Abstract
Age-related macular degeneration, a major cause of blindness for which no satisfactory treatments exist, leads to a gradual decrease in central high acuity vision. The accumulation of fluorescent materials, called lipofuscin, in retinal pigment epithelial cells of the aging retina is most pronounced in the macula. One of the fluorophores of retinal pigment epithelial lipofuscin has been characterized as A2E, a pyridinium bis-retinoid, which is derived from two molecules of vitamin A aldehyde and one molecule of ethanolamine. An investigation aimed at optimizing the in vitro synthesis of A2E has resulted in the one-step biomimetic preparation of this pigment in 49% yield, readily producing more than 50 mg in one step. These results have allowed for the optimization of HPLC conditions so that nanogram quantities of A2E can be detected from extracts of tissue samples. By using 5% of the extract from individual aged human eyes, this protocol has led to the quantification of A2E and the characterization of iso-A2E, a new A2E double bond isomer; all-trans-retinol and 13-cis-retinol also have been identified in these HPLC chromatograms. Exposure of either A2E or iso-A2E to light gives rise to 4:1 A2E:iso-A2E equilibrium mixtures, similar to the composition of these two pigments in eye extracts. A2E and iso-A2E may exhibit surfactant properties arising from their unique wedge-shaped structures.
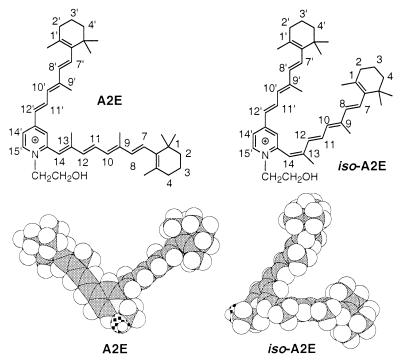
Aging changes in retinal pigment epithelial (RPE) cells are generally assumed to contribute to the pathogenesis of age-related macular degeneration (AMD) (1–3), the leading cause of acquired visual loss in persons older than 65 years of age (4–7) for which no cure exists. The most characteristic feature of aging in the RPE is the progressive cellular accumulation of lipofuscin (2, 3, 8). The deposition of this autofluorescent material is known to occur as a consequence of the role of RPE cells in phagocytosing packets of membrane that are shed by photoreceptor cells as part of the process by which photoreceptor outer segments undergo complete turnover every 2 weeks (9–11). In 1993, Eldred and coworkers (12, 13) proposed a bis-Schiff base structure (N-retinyl-N-retinylidene-ethanolamine) as one of the fluorescent pigments isolated from the pooled lipofuscin of a large number of aged human eyes. We later revised the proposed structure as the pyridinium bis-retinoid A2E (Fig. 1) (14) and succeeded in confirming this structure by a total chemical synthesis, which involved the coupling of the pyridine framework with the retinoid side chains (15).
Figure 1.
Structures of A2E and iso-A2E. Molecular modeling on each compound was performed by optimizing (MOPAC 93, AM1) initial conformers, which were constructed according to the NOE data.
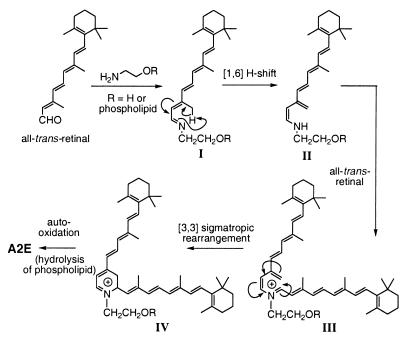
The genesis of A2E (Fig. 2), although speculative, could involve initial Schiff base formation between all-trans-retinal and ethanolamine or phosphatidylethanolamine to give (I), a [1,6]-proton tautomerization to enamine (II) followed by Schiff base generation with a second molecule of retinal to (III). This intermediate would undergo a [3,3]-sigmatropic rearrangement to (IV) and subsequent autooxidation to yield fluorescent pigment A2E or its phosphatidylethanolamine-linked adduct (which would then be hydrolyzed to A2E). The formation of A2E has been simulated in vitro by mixing two equivalents of all-trans-retinal with ethanolamine in organic solvent (14), although the yield was quite low (<1%) (13). Retinal and ethanolamine are both components of photoreceptor outer segment membranes with 11-cis-retinal serving as the chromophore of the visual pigment rhodopsin and phosphatidylethanolamine being an abundant membrane phospholipid. In vivo, absorption of light leads to the isomerization of 11-cis-retinal to all-trans-retinal followed by release of the latter from rhodopsin and its rapid reduction to all-trans-retinol. Thus, only all-trans-retinal, which evades reduction, would be available for A2E formation.
