Abstract
The nucleotide analog 7-deazaguanosine has not previously been reported to possess biological (antiviral or antitumor) properties in cell culture or in vivo. Up to 10(5) U of interferon per ml was detected in mouse sera 1 to 4 h following oral (200-mg/kg of body weight) and intraperitoneal (50-mg/kg) doses of the compound. 7-Deazaguanosine also caused significant activation of natural killer and phagocytic cells but did not augment T- and B-cell blastogenesis. Intraperitoneal treatments of 50, 100, and 200 mg/kg/day administered 24 and 18 h before virus inoculation were highly protective in mice inoculated with lethal doses of Semliki Forest or San Angelo viruses. Less but still significant survivor increases were evident in treated mice infected with banzi or encephalomyocarditis viruses. In most cases, the degree of antiviral activity was similar to that exhibited by the biological response modifier 7-thia-8-oxoguanosine. 7-Thia-8-oxoguanosine was more potent than 7-deazaguanosine against encephalomyocarditis virus in mice, however. Oral efficacy was achieved with 7-deazaguanosine treatments of greater than or equal to 100 mg/kg against all virus infections, whereas 7-thia-8-oxoguanosine is reported to be devoid of oral activity in rodents. Thus, 7-deazaguanosine represents the first reported orally active nucleoside biological response modifier exhibiting broad-spectrum antiviral activity against particular types of RNA viruses.
Full text
PDF
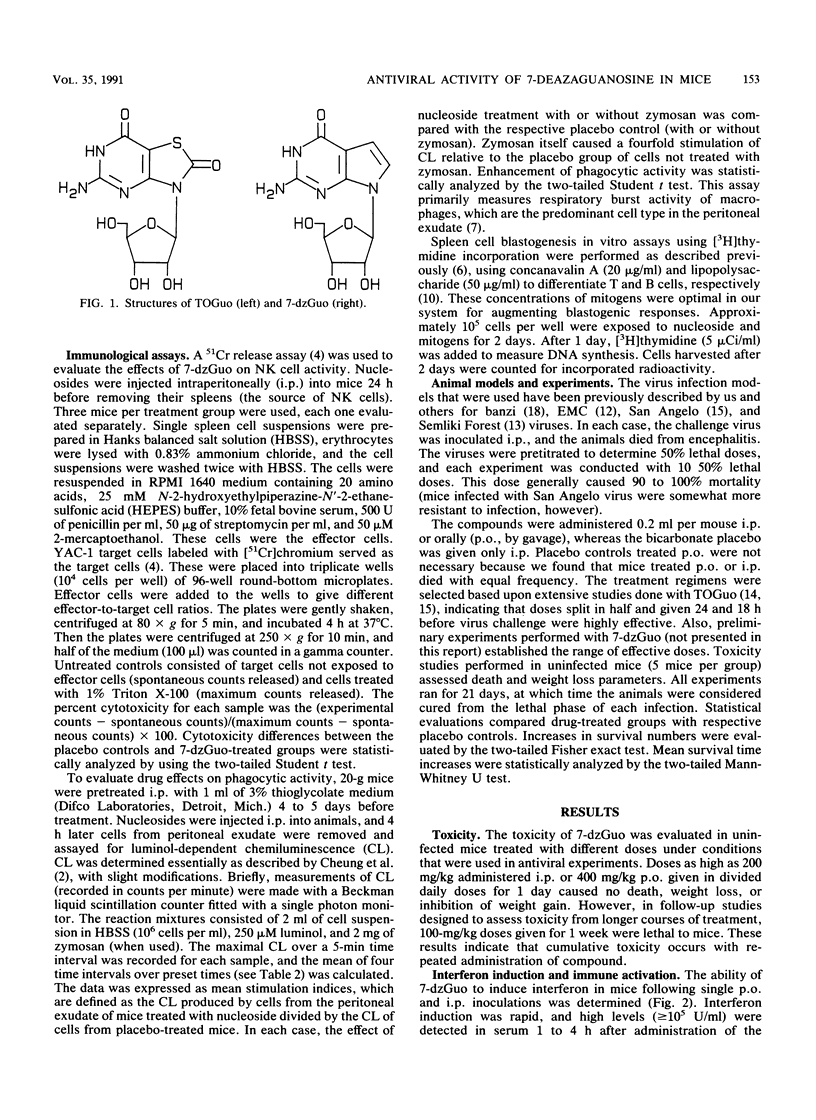
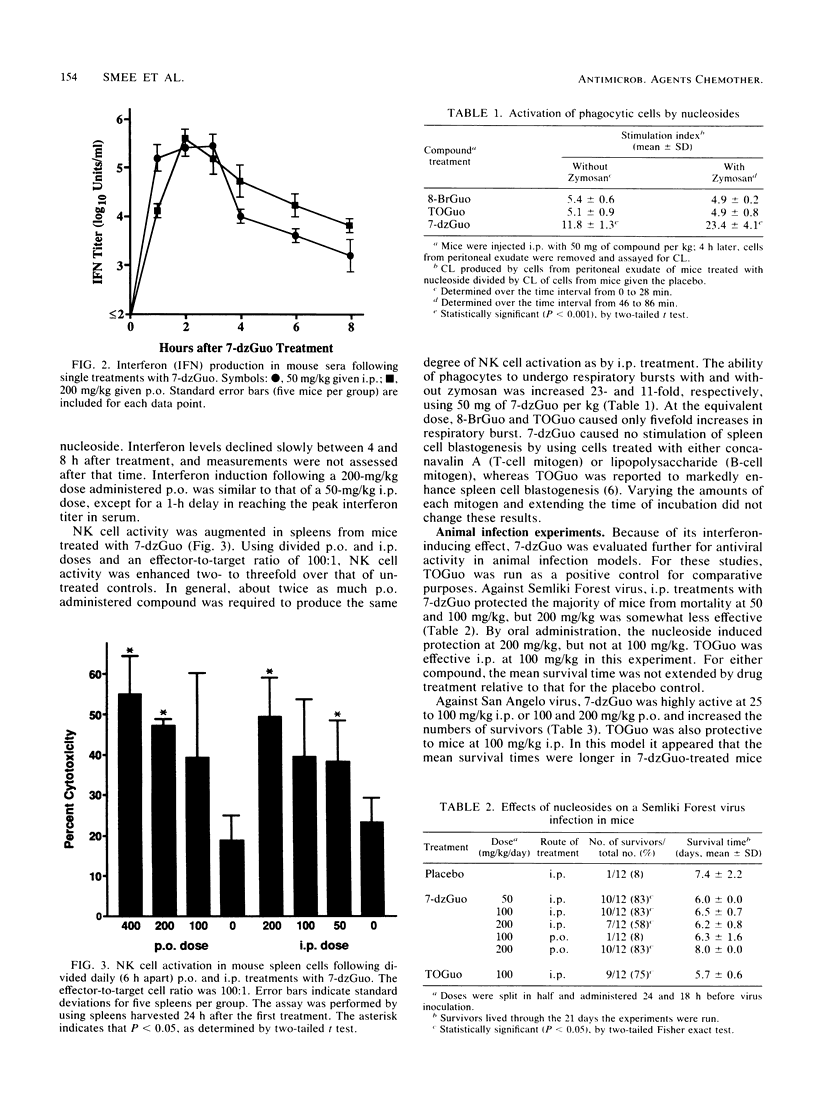
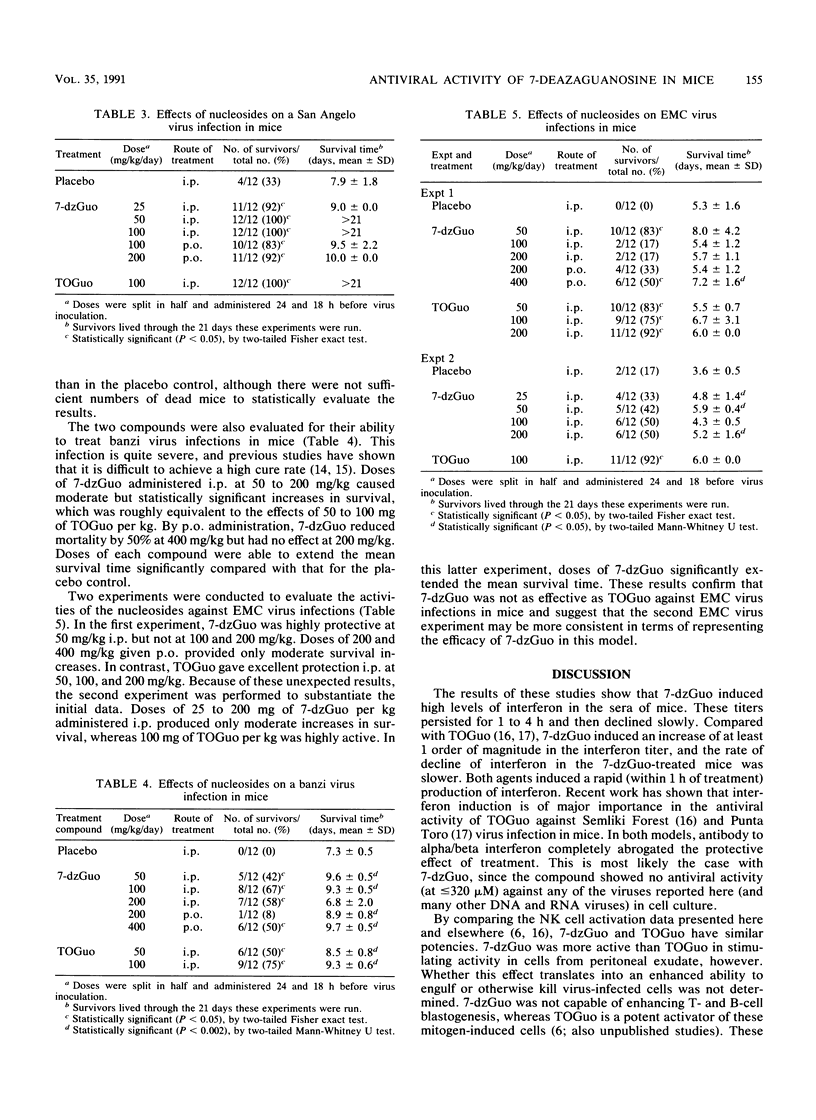


