Abstract
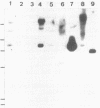
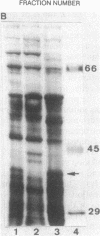
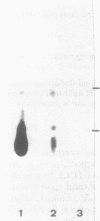
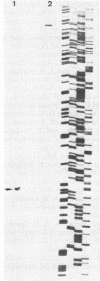
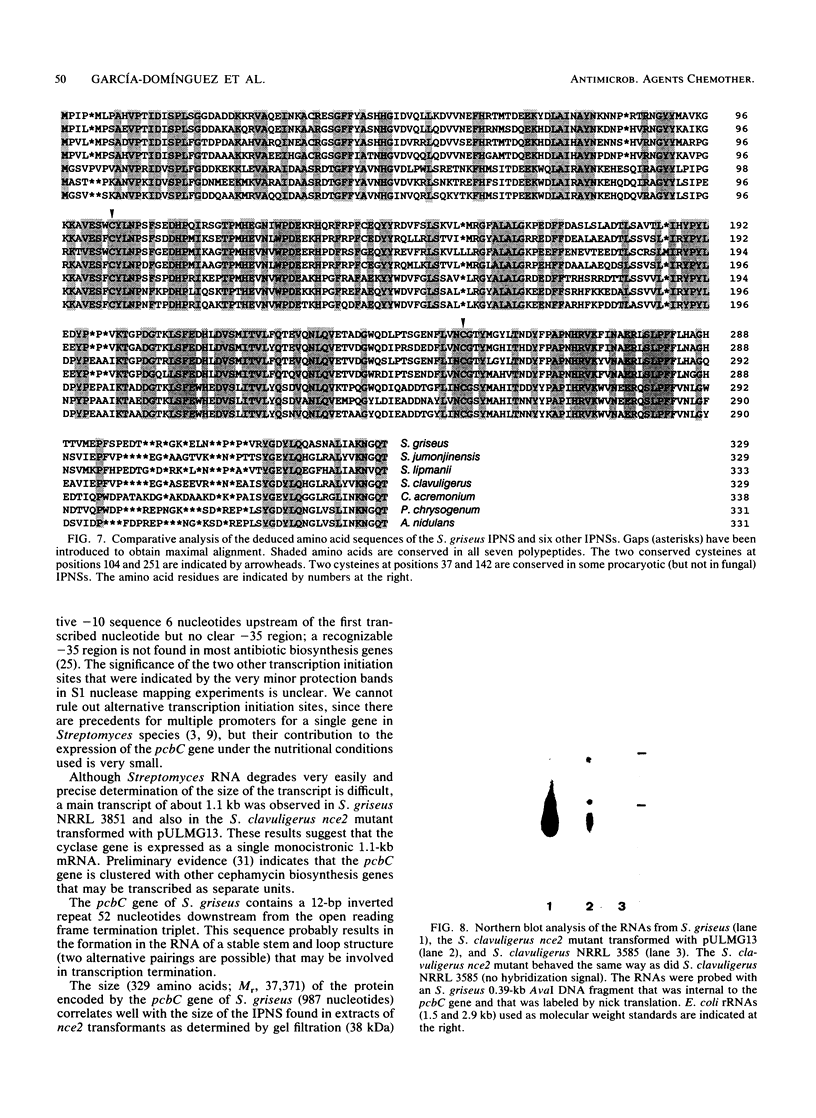
A gene, pcbC, encoding the isopenicillin N synthase of Streptomyces griseus NRRL 3851, has been cloned in a 6.4-kb Bg/II DNA fragment and located in an internal 1.55-kb PvuII segment by hybridization with the Penicillium chrysogenum pcbC gene. Hybridization studies revealed the presence of homologous sequences in the DNAs of several Streptomyces strains and Nocardia lactamdurans. The S. griseus pcbC gene was not expressed in Streptomyces lividans but was expressed in Streptomyces clavuligerus and complemented a mutation, nce2, that impaired isopenicillin N synthase and cephamycin biosynthesis. The pcbC gene contained an open reading frame of 990 nucleotides that encodes a protein of 329 amino acids with a deduced Mr of 37,371. The isopenicillin N synthase formed after expression of the pcbC gene in the S. clavuligerus nce2 mutant strain was found to have an Mr of 38,000 by gel filtration. A protein of about 38 kDa was observed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels of extracts of a transformant of the nce2 mutant strain; this protein was absent from the untransformed mutant strain. The G+C content of the pcbC gene was 63.6%, and the strongly biased codon usage was typical of that of Streptomyces strains. A transcription initiation site was found 44 nucleotides upstream of the ATG translation initiation triplet. A transcript of 1.1 kb was observed in the donor S. griseus strain and also in the S. clavuligerus nce2 mutant strain transformed with the pcbC gene, suggesting that it is transcribed as a monocistronic mRNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez E., Cantoral J. M., Barredo J. L., Díez B., Martín J. F. Purification to homogeneity and characterization of acyl coenzyme A:6-aminopenicillanic acid acyltransferase of Penicillium chrysogenum. Antimicrob Agents Chemother. 1987 Nov;31(11):1675–1682. doi: 10.1128/aac.31.11.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barredo J. L., Cantoral J. M., Alvarez E., Díez B., Martín J. F. Cloning, sequence analysis and transcriptional study of the isopenicillin N synthase of Penicillium chrysogenum AS-P-78. Mol Gen Genet. 1989 Mar;216(1):91–98. doi: 10.1007/BF00332235. [DOI] [PubMed] [Google Scholar]
- Buttner M. J., Smith A. M., Bibb M. J. At least three different RNA polymerase holoenzymes direct transcription of the agarase gene (dagA) of Streptomyces coelicolor A3(2). Cell. 1988 Feb 26;52(4):599–607. doi: 10.1016/0092-8674(88)90472-2. [DOI] [PubMed] [Google Scholar]
- Carr L. G., Skatrud P. L., Scheetz M. E., 2nd, Queener S. W., Ingolia T. D. Cloning and expression of the isopenicillin N synthetase gene from Penicillium chrysogenum. Gene. 1986;48(2-3):257–266. doi: 10.1016/0378-1119(86)90084-3. [DOI] [PubMed] [Google Scholar]