Figure 2.
Biogenesis of A2E from retinal and (phosphatidyl)ethanolamine.
Because the biological properties of A2E and its relationship to AMD have yet to be clarified, the availability of an efficient and simple method for the preparation of large quantities of A2E becomes critical for further chemical and biological studies. Although A2E has been prepared by both biomometic (14) and total chemical syntheses (15), the yield according to the former route was not only <1% but also required extensive chromatography, whereas the latter was labor-intensive, required several chemical transformations, and provided only modest overall yields. In the following, we report a biomimetic preparation of A2E that directly yields substantial quantities of this fluorophore and can be used for extensive studies aimed at clarifying the role of this pigment in AMD.
The optimization of HPLC conditions, which can detect as little as 5 ng has been accomplished. This HPLC protocol has led to the isolation and quantification of A2E in human eyes by using one-twentieth of the extract from a single eye. In addition, these experiments have allowed for the isolation of an additional fluorophore from RPE lipofuscin, a double bond isomer of A2E, iso-A2E. Preliminary studies on the photochemistry of A2E and iso-A2E indicate that they exist in a photoequilibrium of 4:1 A2E:iso-A2E and that this equilibrium most probably exists under physiological conditions.
MATERIALS AND METHODS
A2E and iso-A2E from all-trans-Retinal and Ethanolamine.
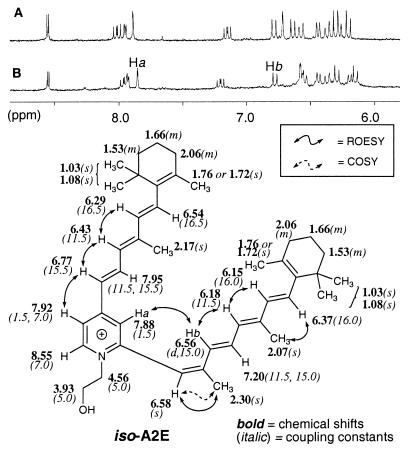
A mixture of all-trans-retinal (100 mg, 352 μmol) and ethanolamine (9.5 mg, 155 μmol) in ethanol (EtOH) (3.0 ml) was stirred in the presence of acetic acid (9.3 μl, 155 μmol) at room temperature with a sealed cap in the dark for 2 days. After the mixture was concentrated in vacuo, the residue was purified by silica gel column chromatography. After elution with methanol (MeOH):CH2Cl2 (5:95), further elution with MeOH:CH2Cl2:trifluoroacetic acid (TFA) (8:92:0.001) gave A2E (53.8 mg, 76.3 μmol, 49%), which contained ≈5% iso-A2E as estimated by 1H-NMR. Pure samples were obtained by HPLC purification [Cosmosil C18, 4.6 × 150 mm, 85–96% H2O/MeOH (0.1% TFA) for 20 min, 1.0 ml/min flow detected at UV 430 nm]. A2E and iso-A2E were detected at retention time (tR) = 16.7 min and tR =18.8 min, respectively. Collection of each fraction provided pure A2E and iso-A2E for further analysis. A2E was identical in all respects to material previously described (14). Because A2E appeared to be sensitive to trace hydrochloric acid, which may be present in CDCl3, NMR analysis in CD3OD was preferable. A2E: 1H-NMR (CD3OD, 500 MHz) δ 1.09, 1.10 (each 6H, s, C5-(CH3)2 and