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bukowski J. F., McIntyre K. W., Yang H., Welsh R. M. Natural killer cells are not required for interferon-mediated prophylaxis against vaccinia or murine cytomegalovirus infections. J Gen Virol. 1987 Aug;68(Pt 8):2219–2222. doi: 10.1099/0022-1317-68-8-2219. [DOI] [PubMed] [Google Scholar]
- Cheung K., Archibald A. C., Robinson M. F. The origin of chemiluminescence produced by neutrophils stimulated by opsonized zymosan. J Immunol. 1983 May;130(5):2324–2329. [PubMed] [Google Scholar]
- Jin A., Mhaskar S., Jolley W. B., Robins R. K., Ojo-Amaize E. A. A novel guanosine analog, 7-thia-8-oxoguanosine, enhances macrophage and lymphocyte antibody-dependent cell-mediated cytotoxicity. Cell Immunol. 1990 Apr 1;126(2):414–419. doi: 10.1016/0008-8749(90)90332-l. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Nagahara K., Anderson J. D., Kini G. D., Dalley N. K., Larson S. B., Smee D. F., Jin A., Sharma B. S., Jolley W. B., Robins R. K. Thiazolo[4,5-d]pyrimidine nucleosides. The synthesis of certain 3-beta-D-ribofuranosylthiazolo[4,5-d]pyrimidines as potential immunotherapeutic agents. J Med Chem. 1990 Jan;33(1):407–415. doi: 10.1021/jm00163a064. [DOI] [PubMed] [Google Scholar]
- Ojo-Amaize E. A., Rubalcava B., Avery T. L., Cottam H. B., Matsumoto S. S., Jolley W. B., Robins R. K. Activation of the respiratory burst in murine phagocytes by certain guanine ribonucleosides modified at the 7 and 8 positions: possible involvement of a pertussis toxin-sensitive G-protein. Immunol Lett. 1990 Jan;23(3):173–178. doi: 10.1016/0165-2478(90)90187-u. [DOI] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Selgrade M. K., Ahmed A., Sell K. W., Gershwin M. E., Steinberg A. D. Effect of murine cytomegalovirus on the in vitro responses of T and B cells to mitogens. J Immunol. 