Abstract
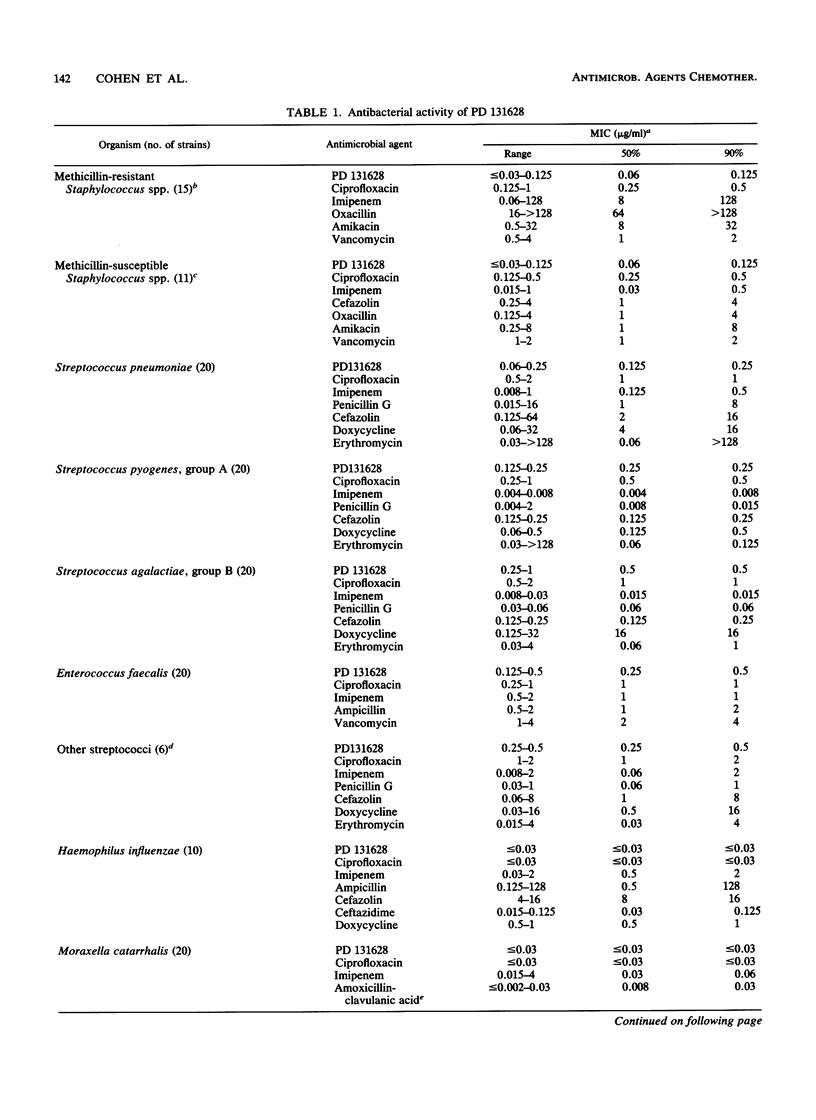
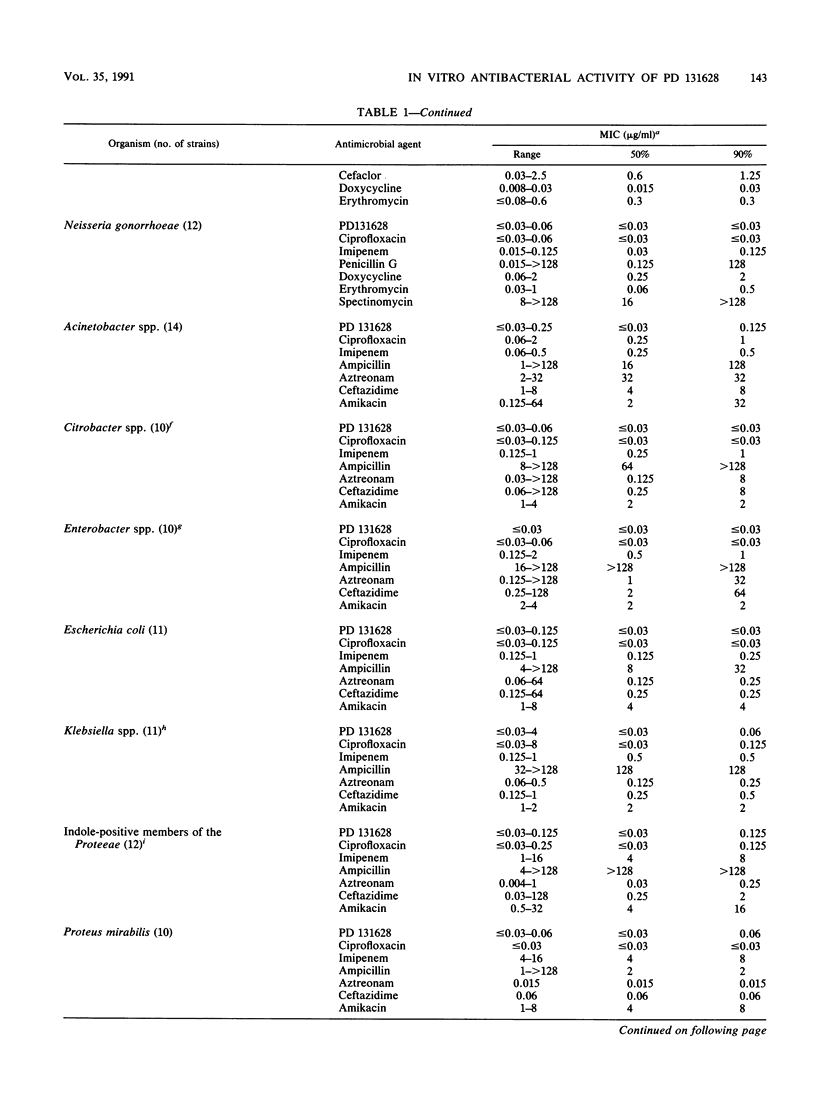
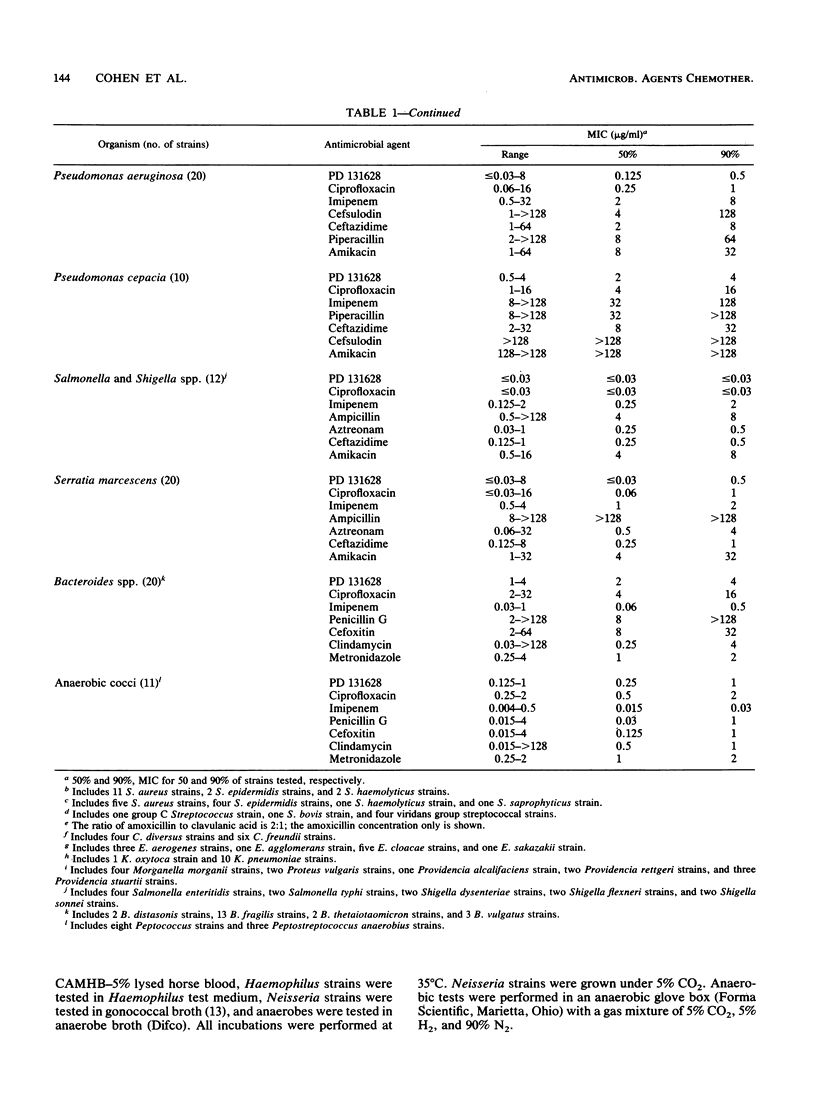
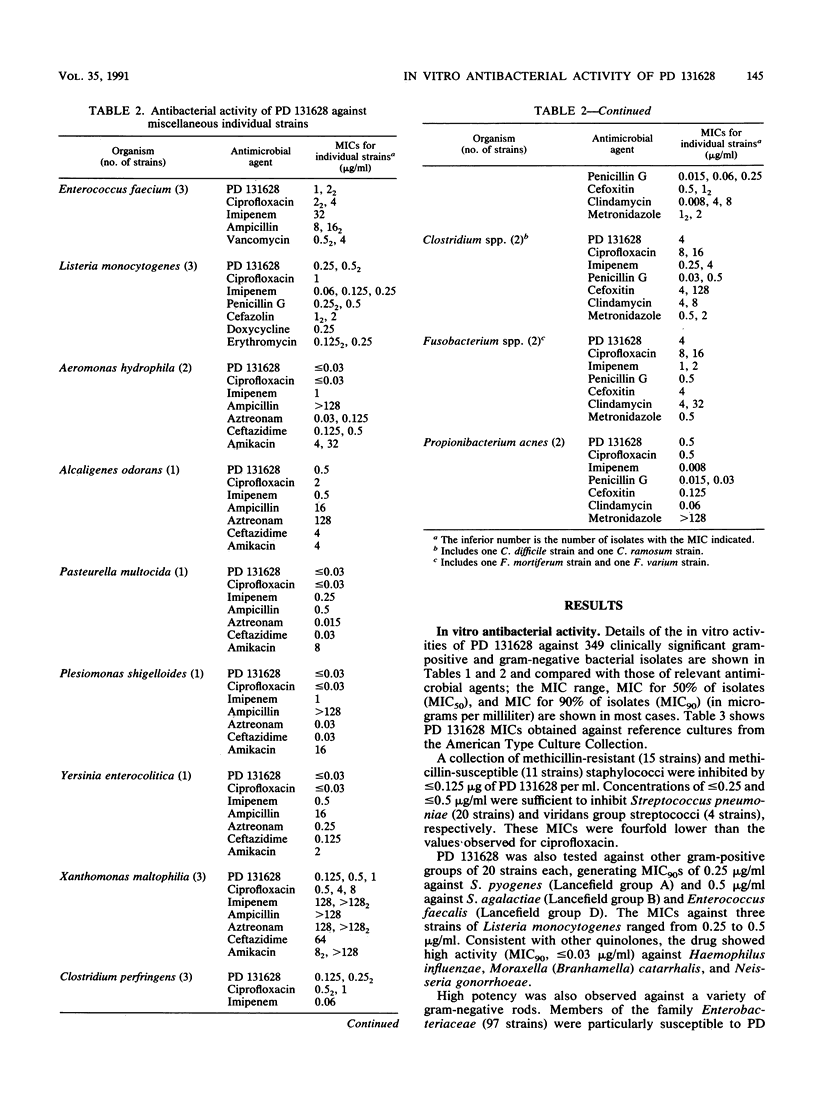
PD 131628 is a new aminopyrrolidine-substituted fluorocyclopropyl naphthyridine quinolone which possesses high in vitro activity against a wide spectrum of bacterial species. The MICs for greater than or equal to 90% of strains were 0.125 to 0.25 microgram/ml for staphylococci, Streptococcus pyogenes, and S. pneumoniae; 0.5 micrograms/ml for S. agalactiae and Enterococcus faecalis; 0.125 micrograms/ml for members of the family Enterobacteriaceae and Acinetobacter spp.; 0.5 micrograms/ml for Pseudomonas aeruginosa; and less than or equal to 0.03 micrograms/ml for Haemophilus influenzae, Moraxella (Branhamella) catarrhalis, and