C5′-[(CH3)2], 1.53 (4H, m, C2-H2 and C2′-H2), 1.68 (4H, m, C3-H2 C3′-H2), 1.75, 1.77 (each 3H, s, C1-CH3 and C1′-CH3), 2.07 (3H, s, C9-CH3), 2.10 (4H, m, C4-H2 and C4′-H2), 2.19 (3H, s, C13-CH3), 2.20 (3H, s, C9′-CH3), 3.94 (2H, t, J = 5.0 Hz, CH2-O), 4.56 (2H, t, J = 5.0 Hz, N-CH2), 6.20 (1H, d, J = 16.0 Hz, C8-H), 6.27 (1H, J = 11.5 Hz, C10-H), 6.30 (1H, d, J = 16.5 Hz, C8′-H), 6.37 (1H, br d, J = 16.0 Hz, C7-H), 6.43 (1H, d, J = 11.5 Hz, C10′-H), 6.57 (1H, br d, J = 16.5 Hz, C7′-H), 6.63 (1H, d, J = 15.5 Hz, C12-H), 6.71 (1H, s, C14-H), 6.78 (1H, d, J = 15.0 Hz, C12′-H), 7.15 (1H, dd, J = 11.0, 15.0 Hz, C11-H), 7.89 (1H, d, J = 0.8 Hz, C13′-CH), 7.95 (1H, dd, J = 0.8, 7.0 Hz, C14′-H), 8.00 (1H, dd, J = 11.5, 15.5 Hz, C11′-H), and 8.55 (1H, d, J = 7.0 Hz, C15′-H). High resolution fast atom bombardment (HR FAB)-MS (3-nitrobenzylalcohol) m/z = 592.4505 (calculated for C42H58ON, 592.4521, [M]+) iso-A2E: 1H-NMR (500 MHz, CD3OD) is shown in Fig. 5. HR FAB-MS (m/z = 592.4491; calculated for C42H58ON, 592.4521, [M]+). UV (methanol): λmax 430 nm (ɛM 31,000), 335 (ɛM 27,000).
Figure 5.
1H-NMR spectra of A2E and iso-A2E. Region of 1H-NMR (CD3OD, 500 MHz) spectra of A2E (A) and iso-A2E (B) corresponding to aromatic and olefinic protons. The critical ROESY correlation peak between Ha and Hb of iso-A2E is identified. This correlation peak is absent in A2E (14). The assignment of all 1H-NMR chemical shifts as well as the COSY and ROESY correlation peaks, which were important for assigning the configuration of each double bond, are indicated.
Extraction of Human RPE Pigments.
Individual human donor eyes were obtained from the Eye Bank for Sight Restoration (New York). From eyes ranging in age from 44 to 80 years (male and female, all Caucasian, died of cancer or cardiovascular disease, and no significant ophthalmologic history), the RPE and choroidal layers were isolated and homogenized in a tissue homogenizer with a solution of chloroform/methanol/PBS (2.0 ml/1.0 ml). Each extract was filtered through cotton and passed through a reversed phase (C18 Sep-Pak, Millipore) cartridge with 0.1% TFA in methanol. After removing all solvent in vacuo, each sample was redissolved in a small amount of methanol (200 μl) before HPLC analysis.
HPLC Analysis of Eye Extracts.
Samples extracted from human RPE/choroid tissue were injected onto a reversed phase (C18) column (Cosmosil 5C18, Nacalai Tesque, 150 mm × 4.6 mm), and eluted with a gradient of methanol in water (85–96% methanol + 0.1% TFA, 1 ml/min, Waters 600E HPLC system). A photodiode array detector (Waters 996) was used to obtain an UV spectrum of each eluted fraction. Specific wavelength detection was used for monitoring A2E (430 nm) and retinols (320 nm).
