HA) antibodies, proteins were eluted and Western blotting analysis was performed. (c) Mammalian expression vector encoding the Myc-tagged or HA-tagged mVSOP was transfected in tsA201 cells. After the cell extracts were precipitated with anti-Myc (IP:anti-Myc) or anti-HA (IP: anti-HA) antibodies, proteins were eluted and Western blotting analysis was performed.
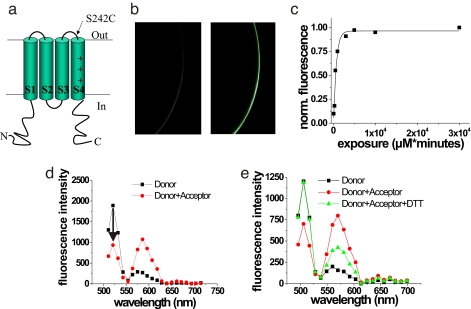
We further used FRET to show that VSOP channels in cell membranes are composed of multiple VSOP subunits. We used FRET to measure distances between ascidian VSOP (Ci-VSOP) subunits that had been fluorescently labeled either with a donor fluorophore (Alexa488) or an acceptor fluorophore (TetraMethylRhodamine; TMR). Ci-VSOP was used in the FRET experiments as this construct is expressed at much higher levels in Xenopus oocytes. In FRET, the energy absorbed by a donor fluorophore can efficiently be transferred to an acceptor fluorophore if the distance between the donor and acceptor fluorophores is <10 nm (11, 12). We used the same method that was previously used to measure distances within human glutamate transporters (11, 12). Briefly, we introduced a single cysteine residue, S242C, into the extracellular region of Ci-VSOP between transmembrane domains S3 and S4 (Fig. 2a). The mutant channels containing the introduced cysteine were expressed in Xenopus oocytes and labeled with Alexa488-maleimide. Alexa488-maleimide labeled oocytes expressing 242C channels substantially more than wild-type VSOP expressing oocytes (Fig. 2b). We measured the labeling time course of S242C for Alexa488-maleimide (Fig. 2c). We then labeled oocytes to a subsaturating level (<20%) of donor fluorophores (Alexa488-maleimide). Fluorescence spectra were measured from individual donor-labeled oocytes, which were subsequently labeled to saturation with acceptor fluorophore {TMR-[2-((5(6)-tetramethylrhodamine) carboxylamino) ethyl-methanethiosulfonate)] (MTS)}, and a second fluorescence spectrum was measured from the same oocyte (Fig. 2d). The addition of the acceptor fluorophore reduced the donor fluorescence significantly, suggesting that VSOP is a multimeric protein. Application of 10 mM DTT, which removes the disulfide-linked acceptor fluorophore (TMR-MTS), restored the donor fluorescence to its original value, showing that the decrease in donor fluorescence was not caused by bleaching (Fig. 2e). The FRET efficiency, measured as donor quenching (13), was 0.65 ± 0.07 (n = 12), corresponding to a distance R = 42.2 ± 1.8 Å (n = 12) between donor and acceptor fluorophores attached to S242C. The FRET efficiency between two fluorophores not only depends on the distance between the fluorophores, but also on the orientation of the fluorophores relative each other. We therefore measured the anisotropy for each fluorophore attached to 242C. The anisotropy (r) was close to 0 for each fluorophore (Alexa488-maleimide: rd = 0.025 ± 0.001; TMR-MTS: ra = 0.026 ± 0.003, n = 5), showing that the fluorophores were free to rotate and that our distance estimate by using the standard orientation factor κ2 = 2/3 was not distorted by the orientation of the fluorophores (see Methods). As concluded from our coimmunoprecipitation results, our FRET results also show that VSOP is expressed as a multimer in the plasma membrane.
Fig. 2.
