Abstract
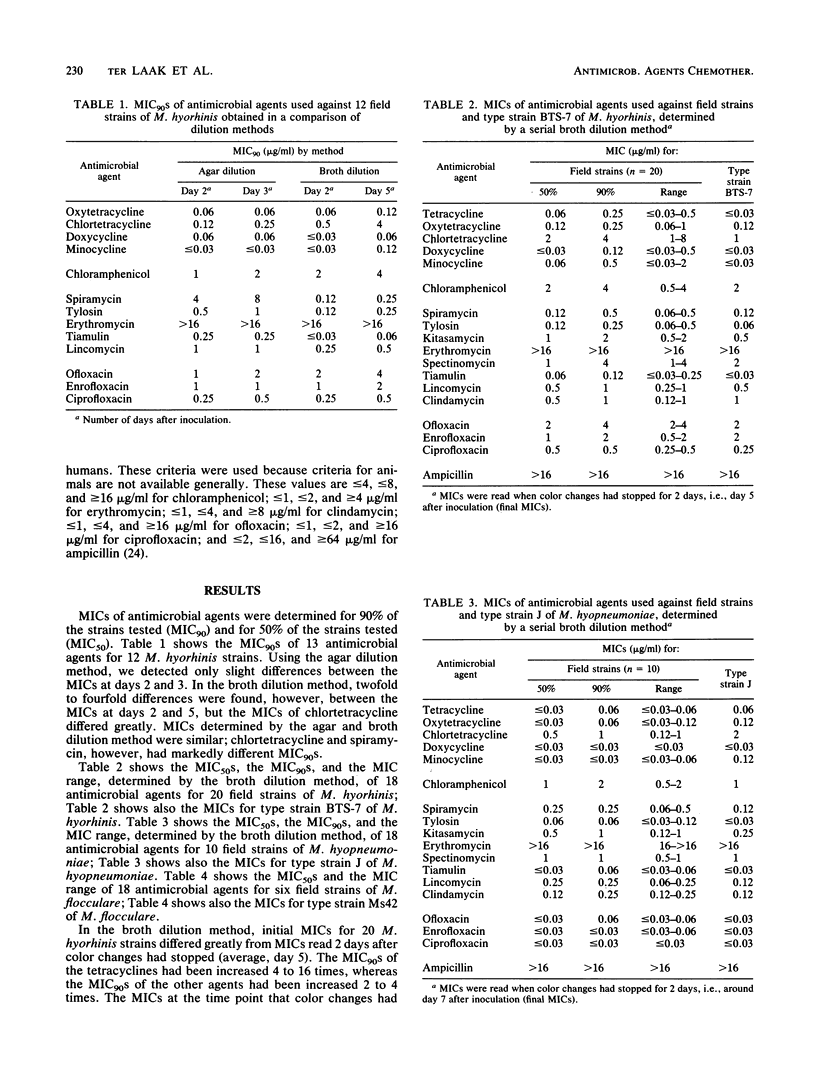
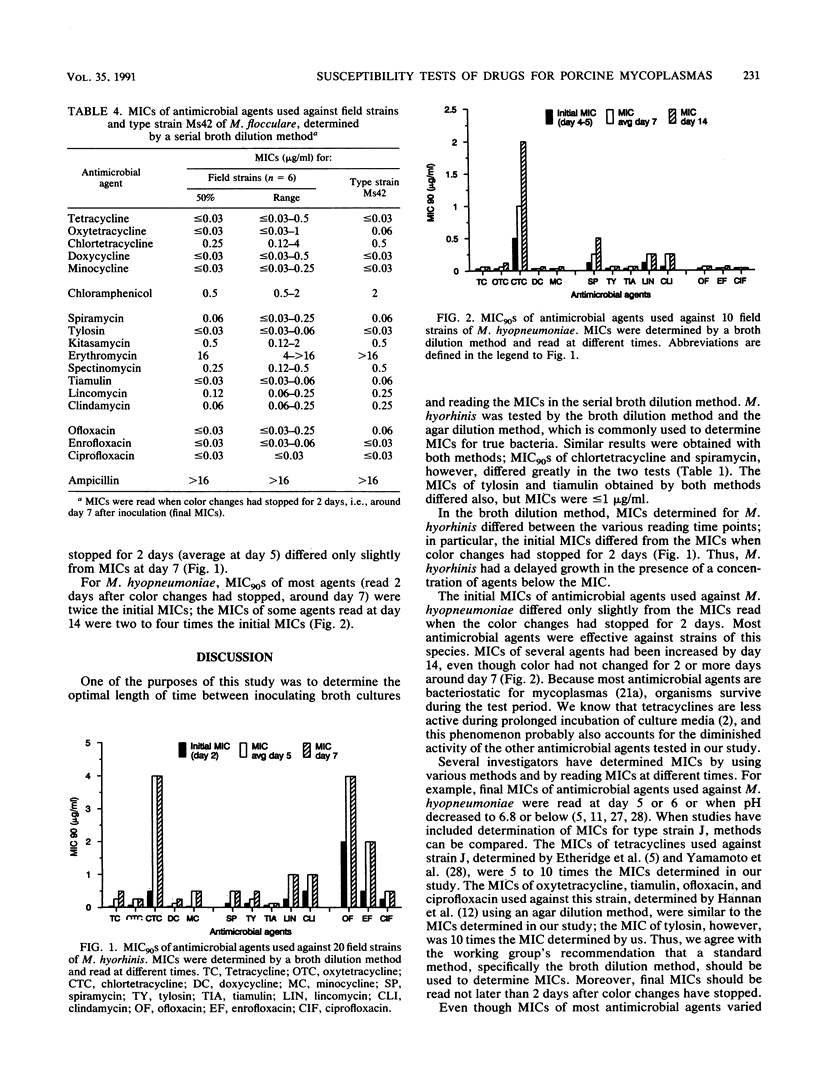
The MICs of 18 antimicrobial agents used against strains of three porcine Mycoplasma species were determined by a serial broth dilution method. Twenty field strains of M. hyorhinis, ten field strains of M. hyopneumoniae, six field strains of M. flocculare, and the type strains of these species were tested. Twelve field strains and the type strain of M. hyorhinis were also tested by an agar dilution method. Tests were read at various time points. When the broth dilution method was used, the final MIC had to be read 2 days after color changes had stopped. MICs of tetracycline, oxytetracycline, doxycycline, and minocycline were low for the three Mycoplasma species tested. MICs of chlortetracycline were 8 to 16 times higher than MICs of the other tetracyclines. Spiramycin, tylosin, kitasamycin, spectinomycin, tiamulin, lincomycin, and clindamycin were effective against all strains of M. hyorhinis and M. hyopneumoniae. The quinolones were highly effective against M. hyopneumoniae but less effective against M. hyorhinis. The susceptibility patterns for M. hyopneumoniae and M. flocculare were similar.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bebear C., Cantet P., Renaudin H., Quentin C. Activité comparée de la minocycline et doxycycline sur les mycoplasmes pathogènes pour l'homme. Pathol Biol (Paris) 1985 Jun;33(5 Pt 2):577–580. [PubMed] [Google Scholar]
- Drews J., Georgopoulos A., Laber G., Schütze E., Unger J. Antimicrobial activities of 81.723 hfu, a new pleuromutilin derivative. Antimicrob Agents Chemother. 1975 May;7(5):507–516. doi: 10.1128/aac.7.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge J. R., Lloyd L. C., Cottew G. S. Resistance of Mycoplasma hyopneumoniae to chlortetracycline. Aust Vet J. 1979 Jan;55(1):40–40. doi: 10.1111/j.1751-0813.1979.tb09552.x. [DOI] [PubMed] [Google Scholar]
- Friis N. F. Mycoplasma suipneumoniae and mycoplasma flocculare in the growth precipitation test. Acta Vet Scand. 1977;18(2):168–175. doi: 10.1186/BF03548445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis N. F. Selective isolation of slowly growing acidifying mycoplasmas from swine and cattle. Acta Vet Scand. 1979;20(4):607–609. doi: 10.1186/BF03546591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis N. F. Some recommendations concerning primary isolation of Mycoplasma suipneumoniae and Mycoplasma flocculare a survey. Nord Vet Med. 1975 Jun;27(6):337–339. [PubMed] [Google Scholar]