Photoisomerization of A2E and iso-A2E.
A solution of HPLC-purified A2E or iso-A2E in methanol (≈40 μM) was subjected to either room light or monochromatic (430 nm) light from a monochromator (5–10 nm slit width). The extent of isomerization at each time point was analyzed by RP-HPLC by using the conditions described above.
RESULTS AND DISCUSSION
An investigation aimed at optimizing the yield of A2E (Fig. 1) from all-trans-retinal and ethanolamine was performed by using a wide range of reaction conditions. Typical results are summarized in Table 1. When this reaction was carried out in the absence of acetic acid, only a trace amount of A2E was observed after 1 week (run 1), whereas conditions using ethanolamine hydrochloride in place of the free amine gave no A2E (run 2). The previously used biomimetic conditions included acetic acid (AcOH) as an additive, and, in fact, A2E was obtained under conditions similar to those initially reported (AcOH, CH2Cl2, 20 min) (13), although this protocol gave the desired product in only ≈0.5% yield. These results indicated that the preparation of A2E is very sensitive to the nature of the acid additive as well as to the overall pH of the reaction mixture. A more detailed analysis of the preparation of A2E and the role of acetic acid in the biomimetic synthesis indicated that improved yields were obtained when only one equivalent of acid was used in the reaction mixture. As shown in run 3 (Table 1), prolonging the reaction time from 20 min to 4 days, under otherwise identical reaction conditions, increased the yield up to 8%.
Table 1.
Optimization of A2E synthesis
| Run* | HOC2H4NH2 | Additives | Solvents | Time | Results† |
|---|---|---|---|---|---|
| 1 | Free amine | None | EtOH | 7 days | trace |
| 2 | HCl salt | None | EtOH | 7 days | 0% |
| 3 | Free amine | AcOH (1 eq) | CH2Cl2 | 4 days | 8% |
| 4 | Free amine | AcOH (1 eq) | EtOH | 2 days | 49% |
All reactions were performed open to the air and under dim red light. The concentration of ethanolamine was 50–100 μmol/ml.
The yields are calculated as TFA salts because they were isolated by silica gel chromatography eluted with TFA-MeOH-CH2Cl2.
The use of ethanol as the solvent in the biomimetic procedure led to a dramatic improvement, affording A2E in 49% yield (>50 mg, run 4); moreover, only a single silica gel column chromatography was required to give essentially pure A2E, which contained only a small amount of an A2E isomer. It appears that the A2E formation cascade (Fig. 2) is not favored under strongly acidic or basic conditions, an observation which is consistent with the fact that A2E is formed under physiologic conditions. The use of these reaction conditions has led to the preparation of 14C-labeled A2E from [1,2-14C]-ethanolamine hydrochloride (data not shown), which will be used in further biochemical studies.
Because the biological properties of A2E and its relationship with AMD are unclear, the development of efficient methods for the microscale analysis of A2E was essential for further biological and chemical studies. The optimization of HPLC conditions for the analysis of A2E was carried out by using the sample previously obtained. It was found that RP- (C18) HPLC by using a linear gradient of methanol and water (85 to 96% methanol) containing 0.1% of TFA provided a sharp peak corresponding to A2E at 16.7 min., the detection limit of A2E under these conditions being ≈5 ng.