Neisseria gonorrhoeae. In these in vitro comparisons with ciprofloxacin, PD 131628 is more active against gram-positive organisms, approximately equivalent against gram-negative organisms, and, like most other quinolones, relatively inactive against gram-negative anaerobes. In most instances, the in vitro potency of PD 131628 exceeded those of widely used compounds: ciprofloxacin, imipenem, ampicillin, penicillin G, oxacillin, cefazolin, ceftazidime, cefoxitin, cefsulodin, aztreonam, piperacillin, amikacin, spectinomycin, doxycycline, erythromycin, metronidazole, and vancomycin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu D. T., Fernandes P. B. Structure-activity relationships of the fluoroquinolones. Antimicrob Agents Chemother. 1989 Feb;33(2):131–135. doi: 10.1128/aac.33.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz C. L., Bien P. A., Cohen M. A., Dombrowski M. E., Griffin T. J., Malta T. E., Sesnie J. C., Shapiro M. A., Wold S. A. Enoxacin: in-vitro and animal evaluation as a parenteral and oral agent against hospital bacterial isolates. J Antimicrob Chemother. 1988 Feb;21 (Suppl B):29–42. doi: 10.1093/jac/21.suppl_b.29. [DOI] [PubMed] [Google Scholar]
- Hoban D. J. Comparative in vitro activity of quinolones. Clin Invest Med. 1989 Feb;12(1):10–13. [PubMed] [Google Scholar]
- Neuman M., Esanu A. Gaps and perspectives of new fluoroquinolones. Drugs Exp Clin Res. 1988;14(6):385–391. [PubMed] [Google Scholar]
- Rosen T., Chu D. T., Lico I. M., Fernandes P. B., Marsh K., Shen L., Cepa V. G., Pernet A. G. Design, synthesis, and properties of (4S)-7-(4-amino-2-substituted-pyrrolidin-1-yl)quinolone-3-carboxylic acids. J Med Chem. 1988 Aug;31(8):1598–1611. doi: 10.1021/jm00403a020. [DOI] [PubMed] [Google Scholar]
- Sanchez J. P., Domagala J. M., Hagen S. E., Heifetz C. L., Hutt M. P., Nichols J. B., Trehan A. K. Quinolone antibacterial agents. Synthesis and structure-activity relationships of 8-substituted quinoline-3-carboxylic acids and 1,8-naphthyridine-3-carboxylic acids. J Med Chem. 1988 May;31(5):983–991. doi: 10.1021/jm00400a016. [DOI] [PubMed] [Google Scholar]
- Shapiro M. A., Heifetz C. L., Sesnie J. C. Comparison of microdilution and agar dilution procedures for testing antibiotic susceptibility of Neisseria gonorrhoeae. J Clin Microbiol. 1984 Oct;20(4):828–830. doi: 10.1128/jcm.20.4.828-830.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]