Associated VSOP subunits in the plasma membrane. (a) VSOP construct containing S242C mutation. (b) Confocal images of oocyte expressing WT Ci-VSOP (Left) and 242C Ci-VSOP (Right) channels labeled with Alexa488-maleimide. (Magnification: × 50.) (c) Labeling time course for Alexa488-maleimide of 242C fitted with an exponential function with τ = 670.5 μM × min. (d) Representative fluorescence spectrum from an oocyte expressing Ci-VSOP S242C channels first labeled with donor fluorophore only (Alexafluor488-maleimide, ≈20% of the subunits labeled; squares) and then labeled to saturation with acceptor fluorophore (TMR-MTS; circles). FRET efficiency was measured as the % decrease in donor fluorescence (arrow). (e) Representative fluorescence spectrum from an oocyte expressing Ci-VSOP S242C channels first labeled with donor fluorophore only (Alexafluor488-maleimide, ≈20% of the subunits labeled; squares), labeled to saturation with acceptor fluorophore (TMR-MTS; circles), and exposed to DTT to restore the donor fluorescence (10 mM DTT for 10 min; triangles).
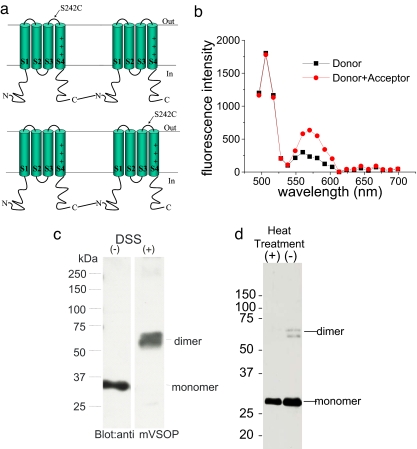
Even though both the coimmunoprecipitation and the FRET results show that VSOP are multimeric proteins, neither the immunoprecipitation data nor the FRET data from the 242C VSOP subunits can tell us with certainty how many subunits there are in a functional VSOP channel. We therefore constructed cDNAs for linked dimers of VSOP subunits containing one WT VSOP subunit and one 242C VSOP subunit, by linking the C terminus of one subunit to the N terminus of the other subunit with a 14-aa linker (Fig. 3a). The covalently linked construct generated robust proton currents similar to WT channels when injected into oocytes (data not shown). We coinjected two constructs, one construct with the 242C subunit first and the WT subunit second, and one construct with the WT subunit first and the 242C subunit second, in equal amounts into oocytes and repeated our FRET measurements on the coinjected channels (Fig. 3b). If VSOP channels were composed of multimeric channels with more than two subunits, then 50% of the resulting channels should have cysteines in two neighboring subunits and fluorophores attached to these channels should undergo FRET to a substantial degree. A simple calculation would predict a total FRET efficiency for these coinjected oocytes of ≈0.25 for any multimeric channel with more than two subunits, assuming random assembly of the two constructs (see Methods). However, if VSOP channels are dimeric channels then each channel will only have one cysteine and the fluorophores will not undergo any FRET. The FRET efficiency for the coinjected oocytes was 0.009 ± .013 (n = 5 cells; Fig. 3b), showing that VSOP channels are not composed of more than two subunits. In addition, the absence of FRET in the coinjected oocytes shows that the FRET measured in oocytes injected with only 242C VSOP subunits is not caused by unspecific aggregation of VSOP subunits. We also tested whether oligomerized VSOP subunits can be detected in Western blot assays by using cross-linkers. Blot of VSOP proteins after treatment with the cross-linker disuccinimidyl suberate (DSS) showed positive bands at the position corresponding to a dimer of VSOP subunits and a weaker band at the monomer size, but not at higher molecular weights, consistent with the idea that VSOP forms dimers (Fig. 3c). We sometimes detected a faint band at the position of dimers in the control condition (no DSS treatment). This band disappeared when the samples were boiled (Fig. 3d), suggesting that this high molecular weight band may be caused by noncovalent associations, such as coiled-coil interactions, between subunits (see Discussion).
Fig. 3.