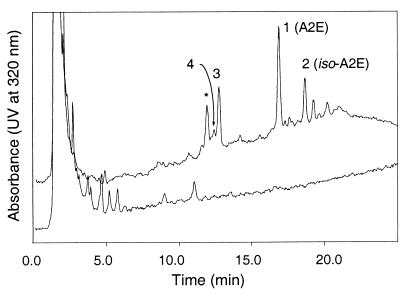
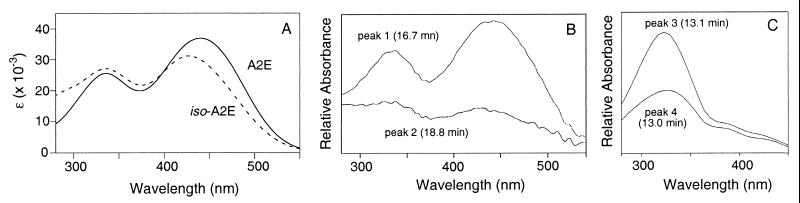
Because the reported isolation from a pool of 250 aged human eyes provided ≈100 μg of A2E (13), it was reasonable to expect that the quantity of A2E from a single aged eye would be more than sufficient to be detected by HPLC. The RPE and choroidal layers were isolated from individual human eyes, the donors of which were greater than forty years of age. Each sample was extracted with chloroform/methanol mixtures to obtain the lipophilic materials present in the tissue. The entire extraction was performed under dim light to prevent photochemical degradation of fluorophores. This simple procedure minimizes the extent of degradation, as well as the amount of sample loss, which might occur during extended protocols. As expected, the HPLC conditions thus developed were able to detect the A2E isolated from 5% of the sample from one aged eye. An example of the HPLC profile is shown in Fig. 3. The major component of all samples was A2E (peak 1) based on UV absorbance. The UV spectrum of synthetic A2E in methanol (Fig. 4) contained two peaks with λmax 439 nm (ɛM 36, 900) and 334 nm (ɛM 25, 600). The analysis at various other wavelengths between 300 and 450 nm provided similar chromatograms with slight alterations in peak intensity. While A2E can be observed with UV detection over 400 nm, retinols (λmax = 325 nm) and retinals (λmax = 365 nm) are only observed at shorter wavelengths. In addition, because retention times may vary slightly between injections, coinjection of authentic material with the eye extract was used to confirm the identity of the A2E peak.
Figure 3.
HPLC of aged and fetal eye extracts. Samples extracted from human RPE/choroid tissue were injected onto a reversed phase (C18) column (Cosmosil 5C18, Nacalai Tesque, 150 mm × 4.6 mm), eluting with a gradient of methanol in water (85–96% methanol + 0.1% TFA, 1 ml/min). The displayed chromatograms correspond to absorbances at 320 nm. Upper trace: The HPLC of extracted pigments (10 μl of a 200 μl extract) from an 80-year-old eye contained a mixture of pigments, which have been partially identified: peak 1, A2E; peak 2, iso-A2E; peak 3, all-trans-retinol; peak 4, 13-cis-retinol; and ∗, unidentified pigment. All-trans-retinal has a retention time of 14.5 min under these conditions. Lower trace: The HPLC of fetal eye extracts (entire extract) did not contain any detectable amount of these materials.
Figure 4.
UV spectra of synthetic and extracted pigments. (A) UV spectra of A2E (solid line) and iso-A2E (dashed line) in methanol. A2E: λmax 439 nm (ɛM 36, 900), 336 (ɛM 25, 600); iso-A2E: λmax 426 nm (ɛM 31,000), 335 (ɛM 27,000); (B) UV spectra of peak 1 (A2E) and peak 2 (iso-A2E) from eye extracts. The weak iso-A2E spectrum is due to the limited quantity of iso-A2E present in the extracts. (C) UV spectra of peak 3 (all-trans-retinol) and peak 4 (13-cis-retinol) from eye extracts. The λmax of retinol is ≈325 nm. The spectra provided in B and C were obtained by PDA detection in the HPLC eluent, ≈10% methanol in water containing 0.1% TFA.