1976 May;116(5):1459–1465. [PubMed] [Google Scholar]
- Sidwell R. W., Huffman J. H. Use of disposable micro tissue culture plates for antiviral and interferon induction studies. Appl Microbiol. 1971 Nov;22(5):797–801. doi: 10.1128/am.22.5.797-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim I. S., Cerruti R. L. Recombinant interferons alpha and gamma: comparative antiviral activity and synergistic interaction in encephalomyocarditis virus infection of mice. Antiviral Res. 1987 Nov;8(4):209–221. doi: 10.1016/0166-3542(87)90075-1. [DOI] [PubMed] [Google Scholar]
- Skulnick H. I., Weed S. D., Eidson E. E., Renis H. E., Wierenga W., Stringfellow D. A. Pyrimidinones. 1. 2-Amino-5-halo-6-aryl-4(3H)-pyrimidinones. Interferon-inducing antiviral agents. J Med Chem. 1985 Dec;28(12):1864–1869. doi: 10.1021/jm00150a018. [DOI] [PubMed] [Google Scholar]
- Smee D. F., Alaghamandan H. A., Cottam H. B., Jolley W. B., Robins R. K. Antiviral activity of the novel immune modulator 7-thia-8-oxoguanosine. J Biol Response Mod. 1990 Feb;9(1):24–32. [PubMed] [Google Scholar]
- Smee D. F., Alaghamandan H. A., Cottam H. B., Sharma B. S., Jolley W. B., Robins R. K. Broad-spectrum in vivo antiviral activity of 7-thia-8-oxoguanosine, a novel immunopotentiating agent. Antimicrob Agents Chemother. 1989 Sep;33(9):1487–1492. doi: 10.1128/aac.33.