VSOP subunits expressed as dimers in the plasma membrane. (a) WT Ci-VSOP and 242C Ci-VSOP subunits were linked together with a 14-aa linker, as WT-242C or 242C-WT constructs. (b) Representative fluorescence spectrum from an oocyte coexpressing WT-S242C and S242C-WT constructs labeled with donor fluorophore only (Alexafluor488-maleimide, ≈20% of the subunits labeled; squares) and then labeled to saturation with acceptor fluorophore (TMR-MTS; circles). No changes in the donor fluorescence were detected, showing that no FRET occurred. (c) mVSOP was expressed in tsA201 cells, washed with PBS, and treated with 1 mM DSS. Proteins were separated on PAGE under nonreducing conditions and subjected to immunoblotting with anti-mVSOP antibody. (d) Western blot analysis with heterologously expressed VSOP proteins as in c, but without DSS treatment. We sometimes detected faint band at higher molecular weight region (−), in addition to the major band corresponding to the molecular weight of the monomer. This band disappears when samples were boiled (+).
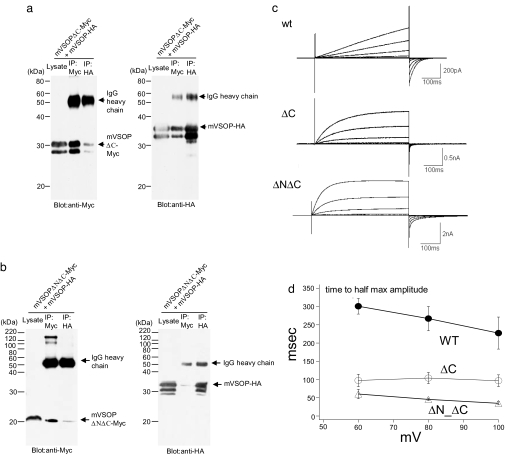
To gain more information about the molecular nature of the dimer interactions of VSOP subunits, we conducted coimmunoprecipitation experiments with VSOP subunits in which the cytoplasmic regions were truncated. One construct lacks only the C-terminal region (mVSOP-DeltaC) with a stop codon introduced 10 amino acids after the last transmembrane domain, S4. Another construct lacks both the N terminus region and the C-terminal region (mVSOP-DeltaN-DeltaC). Deleting the C terminus reduced the coimmunoprecipitation of HA- and Myc-tagged VSOP subunits (Fig. 4a). Deletion of the N terminus further reduced coimmunoprecipitation (Fig. 4b), suggesting that the main dimer interaction involves both the N terminus and the C terminus. To test whether VSOP still functions without dimerization, proton currents were measured from these truncated forms. Both mVSOP-DeltaC and mVSOP-DeltaN-DeltaC exhibited robust Hv currents (71.3 ± 32.2 pA/pF, n = 6; 127.7 ± 74.7 pA/pF, n = 6; 71 ± 26.4 pA/pF, n = 5 for mVSOP-DeltaC, mVSOP-DeltaN-DeltaC, and WT, respectively; Fig. 4c). These currents showed a similar pH-sensitive shift of voltage-dependent gating as the WT protein (data not shown). These data indicate that dimerization is not essential for the primary properties of Hv channels, i.e., proton permeation and voltage-dependent gating. However, closer examination of current traces showed that the kinetics of activation is significantly faster in both truncated forms than in the full-length protein (WT) (Fig. 4d). Together, these results suggest that proton channels can operate as monomers and that the presence of the cytosolic domains and/or dimerization make the channels more difficult to open by slowing the activation of the channels.
Fig. 4.
Cytoplasmic regions are important for coimmunoprecipitation of VSOP channel subunits. (a and b) Coimmunoprecipitation of N terminus- and/or C terminus-truncated mVSOP channels. mVSOP subunits were truncated at position 216 at C terminus (mVSOP-ΔC), or both at 216 for C terminus and 77 for N terminus (mVSOP-ΔN-ΔC). (c) Currents from N terminus- and/or C terminus-truncated mVSOP channels in response to voltage steps from 40 to 100 mV in comparison with those from the full-length wild-type (WT). Holding potential was −80 mV. (d) Activation kinetics as measured by time to half of maximum magnitude during a depolarizing step to 40, 60, 80, and 100 mV for 500 ms.