HR FAB-MS of peak 1 (Fig. 3) collected from an injection of the extracted material (10% of the total solution) provided a molecular ion peak of m/z = 592.4505 (calculated for C42H58ON, 592.4521). The UV of peak 1 as obtained from the photodiode array detector (Fig. 4B) was identical to that obtained from synthetic A2E (Fig. 4A). All eyes from seven adult donors contained substantial amounts of A2E. The quantity of A2E isolated from each eye, as determined from integrated peak intensities was found to range from 200 to 800 ng. No direct comparison between the amount of A2E and age has been made due to the small sample size and the lack of appropriate control eyes. However, this protocol would allow for a thorough analysis of the relationship between A2E and age in a more extensive study. The isolated amounts of A2E corresponded well with the estimate made from the previous protocol (13). In contrast to the adult eyes, the extracts of choroid/RPE isolated from two fetal eyes (18 and 20 weeks of gestation; Anatomic Gift Foundation, White Oaks, GA) did not contain A2E. Even when the entire fetal eye sample was injected, none of this pigment was observed (Fig. 3), indicating that A2E accumulates with increasing age. Recently, rat eye extracts have been analyzed for A2E by HPLC at the short wavelength of 200 nm, a nonselective wavelength where pigmented as well as other compounds would be detected (16). However, those conditions do not provide a clear indication of the quantity of A2E present in the rat samples due to the short wavelength (200 nm) used for detection.
Because A2E can be generated efficiently from all-trans-retinal and ethanolamine under biomimetic conditions, it was necessary to exclude the possibility that the pigment was being generated during isolation. Thus, we sought to determine whether retinal was present in the eye extracts. If A2E was being generated after extraction of the eye tissues, significant quantities of retinal would be observed in these samples. First, it was established that all-trans-retinal could pass through a reversed phase (C18) cartridge under conditions used in the extraction procedure. While the retention time of authentic all-trans-retinal (λmax = 365 nm) was 14.5 min under these HPLC conditions, none was observed in any eye sample. The absence of all-trans-retinal from RPE/choroid was not surprising since all-trans-retinal is reduced to all-trans-retinol within the photoreceptor outer segment before being transported to the RPE (17). All-trans-retinol (Fig. 3, peak 3, tR = 13.1 min) and 13-cis-retinol (Fig. 3, peak 4, tR = 13.0) were identified in the HPLC profile by their UV (λmax = 325) and by coinjection with authentic samples. The single maximum of these UV (Fig. 4C) clearly indicates that these fractions correspond to retinols and not structures that are analogous to A2E. The retinol peaks could be detected in five of the seven aged eyes that have been investigated to this point. It was expected that retinyl palmitate would be present in the RPE/choroid extract (12). However, since authentic retinyl palmitate was retained on the C18 column under these conditions, the presence of this material in the eye extracts can only be confirmed by using a modified HPLC solvent system.
In addition to A2E, a second peak with considerable peak height, corresponding to a slightly less polar pigment, was visible in all aged eye extracts (Fig. 3, peak 2, tR = 18.8 min). The UV spectrum of this fraction (Fig. 4B) contained two peaks with λmax = 430 and 330 nm, similar to that of A2E, but different in relative intensity. HR FAB-MS (m/z = 592.4491; calculated for C42H58ON, 592.4521) indicated that this compound was an isomer of A2E. However, it was not possible to obtain an interpretable 1H-NMR of this compound when isolated from donor eyes since <100 ng could be obtained from each extract. Interestingly, it was found that the HPLC chromatogram of the biomimetic synthesis of A2E described earlier (see above) contained material, which was identical to peak 2 of the eye extract as judged by HPLC coinjection, UV (Fig. 4A) and FAB-MS. Therefore, the synthetic sample was studied by 1H-NMR and identified as iso-A2E, the Z-isomer of A2E at the C13–C14 double bond (Fig. 5). The UV spectrum of iso-A2E in methanol (Fig. 4A) contained two peaks with λmax 426 nm (ɛM 31,000) and 335 nm (ɛM 27,000). The 1H-NMR spectrum was very similar to, but not identical to, A2E (Fig. 5). 1H-1H COSY (homonuclear shift correlation spectroscopy) allowed for the complete identification of methyl and olefinic proton resonances. A significant correlation peak was observed between Ha (7.88 ppm, d, J = 1.5 Hz) and Hb (6.56 ppm, d, J = 15.0 Hz) by rotating frame nuclear Overhauser and exchange spectroscopy (ROESY) suggesting a C13-C14 Z-geometry. This correlation peak was not observed in A2E when the structure was fully described by ROE techniques (14). All other double bonds were identified as being in E configurations based on large coupling constants (J7, 8 = 16.0 Hz, J11, 12 = 15.0, J7′, 8′ = 16.5, J11′, 12′ = 15.5) and ROESY correlations (8-H′10-H, 13-CH3′14-H, 8′-H′10′-H) (see numbering in Fig. 1).
