Abstract
An efficient monoclonal aldolase antibody that proceeds by an enamine mechanism was generated by reactive immunization. Here, this catalyst has been used in the total synthesis of epothilones A (1) and C (3). The starting materials for the synthesis of these molecules have been obtained by using antibody-catalyzed aldol and retro-aldol reactions. These precursors were then converted to epothilones A (1) and C (3) to complete the total synthesis.
Natural product synthesis is the centerpiece of organic chemistry. Much of what we know about organic reaction mechanisms has come from attempts to carry out selective chemical transformations en route to the construction of these often very complex molecules. In spite of many successes, there is a continual search for methods to improve the process for developing important drugs and other chemicals. One of the main goals of the field of antibody catalysis (1–3) has been to learn how to make custom catalysts for synthetic schemes, thereby opening routes that are otherwise inaccessible or cumbersome. In this way, the overall yield of a synthesis to allow practical construction of more totally synthetic drugs and other important natural products can be improved. In general, most antibody-catalyzed reactions are enantioselective (4) and regioselective (5). Even reversal of chemoselectivity has been observed (6). Recently, two aldolase mAbs, 38C2 and 33F12, that proceed by an enamine mechanism were generated against a β-diketone hapten, 6-(4-glutaramidophenyl)hexane-2,4-dione by using reactive immunization (7). These antibody catalysts were found to be very useful for synthetic organic chemistry (8, 9) in that they have all of the good properties of natural aldolases with the added advantage that they accept a much broader range of substrates (10). In reactive immunization (11), unlike normal immunization, highly reactive chemicals are used as immunogens so that a chemical reaction occurs in the binding site of an antibody during its induction. An analogue of this chemical reaction will later become part of a catalytic event. The broad scope arises when the chemical event is covalent and occurs early in the process of the antibody binding-site refinement. After the covalent event has evolved, further improvement of the binding site via noncovalent bonds cannot meaningfully increase the binding energy, and mutations are no longer selectable (10, 12). Thus, reactive immunizations often yield antibody-binding sites into which an efficient chemical mechanism has been installed but are not otherwise highly refined. Such catalysts can be expected to be both efficient and promiscuous. To demonstrate the efficacy of antibody catalysis for natural product synthesis, we now use one of these antibody aldol catalysts in the total synthesis of epothilones.
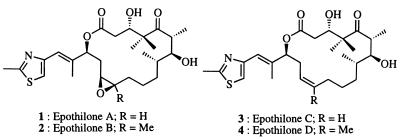
Epothilones are molecules of current interest (see ref. 13 for a recent review on chemical biology of epothilones, and refs. 14–57) to organic chemists because of their medical promise and synthetic challenges. Epothilones A (1) and B (2) (Fig. 1) are powerful cytotoxic agents isolated from myxobacteria (Sorangium cellulosum strain 90; refs. 14–16). They exhibit a taxol-like mode of action, functioning through stabilization of cellular microtubules, and are cytotoxic even in taxol-resistant cell lines (14, 45–51). Epothilone B has been reported to be about 3,400 times more active than taxol against the resistant human leukemia cell line CCRF-CEM/VBL in cell-culture cytotoxicity studies. Desoxy precursors of 1 and 2, epothilones C (3) and D (4), also possess comparable biological properties, particularly the tubulin polymerization activity. Epothilones A–D (1–4) were first synthesized by Danishefsky and coworkers (17, 21). Besides epothilones A–E, many analogs of these compounds have been synthesized and studied for their effects on tubulin polymerization in vitro and in vivo.
Figure 1.
Structures of epothilones A–D (1–4).
MATERIALS AND METHODS
1H- and 13C-NMR spectra were measured in CDCl3 at 400 and 100 MHz, respectively. Positive-ion mass spectra, using the fast-ion bombardment technique, were obtained on a VG ZAB-VSE double-focusing, high-resolution mass spectrometer equipped with either a cesium or a sodium ion gun. Optical rotations were measured in a 1-dm (1.3-ml) cell using an Autopol III automatic polarimeter. TLC was performed on glass sheets precoated with silica gel (Merck, Kieselgel 60, F254, Art. 5715). Column chromatographic separations were performed on silica gel (Merck, Kieselgel 60, 230–400 mesh, Art. 9385) under pressure. Tetrahydrofuran (THF) was dried and distilled over sodium ketyl. 4-Methoxy-α-methylcinnamaldehyde, pentan-3-one, dibutylborontriflate, and lithium diisopropylamide (LDA) were purchased from Aldrich. Aldehyde 7 was prepared by using a known procedure (39).
Synthesis of Aldol (±)-syn-5.
Aldol (±)-syn-5 was prepared by the Evans method (58) for syn-selective aldol reaction of Z-pent-2-en-3-olyl dibutylborane (prepared from dibutylborontriflate and pentan-3-one at 0°C) with 4-methoxy-α-methylcinnamaldehyde at 0°C. 1H NMR: δ 7.20 (d, J = 8.7 Hz, 2H), 6.86 (d, J = 8.7 Hz, 2H), 6.51 (s, 1H), 4.43 (br s, 1H), 3.79 (s, 3H), 2.94 (d, J = 2.8 Hz, 1H), 2.83 (m, 1H), 2.56 (m, 2H), 1.81 (s, 3H), 1.12 (d, J = 7.2 Hz, 3H), 1.04 (t, J = 7.2 Hz, 3H); 13C NMR: δ 216.0, 158.0, 134.9, 130.1, 125.5, 75.9, 55.2, 48.3, 35.1, 15.1, 10.4, 7.6. MS (FAB): 262 (M+), 285 (MNa+).