Discussion
Most members in the superfamily of voltage-gated ion channels (i.e., Na+, Ca2+, and K+ channels) are composed of four subunits or domains, each with six transmembrane segments. The first four transmembrane segments of each subunit make up a separate VSD. The two last transmembrane segments from each subunit come together to make up one common, centrally located pore domain (14). Hv/VSOP channels were found to be homologous to voltage-gated K channels, except that Hv/VSOP channels only have the first four transmembrane segments. When Hv/VSOP channels were first sequenced, it was not clear how Hv/VSOP channels could make a functional channel without an obvious pore domain. It was also not known how many subunits were necessary to make a functional Hv channel.
It has previously been shown that mutations in S4 of the VSD of a Na+ or K+ channel introduced a proton or cation pore in the VSD, and that this cation pore is independent of the centrally located pore of the channel (5–8). It was therefore suggested that VSOP, which lacks a typical pore domain, functions as a single VSD (i.e., that the VSOP channel is a monomer) and that the pore is composed of some pathway through the middle of the VSD (9, 10). In addition, it was recently shown that the related VSP, which is a voltage-sensitive phosphatase composed of a single VSD coupled to a phosphatase, is functionally and structurally a monomer (15). In contrast, in the present work we have shown that VSOP channels are expressed as dimeric proteins. Our results from the experiments on C terminus- and N terminus-truncated VSOP suggest that the presence of the cytoplasmic domains is important for the dimerization of VSOP. In fact, the C terminus sequence exhibits a coiled-coil structure that is known to mediate subunit interactions in many examples of ion channel assembly (16–18). However, because the C terminus- and N terminus-truncated VSOP construct still conduct protons, the dimerization does not seem to be necessary for VSOP to function as a proton channel. Therefore, the proton-permeating pathway must be located within the VSOP protein rather than at the interface between two neighboring subunits. We therefore propose that VSOP is a dimer channel with two pores, similar to voltage-gated Clc chloride channels (19).
Given that the basic properties of a proton channel are seemingly inherent within a monomer, what then is the physiological significance of the dimer formation? We showed that the C terminus- and N terminus-truncated VSOP exhibited significantly reduced dimerization as seen by the coimmunoprecipitation experiments. These channels exhibited faster kinetics of activation and deactivation as compared with the full-length protein. It is known that voltage-gated proton channels in activated phagocytes exhibit a lower activation voltage and more rapid kinetics compared with proton channels in resting cells (20, 21). In addition, the same voltage-gated proton channels in different cell types exhibit a diverse range of channel kinetics (22). It is possible that a mixed population of VSOP channels in either dimerized or monomeric form could account at least in part for such kinetic diversities of native voltage-gated proton channels. However, the faster kinetics in the C terminus- and N terminus-truncated VSOP channels could also be caused directly by the lack of the C and N terminus and not by dimerization per se. This hypothesis will be tested in future experiments when other means of breaking the dimerization have been identified. It will be intriguing to address whether a transition between monomers and dimers could occur in any physiological context, for example modulated by the state of “respiratory burst” that is known to be the key event in phagocytosis.
Methods
Mutagenesis, Expression, and Two-Electrode Voltage Clamp (TEVC) Recording of VSOP Channels.
Site-directed mutagenesis, in vitro transcription of cRNA, and injection of cRNA encoding the Ciona Ci-VSOP into Xenopus laevis oocytes, and TEVC recordings were performed as described (3, 23). Site-directed mutagenesis of mVSOP and transfection into HEK tsA201 cells with polyFect (Qiagen) were performed as described (3). Whole-cell mVSOP currents were recorded from HEK tsA201 cells as described (3). Briefly, cells were transfected with pIRES2-EGFP containing the cDNA for mVSOP or its deleted version, and whole-cell patch recording was performed. Patch pipettes had a resistance <10 Mohm. Series resistance compensation was done up to 75% correction to reduce the voltage error. Recording was done at 25–27° C. External solution contained 75 mM N-methyl-d-glucamine (NMDG), 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 180 mM Hepes (pH 7.0). Pipette solution contained 65 mM NMDG, 3 mM MgCl2, 1 mM EGTA, and 183 mM Hepes (pH 7.0).