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee D. F., Alaghamandan H. A., Jin A., Sharma B. S., Jolley W. B. Roles of interferon and natural killer cells in the antiviral activity of 7-thia-8-oxoguanosine against Semliki Forest virus infections in mice. Antiviral Res. 1990 Feb;13(2):91–102. doi: 10.1016/0166-3542(90)90025-3. [DOI] [PubMed] [Google Scholar]
- Smee D. F., McKernan P. A., Nord L. D., Willis R. C., Petrie C. R., Riley T. M., Revankar G. R., Robins R. K., Smith R. A. Novel pyrazolo[3,4-d]pyrimidine nucleoside analog with broad-spectrum antiviral activity. Antimicrob Agents Chemother. 1987 Oct;31(10):1535–1541. doi: 10.1128/aac.31.10.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringfellow D. A., Kern E. R., Kelsey D. K., Glasgow L. A. Suppressed response to interferon inducation in mice infected with encephalomyocarditis virus, Semliki forest virus, influenza A2 virus, Herpesvirus hominis type 2, or murine cytomegalovirus. J Infect Dis. 1977 Apr;135(4):540–551. doi: 10.1093/infdis/135.4.540. [DOI] [PubMed] [Google Scholar]
- Stringfellow D. A., Weed S. D., Underwood G. E. Antiviral and interferon-inducing properties of 1,5-diamino anthraquinones. Antimicrob Agents Chemother. 1979 Jan;15(1):111–118. doi: 10.1128/aac.15.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M. Regulation of virus infections by natural killer cells. A review. Nat Immun Cell Growth Regul. 1986;5(4):169–199. [PubMed] [Google Scholar]
- Wierenga W. Antiviral and other bioactivities of pyrimidinones. Pharmacol Ther. 1985;30(1):67–89. doi: 10.1016/0163-7258(85)90048-8. [DOI] [PubMed] [Google Scholar]