Synthesis of Aldol (±)-6.
Compound (±)-6 was prepared by aldol reaction of the lithium enolate of acetone (generated by addition of acetone to LDA at −78°C for 2 hr) with aldehyde 7. 1H NMR: δ 6.93 (s, 1H), 6.57 (s, 1H), 4.61 (dd, J = 8.0, 4.2 Hz, 1H), 3.07 (br s, 1H), 2.74 (m, 2H), 2.69 (s, 3H), 2.21 (s, 3H), 2.03 (s, 3H); MS (FAB): 226 (MH+), 248 (MNa+).
RESULTS
Monoclonal catalytic antibodies have been used effectively in the total synthesis of natural products, as demonstrated by the construction of multistriatin (59) and brevicomins (10). In these cases, the antibody catalysts were used to catalyze stereoselective reactions at high rates, thereby yielding precursors to these molecules. The stereochemistry obtained by the antibody-catalyzed reactions guided the rest of the configurations of the molecule. Here, applying the same principle, aldolase antibodies 38C2 were used to catalyze the formation of starting materials for the total synthesis of epothilones A–D (1–4) and their analogs.
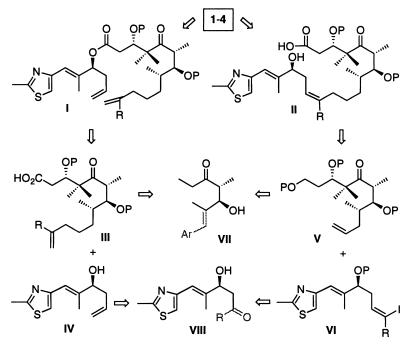
Retrosynthetically, compounds 1–4 could be obtained by the metathesis of I [and its analogs, where R is tert-butyldimethylsilyl (TBS) and/or H] or the macrolactonization of II (Scheme S1). Intermediates I and II (R = H, Me) could be synthesized from two building blocks by reacting III (R = H, Me) with IV or V with VI (R = H, Me). In fact, in many syntheses of 1–4 and their analogs, intermediates I and II or similar compounds have been employed. Intermediates III and V could be obtained from a common precursor VII and similarly, compounds IV and VI could be obtained from VIII.
Scheme 1.
Retrosynthesis of epothilones A–D (1–4)
We have recently found that it is possible to obtain both enantiomers of an aldol compound by antibody 38C2-catalyzed reactions (60). Antibody 38C2 catalyzes the enantioselective retro-aldol reactions of a wide variety of β-hydroxyketones, including ones that are similar to ent-VII and thus leaving behind VII or its analog in the reaction mixture. Also, the same antibody catalyzes the aldol reaction to produce β-hydroxy ketones that are analogous to VIII. Hence, we anticipated that the enantiomerically highly enriched analogs of VII and VIII could be obtained from the aldolase antibody 38C2-catalyzed reactions and that they could be used to prepare the intermediates I and II. Here, we synthesized 1 and 3 by using the metathesis and macrolactonization approaches starting from 5 and 6 (see below) that are analogs of VII and VIII, respectively.
Generation of Key Precursors by Antibody-Catalyzed Aldol and Retro-Aldol Reaction.
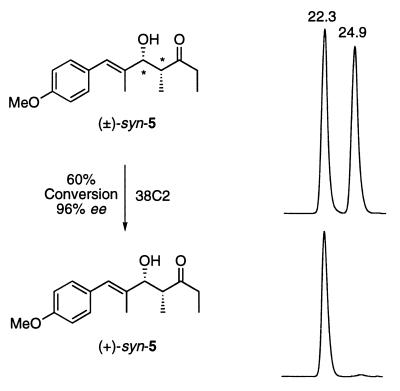
Based on our previous studies compound (±)-syn-5 was chosen as the potential substrate for the retro-aldol reaction with antibody 38C2 (60). In basic medium (pH 7.4, phosphate buffer), aldol (±)-syn-5 undergoes a retro-aldol reaction to give 4-methoxy-α-methylcinnamaldehyde and pentan-3-one. When (±)-syn-5 was subjected to the reaction with antibody 38C2, a sharp increase in the initial rate was observed qualitatively by HPLC analysis. Hence, (±)-syn-5 was treated with 38C2 (0.125 mol %) at room temperature, and the progress of reaction was monitored by HPLC equipped with a reverse-phase chiral column and a UV detector. At 60% conversion, the reaction mixture was analyzed and found to be at a 98:2 ratio of the two enantiomers of 5 (Fig. 2) besides the produced aldehyde. At this point, the reaction was interrupted and purified from the aldehyde to give a pure, kinetically resolved product. The absolute structure of the remaining compound after retro-aldol reaction was identified as (+)-syn-5 by a comparison with the synthetic sample of (+)-syn-5† (61).
Figure 2.