HA- and Myc-Tagged Subunits, Linked Dimer Constructs, and C-Terminal Truncated VSOP Subunits.
The sequences for HA and Myc tags, YPYDVPDYA and ASMQKLISEEDL, respectively, were added to the C terminus by PCR-based subcloning. Linked dimers of WT and 242C Ci-VSOP subunits were constructed by changing the stop codon in one subunit to an AgeI site and introducing an AgeI site 39 bases before the starting codon in the other subunit. Both constructs were digested with AgeI and SacI, and the appropriate DNA segments were purified and ligated. This process resulted in that the subunits in the linked dimers were linked by a 14-aa linker consisting of the amino acids QPVIEFLQPGGSAT. The C terminus was deleted by introducing a stop codon in mVSOP at Val-216. The N terminus was deleted by using PCR primer covering residue 78 as the initiator methionine instead of the native proline residue.
Biochemistry.
For coimmunoprecipitation assays, tsA201 cells expressing mVSOP-Myc and–HA were homogenized in solubilization buffer [20 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, and 1% n-dodecyl-β-d-maltoside] and protease inhibitor mixture. Homogenates were incubated for 20 min on ice and centrifuged for 30 min at 20,000 × g. Protein A agarose beads were incubated with 5 μg/ml of anti-Myc or anti-HA polyclonal antibody for 1 h at 4°C with gentle rotation. The soluble fraction was incubated with Protein A agarose beads for 1 h at 4°C with gentle rotation. The beads were washed four times with wash buffer [20 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, and 0.1% n-dodecyl-β-d-maltoside], and bound proteins were released by boiling for 3 min with SDS/PAGE sample buffer. Western blot analysis was performed by detecting precipitated proteins with antibodies.
For DSS cross-linking of mVSOP proteins in tsA201 cells, cells were washed with PBS and treated with 1 mM DSS. The DSS reaction was quenched by 100 mM Tris·HCl, pH 8.0. Proteins were separated on 10% SDS/PAGE under nonreducing conditions and electrophoretically transferred to Immobilon-P (Millipore). The membrane was incubated in 10% skimmed milk (Difco) dissolved in PBS-Tween20 (PBS-T; 0.1%) for 1 h at room temperature (RT). After washing with PBS-T, the membranes were incubated with anti-mVSOP antibody (0.5 μg/ml, final concentration) for 1 h at RT. After washing with PBS-T, the membrane was incubated with the secondary antibody (1:2,000; horseradish peroxidase-conjugated anti-rabbit Ig from donkey; Amersham Pharmacia) for 1 h at RT. Polyclonal anti-mVSOP antibody was generated in rabbit against GST-fused polypeptide corresponding to amino acids Thr-218–Asn-269 of the C terminus of mVSOP.
FRET Measurements.