- Castro J. M., Liras P., Laíz L., Cortés J., Martín J. F. Purification and characterization of the isopenicillin N synthase of Streptomyces lactamdurans. J Gen Microbiol. 1988 Jan;134(1):133–141. doi: 10.1099/00221287-134-1-133. [DOI] [PubMed] [Google Scholar]
- Cox K. L., Fishman S. E., Larson J. L., Stanzak R., Reynolds P. A., Yeh W. K., van Frank R. M., Birmingham V. A., Hershberger C. L., Seno E. T. The use of recombinant DNA techniques to study tylosin biosynthesis and resistance in Streptomyces fradiae. J Nat Prod. 1986 Nov-Dec;49(6):971–980. doi: 10.1021/np50048a002. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Fishman S. E., Cox K., Larson J. L., Reynolds P. A., Seno E. T., Yeh W. K., Van Frank R., Hershberger C. L. Cloning genes for the biosynthesis of a macrolide antibiotic. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8248–8252. doi: 10.1073/pnas.84.23.8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornwald J. A., Schmidt F. J., Adams C. W., Rosenberg M., Brawner M. E. Two promoters, one inducible and one constitutive, control transcription of the Streptomyces lividans galactose operon. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2130–2134. doi: 10.1073/pnas.84.8.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Dominguez M., Martin J. F., Mahro B., Demain A. L., Liras P. Efficient plasmid transformation of the beta-lactam producer Streptomyces clavuligerus. Appl Environ Microbiol. 1987 Jun;53(6):1376–1381. doi: 10.1128/aem.53.6.1376-1381.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Malpartida F., Hopwood D. A., Beppu T. afsB stimulates transcription of the actinorhodin biosynthetic pathway in Streptomyces coelicolor A3(2) and Streptomyces lividans. Mol Gen Genet. 1989 Jan;215(2):355–357. doi: 10.1007/BF00339742. [DOI] [PubMed] [Google Scholar]
- Jensen S. E., Leskiw B. K., Vining L. C., Aharonowitz Y., Westlake D. W., Wolfe S. Purification of isopenicillin N synthetase from Streptomyces clavuligerus. Can J Microbiol. 1986 Dec;32(12):953–958. doi: 10.1139/m86-176. [DOI] [PubMed] [Google Scholar]
- Jones G. H., Hopwood D. A. Activation of phenoxazinone synthase expression in Streptomyces lividans by cloned DNA sequences from Streptomyces antibioticus. J Biol Chem. 1984 Nov 25;259(22):14158–14164. [PubMed] [Google Scholar]
- Kieser T., Melton R. E. Plasmid pIJ699, a multi-copy positive-selection vector for Streptomyces. Gene. 1988 May 15;65(1):83–91. doi: 10.1016/0378-1119(88)90419-2. [DOI] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Leskiw B. K., Aharonowitz Y., Mevarech M., Wolfe S., Vining L. C., Westlake D. W., Jensen S. E. Cloning and nucleotide sequence determination of the isopenicillin N synthetase gene from Streptomyces clavuligerus. Gene. 1988;62(2):187–196. doi: 10.1016/0378-1119(88)90557-4. [DOI] [PubMed] [Google Scholar]
- Martín J. F., Liras P. Enzymes involved in penicillin, cephalosporin and cephamycin biosynthesis. Adv Biochem Eng Biotechnol. 1989;39:153–187. doi: 10.1007/BFb0051954. [DOI] [PubMed] [Google Scholar]