- Goodwin R. F. In vitro activity of tiamulin against porcine mycoplasmas. Res Vet Sci. 1985 Jan;38(1):124–125. [PubMed] [Google Scholar]
- Hannan P. C., O'Hanlon P. J., Rogers N. H. In vitro evaluation of various quinolone antibacterial agents against veterinary mycoplasmas and porcine respiratory bacterial pathogens. Res Vet Sci. 1989 Mar;46(2):202–211. [PubMed] [Google Scholar]
- Livingston C. W., Jr, Stair E. L., Underdahl N. R., Mebus C. A. Pathogenesis of mycoplasmal pneumonia in swine. Am J Vet Res. 1972 Nov;33(11):2249–2258. [PubMed] [Google Scholar]
- Mårdh P. A. Human respiratory tract infections with mycoplasmas and their in vitro susceptibility to tetracyclines and some other antibiotics. Chemotherapy. 1975;21 (Suppl 1):47–57. doi: 10.1159/000221891. [DOI] [PubMed] [Google Scholar]
- Robertson J. A., Coppola J. E., Heisler O. R. Standardized method for determining antimicrobial susceptibility of strains of Ureaplasma urealyticum and their response to tetracycline, erythromycin, and rosaramicin. Antimicrob Agents Chemother. 1981 Jul;20(1):53–58. doi: 10.1128/aac.20.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Black F. T. Direct and indirect immunofluorescence of unfixed and fixed Mycoplasma colonies. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(4):615–622. doi: 10.1111/j.1699-0463.1972.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Vogelzang A. A., van Klingeren B. Proceedings: Mycoplasmas in cell cultures. Antonie Van Leeuwenhoek. 1974;40(2):316–317. doi: 10.1007/BF00394394. [DOI] [PubMed] [Google Scholar]
- Williams P. P. In vitro susceptibility of Mycoplasma hyopneumoniae and Mycoplasma hyorhinis to fifty-one antimicrobial agents. Antimicrob Agents Chemother. 1978 Aug;14(2):210–213. doi: 10.1128/aac.14.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Koshimizu K., Ogata M. In vitro susceptibility of Mycoplasma hyopneumoniae to antibiotics. Nihon Juigaku Zasshi. 1986 Feb;48(1):1–5. doi: 10.1292/jvms1939.48.1. [DOI] [PubMed] [Google Scholar]