Antibody 38C2-catalyzed enantioselective retro-aldol reactions of racemic (±)-syn-5 (Upper Left) to (+)-syn-5 (Lower Left). HPLC traces of (±) syn-5 (upper right) and resolved product (+)-syn-5 (lower right) [HPLC conditions: λmax-254 nm; Chiralcel OD-R column (250 × 4.6 mm, Daicel Chemical Industries; Chiral Technologies, Exton, PA) using 0.1% trifluoroacetic acid in acetonitrile/water (1:1) at a flow rate of 0.4 ml/min.). In a typical process, compound (±)-syn-5 (0.75 g, 2.85 mmol in 50 ml of CH3CN) was stirred slowly with antibody 38C2 (0.5 g, 0.00357 mmol) in PBS (pH 7.4) in a total volume of 500 ml. At 98% consumption of one enantiomer as judged by HPLC analysis (Lower Right), the reaction mixture was filtered through membranes to recover antibodies. The filtrate was passed through a reverse-phase column (C18) to elute water, and then the compounds were isolated using methanol as eluants. The solvents were removed and the residue was purified over silica gel to afford the corresponding aldehyde (0.25 g, 1.42 mmol) and (+)-syn-5 (0. 30 g, 1.15 mmol).
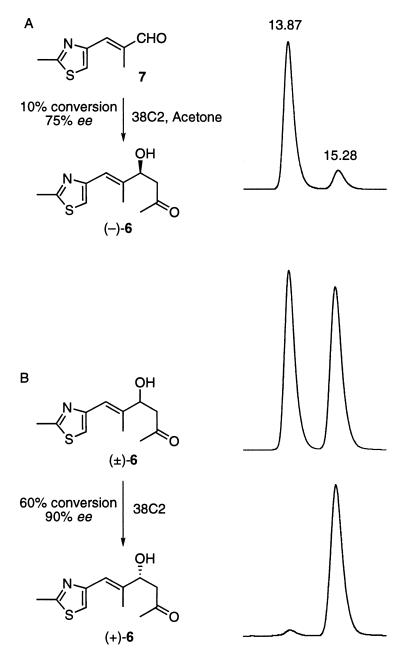
Compound (−)-6 possesses the same stereochemistry as the products of acetone or other ketones added to aldehydes in the aldol reaction catalyzed by antibody 38C2 (7, 10). We expected that the aldol reaction of acetone with aldehyde 7 could be catalyzed with antibody 38C2, to produce compound (−)-6. Hence, we carried out the aldol reaction of acetone with aldehyde 7 in the presence of 0.06 mol % of aldolase antibody 38C2. The reaction was interrupted at 10% conversion of 7 to give a 51% yield of the aldol product (−)-6 (based on the recovered starting material). The product obtained by the antibody-catalyzed aldol reaction gave two enantiomers of 6, i.e., (−)-6 and (+)-6 in the ratio of 7:1 (Fig. 3A). At 20% conversion of 7, the ratio between (−)-6 and (+)-6 dropped to 4:1, because as higher concentrations of the aldol product are achieved, the retro-aldol process starts, which is faster than the forward reaction. Hence, we thought that the enantiomer (+)-6, obtained by an enantioselective antibody-catalyzed retro-aldol process using (±)-6 as a substrate could substitute for formation of (−)-6 by aldol reaction; however, inversion of the hydroxyl group in (+)-6 would be required. The inversion of the stereochemistry of the hydroxyl group in the compound derived from (+)-6 can be achieved by the Mitsunobu reaction during the esterification step, and that compound should be suitable for the synthesis of intermediates I.
Figure 3.
(A) Antibody 38C2-catalyzed enantioselective aldol reaction of acetone with aldehyde 7. HPLC trace of (−)-6 (right). [HPLC conditions: λmax = 254 nm; Chiralcel OD-R (250 × 4.6 mm) column using 0.1% trifluoroacetic acid in acetonitrile/water (3:17) at a flow rate of 0.4 ml/min)]. A mixture of aldehyde 7 (1.28 g, 7.7 mmol) and antibody 38C2 (0.64 g, 0.0046 mmol) in PBS (total volume of 640 ml containing 64 ml of acetone) was stirred slowly at room temperature for 4 days. Antibody was filtered using Amicon membranes. Water solutions were passed through a reverse-phase (C18) column to elute water, and methanol was used as solvent to elute organics. Solvents were evaporated and separated over silica gel (hexane/ethyl acetate) to afford 0.11 g of pure (−)-6 and recovered 7 (1.12 g). (B) Antibody 38C2 catalyzed enantioselective retro-aldol reaction of (±)-6 (upper left) to (+)-6 (lower left). HPLC traces of (±)-6 (upper right) and reolved product (+)-6 (lower right). [HPLC conditions: Same as above.] Compound (±)-6 (1.32 g, 5.85 mmol in 50 ml of acetonitrile) was stirred slowly with antibody 38C2 (0.5 g, 0.00357 mmol) in PBS (pH 7.4) in a total volume of 500 ml. At >90% consumption of one enantiomer as judged by HPLC analysis (Right), the reaction mixture was filtered to recover antibodies and passed through a reverse-phase column as above to afford aldehyde 7 (0.44 g, 2.65 mmol) and (+)-6 (0.49g, 37%).