While iso-A2E in methanol was stable when stored at −70°C for more than 1 month in the dark, exposure of this solution to room light for 30 min at room temperature led to isomerization of most of the compound to A2E. Under these conditions, HPLC-purified A2E and iso-A2E both undergo photoequilibration to provide ≈4:1 mixtures of A2E:iso-A2E without significant decomposition to other products, as determined by HPLC (Fig. 6). This isomerization also was observed when A2E and iso-A2E were exposed to monochromatic (430 nm) light. For example, a gradual conversion of iso-A2E to A2E resulted from increasing light exposure: 15 sec exposure, A2E/iso-A2E 3/97; 2 min, 22/78; 7 min, 58/42; and 15 min, 78/22. Thus, although the human lens absorbs light below 400 nm (18), longer wavelength light reaching the retina would have the capacity to photoisomerize A2E generated in vivo. Furthermore, because iso-A2E was present in an eye extracted in the dark, it appears that this fluorophore is also present in RPE lipofuscin, as opposed to being generated by photoisomerization after isolation. The photochemistry of A2E is such that the generation of radical species could be involved in this isomerization in vivo (19). It also is possible that radicals generated by photoactivated A2E may lead to cellular damage and a decrease in RPE function.
Figure 6.
Photoisomerization of A2E and iso-A2E. Methanolic solutions of purified (A) A2E and (C) iso-A2E were exposed to room light for 30 min. The samples were reanalyzed by HPLC and resulted in similar product mixtures containing approximately 4:1 A2E:iso-A2E: (B) A2E + light; (D) iso-A2E + light. A small impurity (∗) is present in the synthetic iso-A2E sample.
This work describes the optimization of conditions for the single-step preparation of A2E in high yield providing large (>50 mg) quantities of material. An efficient HPLC protocol allows for the identification of the fluorophores present in RPE lipofuscin by using extracts from a single human eye. This procedure has resulted in the identification of one novel pigment (iso-A2E) present in aged eye RPE lipofuscin. The presence of all-trans- and 13-cis-retinol in most extracts also has been confirmed. While the role of A2E in AMD remains unclear, the amount of this natural product in each eye can be easily quantified by this procedure. The ability to determine the amount of A2E in the RPE of an individual is of special interest for epidemiological studies. A substantial source of A2E and iso-A2E will enable the further clarification of the role of these fluorophores in AMD. Preliminary experiments incubating cultured human RPE cells with synthetic A2E have confirmed that A2E is incorporated into the cells (data not shown). The cellular effects of A2E exposure are currently being characterized. In addition to the possibility that these pigments can lead to oxidative/radical damage in cells, the amphiphilic character of the uniquely wedge-shaped A2E and the narrower, more streamlined iso-A2E (Fig. 1, molecular models) would suggest that these detergent-like molecules could readily interact with membranes and perturb membrane integrity. The identification of additional lipofuscin fluorophores, the photochemical properties of A2E, and the effect of this pigment on membrane and cell function are under investigation.