FRET measurements were performed as described (11, 12). Briefly, after having determined the labeling time course for the donor fluorophore Alexa488-Maleimide for Ci-VSOP S242C channels, we labeled ≈20% of the 242C subunits in an oocyte with the donor fluorophore. This procedure ensured that only 10% [0.04/(0.04 + 0.32)] of the VSOP dimers that were labeled with donor fluorophores had two donors, because the probability P of having n donor fluorophores for a dimeric protein are: P[n = 2] = 0.22 = 0.04, P[n = 1] = 2 × 0.2 × 0.8 = 0.32, P[n = 0] = 0.82 = 0.64. The VSOP dimers with two donor fluorophores do not undergo FRET. Therefore, in estimating the FRET efficiencies, the donor fluorescence was corrected for this predicted double labeling of donor fluorophores. A donor-only fluorescence spectrum was measured on a Zeiss LSM 510 inverted confocal microscope with a META spectral detector by using 488-nm exitation. The oocyte was subsequently labeled to saturation with TMR-MTS acceptor fluorophore, and a second fluorescence (donor + acceptor) spectrum was measured. The FRET efficiency E was determined by the donor quenching method measured at 520 nm. The decrease of donor fluorescence was measured at 520 nm because, at this wavelength, the oocyte endogenous fluorescence and the acceptor fluorescence were negligible when excited with a 488-nm laser (11, 12). The distance between acceptor and donor fluorophores was calculated from ref. 13:
R0 (the Förster distance) is given by: R0 = 0.211(κ2n−4QDJ(λ))1/6, where κ is the orientation factor, n is the refractive index of the solvent, Q is the quantum yield, and J(λ)) is the spectral overlap between the donor emission and acceptor absorption. R0 was previously estimated to be 50 Å for Alexa488 and TMR (11, 12). Uncertainties in the κ2 lead to errors in the estimates of R0. We therefore measured the anisotropy to estimate the range of possible values of κ2. κ2 was assumed to be 2/3, because the anisotropy measurements indicated that all residues had a reasonable mobility. The range of possible values for κ2 was 0.55–0.97, corresponding to a worst-case error in R0 of 3 Å. Most likely the errors are smaller because the estimates of κ2 do not take into account the depolarization caused by the FRET process itself (24). The background fluorescence in fluorescent-labeled, uninjected oocytes was subtracted from the fluorescence signals.
Estimating the Number of Subunits from FRET.
If we assume that VSOP was a tetrameric protein, then from the coinjection of the two linked-dimer constructs (WT-242C and 242C-WT) we expect a 50% chance that a 242C subunit would be next to another 242C subunit, assuming that the two constructs assemble randomly. Because we labeled to 20% of the maximum of donor fluorescence, 32% of the putative tetrameric complex would be labeled with one donor fluorophore and 4% would be labeled with two donor fluorophores (which would not undergo FRET). To simplify the calculation, we have assumed that FRET between nonneighboring 242C subunits in the same putative tetrameric complex is negligible. Therefore, 40% [i.e., the number of donor fluorophores with neighboring acceptor fluorophores/total number of donor fluorophores = (0.5 × 32)/(4 × 2 + 32 ×1)] of the donor fluorophores would be next to an acceptor fluorophore in a neighboring subunit and therefore undergo FRET. Thus, if VSOP was tetrameric, then the FRET efficiency in the coinjection experiments would be 40% of the FRET efficiency observed in the 242C FRET experiments between a single donor and acceptor fluorophore. The expected FRET efficiency in the coinjection experiments for a tetrameric VSOP would therefore be 0.22 (i.e., 40% of 0.54; 0.54 is the calculated FRET efficiency between a single donor and acceptor for tetrameric 242C channels with a measured total FRET efficiency of 0.65). Similar arguments can be made for any multimer of VSOP with more than two subunits. In contrast, for a dimeric VSOP, no FRET would be expected.
Anisotropy and κ2 Range.
The anisotropy (r) was measured from 242C-expressing oocytes labeled with only Alexa-488-maleimide (rd) and 242C-expressing oocytes labeled with only TMR-MTS (ra). r = Ill − I⊥/Ill + 2I⊥, where Ill is the parallel and I⊥ is the perpendicular-emitted light with respect to the polarized excitation light. The collimated emission signal was split into parallel and perpendicular components by using a polarizing cube beamsplitter, and the two components were measured simultaneously. The error bound in the distance measurements was calculated by setting upper and lower limits for κ2 by using anisotropy values for the donor (rd) and acceptor (ra):
where, Frd and Fra are defined as (rd/r0)0.5 and (ra/r0)0.5, respectively. r0 is the fundamental anisotropy (24).
Acknowledgments.