Hence, a racemic mixture of 6 was subjected to the reaction with the antibody 38C2. As expected, the retro-aldol reaction of racemic compound 6 was catalyzed by antibody 38C2 (Fig. 3B). At 50% conversion, the ratio of the two enantiomers of 6, i.e., (+)-6 and (−)-6, was found to be 7:1. The enantioselectivity of the remaining β-hydroxyketone, (+)-6, could be increased to 90–95% enantiomer excess at a higher conversion, i.e., 60–65%. The retro-aldol reaction of racemic (±)-6 with 0.06 mol % of antibody 38C2 afforded (+)-6 with an enantiomeric purity of 90%. The absolute stereochemistry of (+)-6 and (−)-6 obtained by antibody-catalyzed reactions was assigned by comparison with synthetic samples of (−)-6 using a chiral ODR (reverse-phase) column.‡
Total Synthesis of Epothilones.
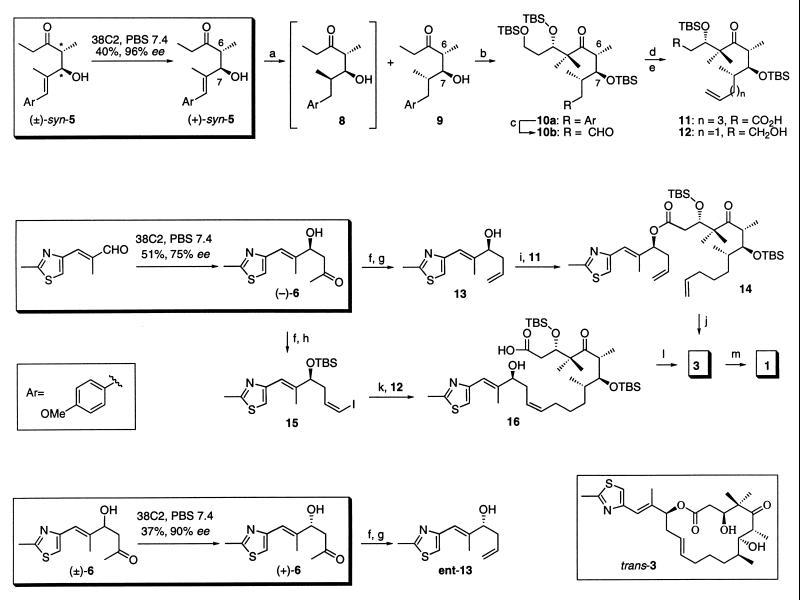
With the enantiomerically enriched compounds (+)-syn-5, (−)-6, and (+)-6 in hand, we converted them to the naturally occurring epothilone A (1) via its desoxy precursor epothilone C (3) using both the metathesis and macrolactonization approach (Scheme S2). Compound 5 possesses two chiral centers (C-6 and C-7) of the required five (C-3, C-6, C-7, C-8, and C-15) of epothilone C (3). Starting from (+)-syn-5, we first incorporated the stereochemistry of the remaining methyl at C-8 of the vicinal methyl-hydroxymethyl system. Nonselective hydrogenation of (+)-syn-5 with Rh-Al2O3 afforded an easily separable 1:1 mixture of compounds 8 and 9. The structure of 9, which now possessed all three vicinal stereogenic centers appropriate for epothilones, was confirmed by x-ray analysis. The free alcohol in 9 was silylated as the tert-butyldimethylsilyl (TBS) ether by using tert-butyldimethylsilyl chloride (TBSCl) and imidazole in dry dimethylformamide (DMF) at 50°C. The silylated compound was then monomethylated at the less-substituted carbon α to the carbonyl function. Aldol reaction of the monomethylated compound with 3-tert-butyldimethylsilyloxypropanal took place at the same α carbon mentioned above, affording the aldol product in a ratio of 2:1 mixture of two diastereomers at C-3 in favor of the desired isomer. The other possible product of aldol reaction at C-6 was not detected. The aldol product thus obtained was silylated as the TBS ether by using TBS trifluoromethanesulfonate (TBSOTf) and purified from its minor 3-epimer to give 10a. The methoxy-substituted aromatic ring was exhaustively degraded to the corresponding carboxylic acid with the RuCl3/NaIO4 system (59, 62). Esterification of the acid was achieved with diazomethane. Reduction of the latter ester with diisobutylaluminum hydride (DIBAL-H) to the corresponding primary alcohol, followed by oxidation with Dess–Martin reagent, gave the aldehyde 10b. Compound 10b was used as a precursor for the synthesis of intermediates in both the metathesis as well as the macrolactonization processes.
Scheme 2.