Acknowledgments
This work was supported by National Institutes of Health Grant 34509 (to K.N.), a National Institutes of Health National Research Service Award postdoctoral fellowship (to C.A.P.), and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology, Columbia University.
ABBREVIATIONS
- AMD
age-related macular degeneration
- A2E
pyridinium bis-retinoid formed from two molecules of vitamin A aldehyde and one molecule of ethanolamine
- iso-A2E
double bond isomer of A2E
- RPE
retinal pigment epithelium
- TFA
trifluoroacetic acid
- HR FAB-MS
high resolution fast atom bombardment mass spectrometry
- tR
retention time
References
- 1.Sahel J A, Brini A, Albert D M. In: Pathology of the Retina and Vitreous. Albert D M, Jakobiec F A, editors. Vol. 4. Philadelphia: Saunders; 1994. pp. 2239–2280. [Google Scholar]
- 2.Feeney-Burns L, Hildebrand E S, Eldridge S. Invest Ophthalmol Visual Sci. 1984;25:195–200. [PubMed] [Google Scholar]
- 3.Dorey C K, Wu G, Ebenstein D, Garsd A, Weiter J J. Invest Ophthalmol Visual Sci. 1989;30:1691–1699. [PubMed] [Google Scholar]
- 4.Klein R, Klein B E K, Linton K L P. Ophthalmology. 1992;99:933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 5.Egan K M, Seddon J M. In: Age-Related Macular Degeneration: Epidemiology. Albert D M, Jakobiec F A, editors. Philadelphia: Saunders; 1994. pp. 1266–1274. [Google Scholar]
- 6.Young R W. Surv Ophthalmol. 1987;31:291–306. doi: 10.1016/0039-6257(87)90115-9. [DOI] [PubMed] [Google Scholar]
- 7.Tso M O M. Ophthalmology. 1985;92:628–635. doi: 10.1016/s0161-6420(85)33992-1. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy C J, Rakoczy P E, Constable I J. Eye. 1995;9:763–771. doi: 10.1038/eye.1995.192. [DOI] [PubMed] [Google Scholar]
- 9.Katz M L, Eldred G E. Invest Ophthalmol Visual Sci. 1989;30:37–43. [PubMed] [Google Scholar]
- 10.Boulton M, McKechnie N M, Breda J, Bayly M, Marshall J. Invest Ophthalmol Visual Sci. 1989;30:82–89. [PubMed] [Google Scholar]
- 11.Feeney-Burns L, Eldred G E. Trans Ophthalmol Soc U K. 1983;103:416–421. [PubMed] [Google Scholar]
- 12.Eldred G E, Katz M L. Exp Eye Res. 1988;47:71–86. doi: 10.1016/0014-4835(88)90025-5. [DOI] [PubMed] [Google Scholar]
- 13.Eldred G E, Lasky M R. Nature (London) 1993;361:724–726. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- 14.Sakai N, Decatur J, Nakanishi K, Eldred G E. J Am Chem Soc. 1996;118:1559–1560. [Google Scholar]
- 15.Ren R X-F, Sakai N, Nakanishi K. J Am Chem Soc. 1997;119:3619–3620. [Google Scholar]
- 16.Reinboth J-J, Gautschi K, Munz K, Eldred G E, Remé C E. Exp Eye Res. 1997;65:639–643. doi: 10.1006/exer.1997.0367. [DOI] [PubMed] [Google Scholar]
- 17.Bok D. J Cell Sci Suppl. 1993;17:189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- 18.Dillon J. J Photochem Photobiol B. 1991;10:23–40. doi: 10.1016/1011-1344(91)80209-z. [DOI] [PubMed] [Google Scholar]
- 19.Reszka K, Eldred G E, Wang R H, Chignell C, Dillon J. Photochem Photobiol. 1995;62:1005–1008. doi: 10.1111/j.1751-1097.1995.tb02400.x. [DOI] [PubMed] [Google Scholar]