- Martín M. F., Liras P. Organization and expression of genes involved in the biosynthesis of antibiotics and other secondary metabolites. Annu Rev Microbiol. 1989;43:173–206. doi: 10.1146/annurev.mi.43.100189.001133. [DOI] [PubMed] [Google Scholar]
- Nüesch J., Heim J., Treichler H. J. The biosynthesis of sulfur-containing beta-lactam antibiotics. Annu Rev Microbiol. 1987;41:51–75. doi: 10.1146/annurev.mi.41.100187.000411. [DOI] [PubMed] [Google Scholar]
- Parag Y. Genetic recombination in Streptomyces griseus. J Bacteriol. 1978 Feb;133(2):1027–1031. doi: 10.1128/jb.133.2.1027-1031.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos F. R., López-Nieto M. J., Martín J. F. Isopenicillin N synthetase of Penicillium chrysogenum, an enzyme that converts delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine to isopenicillin N. Antimicrob Agents Chemother. 1985 Mar;27(3):380–387. doi: 10.1128/aac.27.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón D., Carramolino L., Patiño C., Sánchez F., Peñalva M. A. Cloning and characterization of the isopenicillin N synthetase gene mediating the formation of the beta-lactam ring in Aspergillus nidulans. Gene. 1987;57(2-3):171–181. doi: 10.1016/0378-1119(87)90120-x. [DOI] [PubMed] [Google Scholar]
- Samson S. M., Belagaje R., Blankenship D. T., Chapman J. L., Perry D., Skatrud P. L., VanFrank R. M., Abraham E. P., Baldwin J. E., Queener S. W. Isolation, sequence determination and expression in Escherichia coli of the isopenicillin N synthetase gene from Cephalosporium acremonium. Nature. 1985 Nov 14;318(6042):191–194. doi: 10.1038/318191a0. [DOI] [PubMed] [Google Scholar]
- Samson S. M., Chapman J. L., Belagaje R., Queener S. W., Ingolia T. D. Analysis of the role of cysteine residues in isopenicillin N synthetase activity by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5705–5709. doi: 10.1073/pnas.84.16.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman D., Mevarech M., Jensen S. E., Cohen G., Aharonowitz Y. Cloning and comparative sequence analysis of the gene coding for isopenicillin N synthase in Streptomyces. Mol Gen Genet. 1988 Nov;214(3):562–569. doi: 10.1007/BF00330495. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stapley E. O., Jackson M., Hernandez S., Zimmerman S. B., Currie S. A., Mochales S., Mata J. M., Woodruff H. B., Hendlin D. Cephamycins, a new family of beta-lactam antibiotics. I. Production by actinomycetes, including Streptomyces lactamdurans sp. n. Antimicrob Agents Chemother. 1972 Sep;2(3):122–131. doi: 10.1128/aac.2.3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel B. J., Burgett S. G., Chen V. J., Skatrud P. L., Frolik C. A., Queener S. W., Ingolia T. D. Cloning and expression in Escherichia coli of isopenicillin N synthetase genes from Streptomyces lipmanii and Aspergillus nidulans. J Bacteriol. 1988 Sep;170(9):3817–3826. doi: 10.1128/jb.170.9.3817-3826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]