Total synthesis of epothilones A (1) and C (3) from (±)-syn-5 and aldehyde 7 via antibody-catalyzed retro-aldol and aldol reactions and synthesis of ent-13 from (±)-6 via antibody-catalyzed retro-aldol reaction. (a) H2, Rh/Al2O3, THF, 12 hr. (b) TBSCl, imidazole, DMF, 40–50°C, 48 hr; lithiumdiisopropylamide (LDA), THF, −78°C then MeI, hexamethylphosphoiamide (HMPA), −78°C to 0°C, 4 hr; LDA, THF, −78°C to −30°C, 2–5 hr; TBSOCH2CH2CHO, 0.5 hr (90%; 3S:3R, 2:1); TBSOTf, lutidine, CH2Cl2, −30°C, 3 hr, separation from 3-epi-10a by chromatotrone. (c) RuCl3, NaIO4, CCl4, CH3CN, PBS buffer (pH 7.4), 12 hr; CH2N2, MeOH–Et2O, 12 hr, 0°C; diisobutylaluminum hydride (DIBAL-H), THF, −78°C to −40°C, 2 hr; Dess–Martin reagent, CH2Cl2, 2 hr. (d) For the synthesis of 11: (EtO)2P(O)CH2CO2Et, NaH, THF, 0.5 hr; H2, Rh–Al2O3, THF, 12 hr; DIBAL-H, THF, −78°C to −40°C, 2 hr; Dess–Martin reagent, CH2Cl2, 2 hr; MePPh3I, BuLi, THF, 0°C, 0.5 hr; TsOH, CH2Cl2-MeOH, 0°C, 0.5 hr; Dess–Martin reagent, CH2Cl2, room temperature, 2 hr; NaClO2, tert-butylhydroperoxide (TBHP), 2-methyl-2-butene, NaH2PO4, THF, H2O, room temperature, 2 hr. (e) For the synthesis of 12: MePPh3I, BuLi, THF, 0°C, 0.5 hr; TsOH, CH2Cl2/MeOH, 0°C, 0.5 hr. (f) TBSCl, imidazole, DMF, room temperature, 16 hr; TMSOTf, lutidine, CH2Cl2, −78°C, 3 hr then excess m-CPBA, −78°C, room temperature, 2 hr; NaBH4, EtOH, 0°C, 0.5 hr; Pb(OAc)4, benzene, 0°C, 1 hr. (g) MePPh3I, BuLi, THF, 0°C, 0.5 hr; tetrabutylammonium fluoride (TBAF), THF, 0°C, 2 hr. (h) MePPh3I, BuLi, THF, 0°C, 0.5 hr, then I2, −78°C, 10 min, then NaHMDS, −20°C, 10 min, then aldehyde from (−)-6, 0.5 hr. (i) EDC, CH2Cl2, 0°C, room temperature, 12 hr. (j) Grubb’s catalyst, benzene, room temperature, 16 hr. (k) Reaction 1: compound 12, 9-borabicyclo[3:3:1]nonane (9-BBN), THF, 0°C, room temperature, 4 hr, reaction 2: compound 15, PdCl2(dppf)2, Cs2CO3, Ph3 As, H2O, DMF, room temperature, 1 hr, then reaction 2 added to reaction 1, room temperature, 4 hr; TBAF, THF, room temperature, 8 hr. (l) 2,4,6-Cl3C6H2COCl, Et3N, THF, 0°C, 0.5 hr; diluted with toluene and added dropwise to 4-dimethylaminipyridine (DMAP), toluene, room temperature, 2 hr. (m) Trifluoroacetic acid, CH2Cl2, −20 to 0°C, 2 hr; separation from its trans isomer using chromatotrone; solvent: CH2Cl2/MeOH, 49:1, (44% of 3 and 29% of trans isomer of 3, from 14; 40% of 3 and 19% of trans isomer of 3, from 16); CF3C(O)Me, oxone, acetonitrile, 0°C 1 hr, 55%.
The Metathesis Approach.
Aldehyde 10b was reacted with sodium triethylphosphonoacetate to give an α,β-unsaturated ester. Hydrogenation of alkene catalyzed by Rh/Al2O3 at atmospheric pressure gave the saturated ester. The ester function in the latter compound was first reduced with DIBAL-H and the alcohol thus obtained was oxidized with Dess–Martin reagent to form the corresponding aldehyde, which was then reacted with methyltriphenylphosphorane (generated from the reaction of methyltriphenylphosphonium iodide with BuLi) to afford the terminal alkene. The primary alcohol in the alkene thus obtained was deprotected with TsOH in methanol/CH2Cl2 at 0°C to give the free primary alcohol. The 1H- and 13C-NMR spectra of the protected and deprotected primary alcohol compounds were identical to the same compounds used in the Schinzer synthesis of 1 and 3. Oxidation of the primary alcohol to the acid 11 was achieved in two steps via the aldehyde.
Compound 13 was obtained from (−)-6 as shown in Scheme S2. The aldol product (−)-6 was first protected as the TBS ether. Reaction of the TBS ether with lutidine and trimethylsilytrifluoromethanesulfonate (TMSOTf) at −78°C gave the trimethylsilylenol ether, which on in situ reaction with m-chloroperbenzoic acid (m-CPBA), afforded the primary hydroxyketone. Reduction of the ketone to the vicinal diol followed by cleavage with Pb(OAc)4 gave an aldehyde. Wittig reaction of the aldehyde with methylphosphorane followed by deprotection of the TBS ether afforded the required allylic alcohol 13. Following the same set of reactions, (+)-6 was converted to ent-13.
Esterification of the acid 11 with the thiazole alcohol 13 afforded the known ester 14. The same ester 14 could also be obtained by the reaction of 11 with ent-13 via Mitsunobu inversion; however, this has yet to be confirmed. Compound 14 was converted to 1 via 3 as previously reported (23, 25). Thus, metathesis of 14 with Grubb’s catalyst afforded the cyclized compound along with its trans isomer in a ratio of 3:2. Deprotection of the mixture of the cyclized product and its trans isomer with trifluoroacetic acid followed by separation afforded compounds 3 and trans-3. Epoxidation with methyl(trifluoromethyl)dioxirane afforded 1 and its α-stereoisomer.