We thank Dr. Hirohide Iwasaki for help in preparing GST-fusion protein of the C terminus of mVSOP, Dr. Tom McCormack for his insights into potential roles of cytoplasmic regions, and Mr. Masahiro Takagi for help in patch clamp recording.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Okamura Y. Biodiversity of voltage sensor domain proteins. Pflügers Arch. 2007;454:361–371. doi: 10.1007/s00424-007-0222-6. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- 4.Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 5.Starace DM, Bezanilla F. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature. 2004;427:548–553. doi: 10.1038/nature02270. [DOI] [PubMed] [Google Scholar]
- 6.Tombola F, Pathak MM, Isacoff EY. Voltage-sensing arginines in a potassium channel permeate and occlude cation-selective pores. Neuron. 2005;45:379–388. doi: 10.1016/j.neuron.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 7.Sokolov S, Scheuer T, Catterall WA. Gating pore current in an inherited ion channelopathy. Nature. 2007;446:76–78. doi: 10.1038/nature05598. [DOI] [PubMed] [Google Scholar]
- 8.Sokolov S, Scheuer T, Catterall WA. Ion permeation through a voltage- sensitive gating pore in brain sodium channels having voltage sensor mutations. Neuron. 2005;47:183–189. doi: 10.1016/j.neuron.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Tombola F, Pathak MM, Gorostiza P, Isacoff EY. The twisted ion-permeation pathway of a resting voltage-sensing domain. Nature. 2007;445:546–549. doi: 10.1038/nature05396. [DOI] [PubMed] [Google Scholar]
- 10.Tombola F, Pathak MM, Isacoff EY. How does voltage open an ion channel? Annu Rev Cell Dev Biol. 2006;22:23–52. doi: 10.1146/annurev.cellbio.21.020404.145837. [DOI] [PubMed] [Google Scholar]
- 11.Koch HP, Hubbard JM, Larsson HP. Voltage-independent sodium-binding events reported by the 4B–4C loop in the human glutamate transporter EAAT3. J Biol Chem. 2007;282:24547–24553. doi: 10.1074/jbc.M704087200. [DOI] [PubMed] [Google Scholar]
- 12.Koch HP, Larsson HP. Small-scale molecular motions accomplish glutamate uptake in human glutamate transporters. J Neurosci. 2005;25:1730–1736. doi: 10.1523/JNEUROSCI.4138-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selvin PR. Fluorescence resonance energy transfer. Methods Enzymol. 1995;246:300–334. doi: 10.1016/0076-6879(95)46015-2. [DOI] [PubMed] [Google Scholar]
- 14.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 15.Kohout SC, Ulbrich MH, Bell SC, Isacoff EY. Subunit organization and functional transitions in Ci-VSP. Nat Struct Mol Biol. 2008;15:106–108. doi: 10.1038/nsmb1320. [DOI] [PubMed] [Google Scholar]
- 16.Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- 17.Mei ZZ, Xia R, Beech DJ, Jiang LH. Intracellular coiled-coil domain engaged in subunit interaction and assembly of melastatin-related transient receptor potential channel 2. J Biol Chem. 2006;281:38748–38756. doi: 10.1074/jbc.M607591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiener R, et al. The KCNQ1 (Kv7.1) C terminus, a multi-tiered scaffold for subunit assembly and protein interaction. J Biol Chem. 2007;283:5815–5830. doi: 10.1074/jbc.M707541200. [DOI] [PubMed] [Google Scholar]
- 19.Miller C, White MM. Dimeric structure of single chloride channels from Torpedo electroplax. Proc Natl Acad Sci USA. 1984;81:2772–2775. doi: 10.1073/pnas.81.9.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banfi B, et al. A novel H(+) conductance in eosinophils: Unique characteristics and absence in chronic granulomatous disease. J Exp Med. 1999;190:183–194. doi: 10.1084/jem.190.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan D, et al. Sustained activation of proton channels and NADPH oxidase in human eosinophils and murine granulocytes requires PKC but not cPLA2α activity. J Physiol (London) 2007;579:327–44. doi: 10.1113/jphysiol.2006.124248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Decoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- 23.Bruening-Wright A, Elinder F, Larsson HP. Kinetic relationship between the voltage sensor and the activation gate in spHCN channels. J Gen Physiol. 2007;130:71–81. doi: 10.1085/jgp.200709769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakowicz JR. Principles of Fluorescence Spectroscopy. New York: Kluwer; 1999. [Google Scholar]