The Macrolactonization Approach.
Compound 10b and the aldehyde obtained by thiazolyldiol en route to the synthesis of 13 were used as a precursor for the synthesis of 16, which is an intermediate in the macrolactonization approach to 1 and 3. Thus, compound 10b was first methylenated with methyltriphenylphosphorane and then deprotected at the primary alcohol to give compound 12. The other fragment 15 was obtained by a Wittig reaction of the thiazolylaldehyde mentioned above obtained by cleavage of the vicinal diol with iodomethyltriphenylphosphorane. Compound 15, thus obtained, was contaminated with its trans isomer (20%). Suzuki coupling between 15 and the 9-borabicyclo[3:3:1]nonane derivative of 12 gave the coupling product that was contaminated with its trans isomer in a ratio of 2:1 in favor of the cis isomer. Two-step oxidation of the primary alcohol to acid and selective desilylation of the allylic alcohol gave hydroxy acid 16. Macrolactonization using Yamaguchi’s procedure followed by deprotection gave a mixture of 3 and its trans isomer.
DISCUSSION
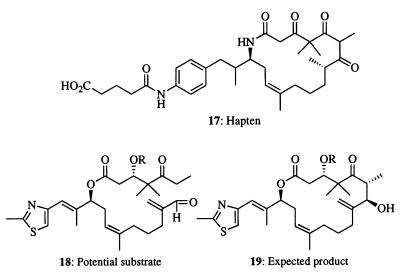
In the work reported here, we have taken advantage of the promiscuous nature of antibody catalysts made by reactive immunization to demonstrate how such catalysts may be used to facilitate two different synthetic routes to a complex natural product. Although the final steps of the synthetic strategies that we employed were designed by others, it is important to realize that a general approach in designing catalysts to improve the overall yield of existing synthetic routes has long been a major goal of organic chemistry. Arguably, this is a more powerful strategy than the reciprocal process of first finding the catalyst and then designing a route to fit it. In separate studies, we have learned that immunization with other 1,3-diketones invariably leads to aldolase antibodies that are widely accepting of appended functionalities. The only requirement is that the organic assemblage does not exceed in size the approximate 3,000-Å3 volume of a typical antibody-binding site. Thus, it should be possible to induce catalysts for new synthetic routes (for example: against hapten 17 for an intramolecular aldol reaction of compound 18 to 19) in which the chance for occurrence of undesired side reactions is minimized, thereby reducing the need for blocking groups. This strategy features the use of more complex 1,3-diketone haptens such that the induced antibody catalysts accept larger portions of the target molecule. In this scheme, we minimize or exclude the requirement for blocking groups, and only two or three steps would be required after the catalytic process to convert 19 to epothilones.
In conclusion, we have achieved a total synthesis of 1 and 3. The synthetic scheme involved two antibody-catalyzed steps affording highly enriched enantiomers (+)-5 and (−)-6. Three stereogenic centers were incorporated into the epothilone molecules by using the antibody-catalyzed steps. To minimize the need for blocking groups in the synthesis of epothilones, a new antibody-catalyzed synthetic route involving an intramolecular aldol reaction for the macrocyclization step is being developed.
Scheme 3.
Structures 17–19.
Acknowledgments
We are grateful to Diane Kubitz of Scripps Research Institute for providing the supply of antibodies. We are thankful to Prof. D. Schinzer for providing us with the spectral data of intermediates and Ms. A. Pervin and T. Yang for technical support.
ABBREVIATIONS
- LDA
lithium diisopropylamide
- THF
tetrahydrofuran
- TSB
tert-butyldimethysilyl
Footnotes
A Commentary on this article begins on page 14590.
A synthetic sample of (+)-syn-5 for comparison was obtained by the reaction of pent-2-en-3-oldiisocampheyl-borinate [prepared from (+)-diisopinocampheylboron triflate [(Ipc)2BOTf] and 3-pentanone] with 4-methoxy-α-methylcinnamaldehyde by the method of Paterson (61) used in the synthesis of analogs of compound 5.
By using the same methodology as Paterson (61), a sample of (−)-6 for comparison was prepared by the reaction of prop-1-en-2-ol diisocampheylborinate [prepared from (+)-(Ipc)2 BOTf and acetone] with aldehyde 7.
References
- 1.Tramontano A, Janda K D, Lerner R A. Science. 1986;234:1566–1570. doi: 10.1126/science.3787261. [DOI] [PubMed] [Google Scholar]
- 2.Pollack S J, Jacobs J W, Schultz P G. Science. 1986;234:1570–1573. doi: 10.1126/science.3787262. [DOI] [PubMed] [Google Scholar]
- 3.Keinan E, Lerner R A. Isr J Chem. 1996;36:113–119. [Google Scholar]
- 4.Keinan E, Sinha S C, Shabat D, Itzhaky H, Reymond J-L. Acta Chem Scand. 1996;50:679–687. doi: 10.3891/acta.chem.scand.50-0679. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh L C, Yonkovich S, Kochersperger L, Schultz P G. Science. 1993;260:337–339. doi: 10.1126/science.10049109. [DOI] [PubMed] [Google Scholar]
- 6.Sinha S C, Keinan E, Reymond J-L. Proc Natl Acad Sci USA. 1993;90:11910–11913. doi: 10.1073/pnas.90.24.11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner J, Lerner R A, Barbas C F., III Science. 1995;270:1797–1800. doi: 10.1126/science.270.5243.1797. [DOI] [PubMed] [Google Scholar]
- 8.Zhong G, Hoffmann T, Lerner R A, Danishefsky S, Barbas C F., III J Am Chem Soc. 1997;119:8131–8132. [Google Scholar]
- 9.List B, Shabat D, Barbas C F, III, Lerner R A. Chem Eur J. 1998;4:881–885. [Google Scholar]
- 10.Hoffmann T, Zhong G, List B, Shabat D, Anderson J, Gramatikova S, Lerner R A, Barbas C F., III J Am Chem Soc. 1998;120:2768–2779. [Google Scholar]
- 11.Wirsching P, Ashley J A, Lo C-H L, Janda K D, Lerner R A. Science. 1995;270:1775–1782. doi: 10.1126/science.270.5243.1775. [DOI] [PubMed] [Google Scholar]
- 12.Barbas C F, III, Heine A, Zhong G, Hoffmann T, Gramatikova S, Bjornestedt R, List B, Anderson J, Stura E A, Wilson E A, et al. Science. 1997;278:2085–2092. doi: 10.1126/science.278.5346.2085. [DOI] [PubMed] [Google Scholar]
- 13.Nicolaou K C, Roschanger F, Vourloumis D. Angew Chem Int Ed Engl. 1998;37:2014–2045. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2014::AID-ANIE2014>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Bollag D M, McQueney P A, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods C M. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 15.Gerth K, Bedorf N, Hofle G, Irschik H, Reichenbach H. J Antibiot. 1996;49:560–563. doi: 10.7164/antibiotics.49.560. [DOI] [PubMed] [Google Scholar]
- 16.Hofle G, Bedorf N, Steinmetz H, Schomburg D, Gerth K, Reichenbach H. Angew Chem Int Ed Engl. 1996;35:1567–1569. [Google Scholar]
- 17.Balog A, Meng D, Kamenecka T, Bertinato P, Su D-S, Sorensen E J, Danishefsky S J. Angew Chem Int Ed Engl. 1996;35:2801–2803. [Google Scholar]
- 18.Yang Z, He Y, Vourloumis D, Vallberg H, Nicolaou K C. Angew Chem Int Ed Engl. 1997;36:166–168. [Google Scholar]
- 19.Schinzer D, Limberg A, Bauer A, Bohm O M, Cordes M. Angew Chem Int Ed Engl. 1997;36:523–524. [Google Scholar]
- 20.Nicolaou K C, Sarabia F, Ninkovic S, Yang Z. Angew Chem Int Ed Engl. 1997;36:525–527. [Google Scholar]
- 21.Su D-S, Meng D, Bertinato P, Balog A, Sorensen E J, Danishefsky S J, Zheng Y-H, Chou T-C, He L, Horwitz S B. Angew Chem Int Ed Engl. 1997;36:757–759. [Google Scholar]
- 22.Meng D, Su D-S, Balog A, Bertinato P, Sorensen E J, Danishefsky S J, Zheng Y-H, Chou T-C, He L, Horwitz S B. J Amer Chem Soc. 1997;119:2733–2734. [Google Scholar]
- 23.Nicolaou K C, He Y, Vourloumis D, Vallberg H, Roschangar F, Sarabia F, Ninkovic S, Yang Z, Trujillo J I. J Am Chem Soc. 1997;119:7960–7973. [Google Scholar]
- 24.Nicolaou K C, Ninkovic S, Sarabia F, Vourloumis D, He Y, Vallberg H, Finlay M R V, Yang Z. J Am Chem Soc. 1997;119:7974–7991. [Google Scholar]
- 25.Meng D, Bertinato P, Balog A, Su D-S, Kamenecka T, Sorensen E J, Danishefsky S J. J Amer Chem Soc. 1997;119:10073–10092. [Google Scholar]
- 26.Nicolaou K C, Winssinger N, Pastor J, Ninkovic S, Sarabia F, He Y, Vourloumis D, Yang Z, Li T, Giannakakou P, et al. Nature (London) 1997;387:268–272. doi: 10.1038/387268a0. [DOI] [PubMed] [Google Scholar]
- 27.Nicolaou K C, He Y, Roschangar F, King N P, Vourloumis D, Li T H. Angew Chem Int Ed Engl. 1998;37:84–87. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2014::AID-ANIE2014>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 28.May, S. A. & Grieco, P. A. (1998) Chem. Commun. 1597–1598.
- 29.Schinzer, D., Bauer, A. & Schieber, J. (1998) Synlett., 861–864.
- 30.Meng D, Sorensen E J, Bertinato P, Danishefsky S J. J Org Chem. 1996;61:7998–7999. doi: 10.1021/jo961673w. [DOI] [PubMed] [Google Scholar]
- 31.Bertinato P, Sorensen E J, Meng D, Danishefsky S J. J Org Chem. 1996;61:8000–8001. doi: 10.1021/jo961674o. [DOI] [PubMed] [Google Scholar]
- 32.Schinzer D, Limberg A, Bohm O M. Chem Eur J. 1996;2:1477–1482. [Google Scholar]
- 33.Nicolaou K C, He Y, Vourloumis D, Vallberg H, Yang Z. Angew Chem Int Ed Engl. 1996;35:2399–2401. [Google Scholar]
- 34.Mulzer J, Mantoulidis A. Tetrahedron Lett. 1996;37:9179–9182. [Google Scholar]
- 35.Debrabander J, Rosset S, Bernardinelli G. Synlett. 1997;7:824–826. [Google Scholar]
- 36.Liu, Z. Y., Yu, C. Z. & Yang, J. D. (1997) Synlett 1383–1384.
- 37.Claus E, Pahl A, Jones P G, Meyer H M, Kalesse M. Tetrahedron Lett. 1997;38:1359–1362. [Google Scholar]
- 38.Gabriel T, Wessjohann L. Tetrahedron Lett. 1997;38:1363–1366. [Google Scholar]
- 39.Taylor R E, Haley J D. Tetrahedron Lett. 1997;38:2061–2064. [Google Scholar]
- 40.Mulzer J, Mantoulidis A, Ohler E. Tetrahedron Lett. 1997;38:7725–7728. [Google Scholar]
- 41.Bornscheuer U T, Altenbuchner J, Meyer H H. Biotech Bioeng. 1998;58:554–559. doi: 10.1002/(sici)1097-0290(19980605)58:5<554::aid-bit12>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 42.Chakraborty T K, Dutta S. Tetrahedron Lett. 1998;39:101–104. [Google Scholar]
- 43.Bijoy P, Avery M A. Tetrahedron Lett. 1998;39:209–212. [Google Scholar]
- 44.Liu Z Y, Yu C Z, Wang R F, Li G. Tetrahedron Lett. 1998;39:5261–5264. [Google Scholar]
- 45.Muhlradt P F, Sasse F. Cancer Res. 1997;57:3344–3346. [PubMed] [Google Scholar]
- 46.Wolff A, Technau A, Brandner G. Int J Oncol. 1997;11:123–126. doi: 10.3892/ijo.11.1.123. [DOI] [PubMed] [Google Scholar]
- 47.Kowalski R J, Giannakakou P, Hamel E. J Biol Chem. 1997;272:2534–2541. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- 48.Moasser M M, Sepp-Lorenzino L, Kohl N E, Oliff A, Balog A, Su D-S, Danishefsky S J, Rosen N. Proc Natl Acad Sci USA. 1998;95:1369–1374. doi: 10.1073/pnas.95.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chou T-C, Zhang X-G, Balog A, Su D-S, Meng D, Savin K, Bertino J R, Danishefsky S J. Proc Natl Acad Sci USA. 1998;95:9642–9647. doi: 10.1073/pnas.95.16.9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Su D-S, Balog A, Meng D, Bertinato P, Danishefsky S J, Zheng Y-H, Chou T-C, He L, Horwitz S B. Angew Chem Int Ed Engl. 1997;36:2093–2096. [Google Scholar]
- 51.Nicolaou K C, Vourloumis D, Li T, Pastor J, Winssinger N, He Y, Ninkovic S, Sarabia F, Vallberg H, Roschangar F, et al. Angew Chem Int Ed Engl. 1997;36:2097–2103. [Google Scholar]
- 52.Nicolaou K C, Finlay M R V, Ninkovic S, Sarabia F. Tetrahedron. 1998;54:7127–7166. [Google Scholar]
- 53.Nicolaou K C, Vallberg H, King N P, Roschangar F, He Y, Vourloumis D, Nicolaou C G. Chem Eur J. 1997;3:1957–1970. [Google Scholar]
- 54.Nicolaou K C, Sarabia F, Finlay M R V, Ninkovic S, King N P, Vourloumis D, He Y. Chem Eur J. 1997;3:1971–1986. [Google Scholar]
- 55.Nicolaou K C, Finlay M R V, Ninkovic S, King N P, He Y, Li T H, Sarabia F, Vourloumis D. Chem & Biol. 1997;5:365–372. doi: 10.1016/s1074-5521(98)90070-9. [DOI] [PubMed] [Google Scholar]
- 56.Nicolaou K C, Sarabia F, Ninkovic S, Finlay M R V, Boddy C N C. Angew Chem Int Ed Engl. 1998;37:81–84. [Google Scholar]
- 57.Balog A, Bertinato P, Su D-S, Meng D, Sorensen E, Danishefsky S J, Zheng Y-H, Chou T-C, He L, Horwitz S B. Tetrahedron Lett. 1997;38:4529–4532. [Google Scholar]
- 58.Evans D A, Vogel E, Nelson J V. J Am Chem Soc. 1979;101:6120–6123. [Google Scholar]
- 59.Sinha S C, Keinan E. J Am Chem Soc. 1995;117:3653–3654. [Google Scholar]
- 60.Zhong G, Shabat D, List B, Anderson J, Sinha S C, Lerner R A, Barbas C F., III Angew Chem Int Ed Engl. 1998;37:2481–2484. doi: 10.1002/(SICI)1521-3773(19981002)37:18<2481::AID-ANIE2481>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 61.Paterson I, Goodman J M, Lister M A, Schumann R C, McClure C K, Norcross R D. Tetrahedron. 1990;46:4663–4684. [Google Scholar]
- 62.Carlsen P H J, Katsuki T, Martin V S, Sharpless K B. J Org Chem. 1981;46:3936. [Google Scholar]