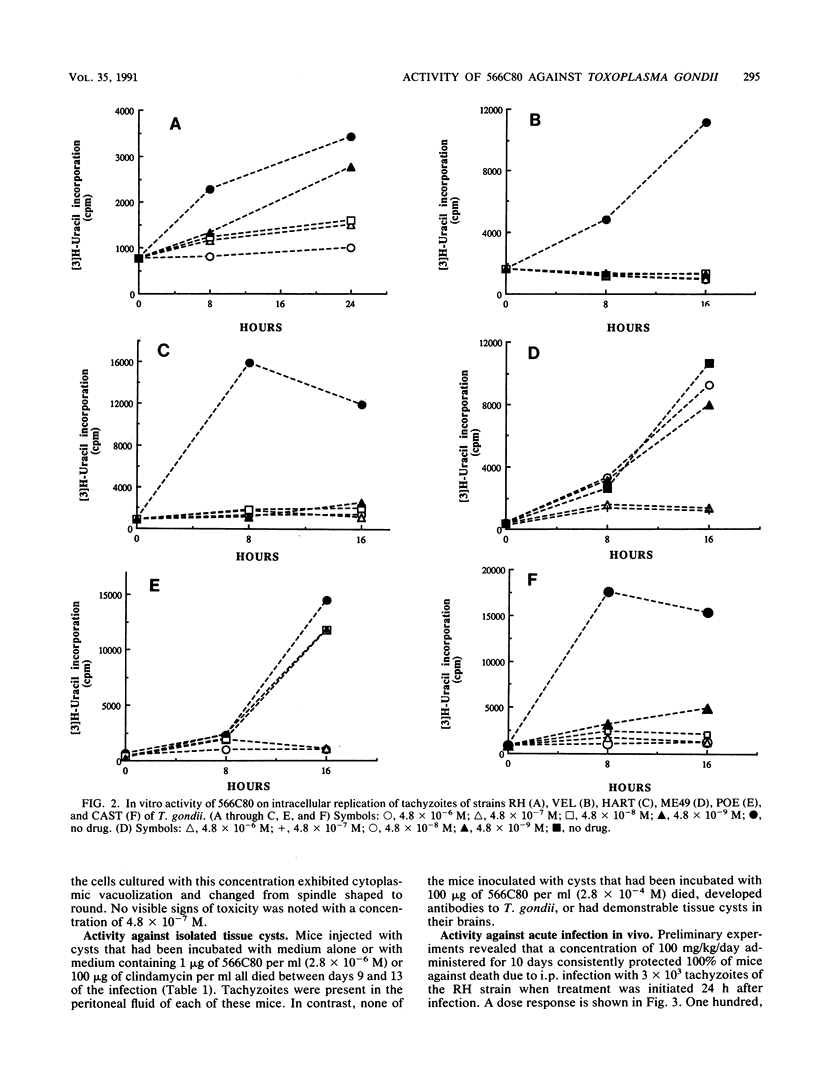
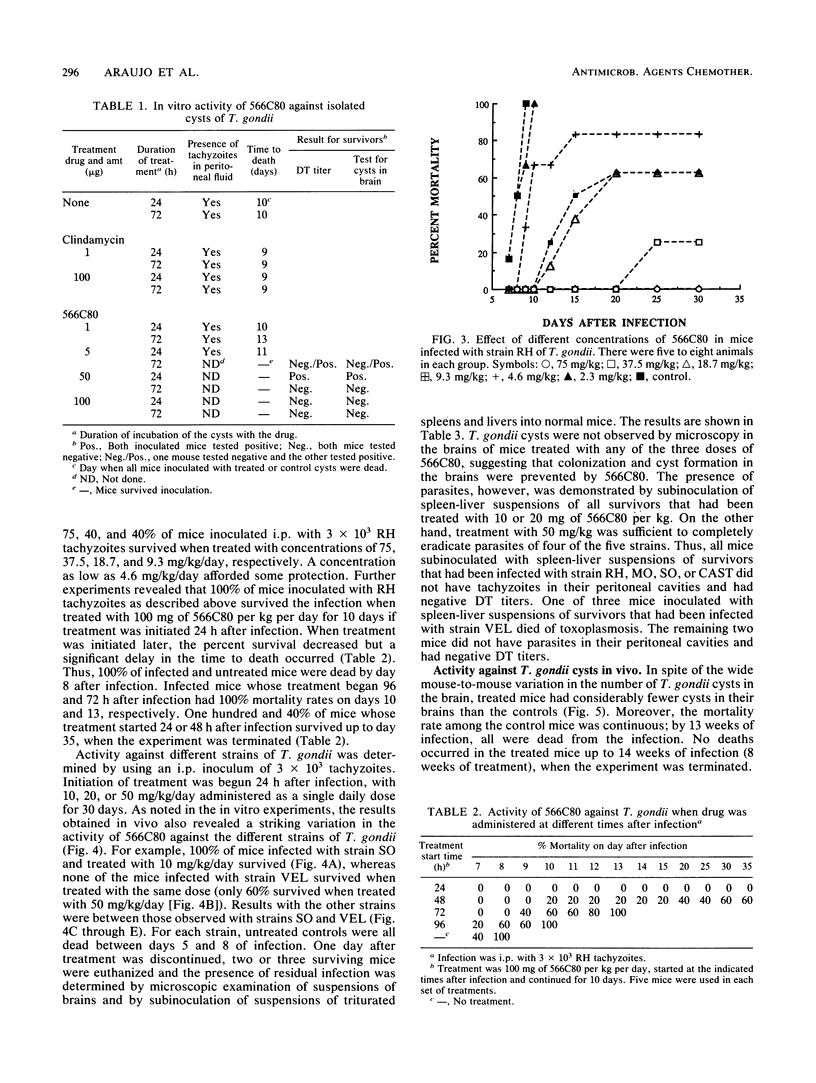
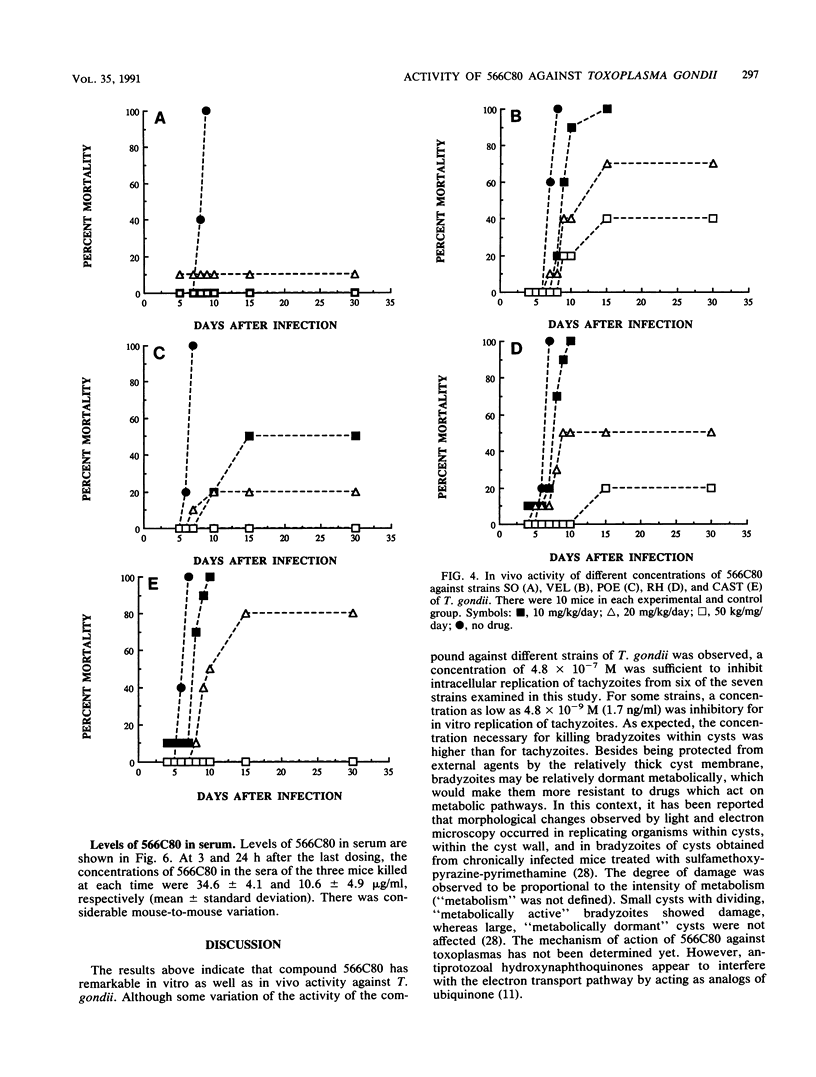
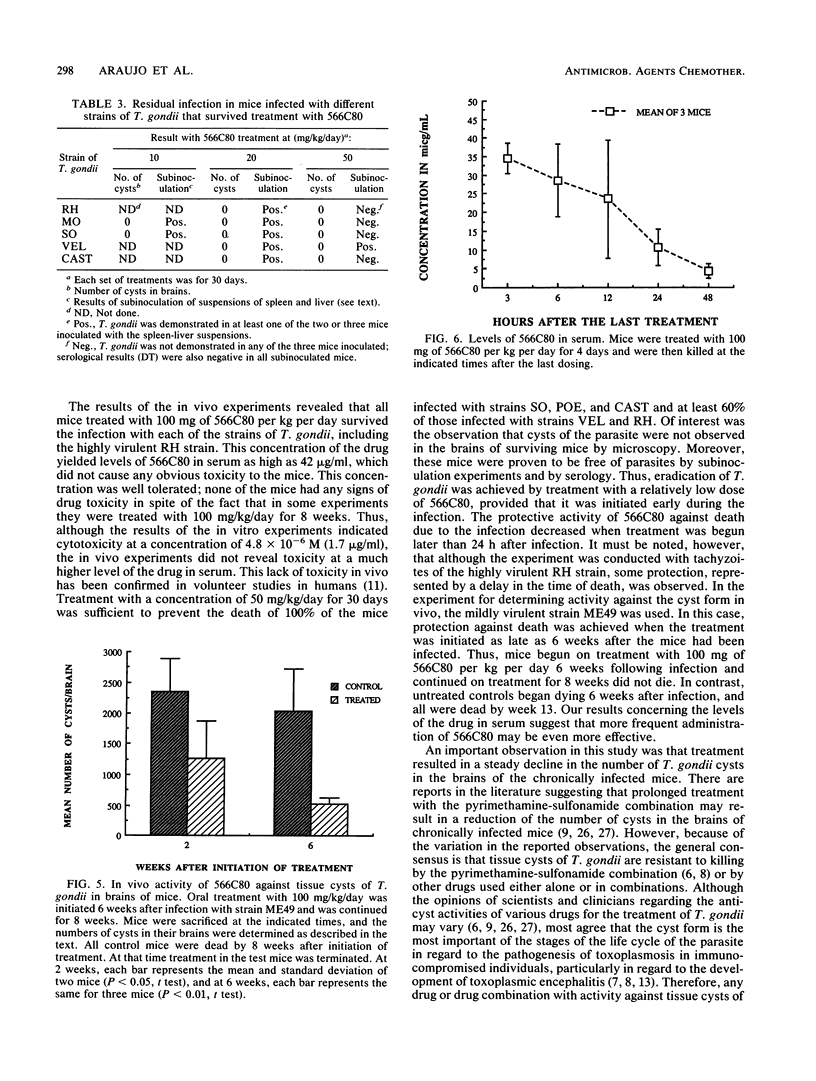
Abstract
Compound 566C80, 2-[trans-4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone, was studied for its in vitro and in vivo activities against Toxoplasma gondii. Replication within human foreskin fibroblasts of tachyzoites of seven different strains, five of them isolated from AIDS patients, was inhibited by concentrations as low as 4.8 x 10(-9) M. In vivo, a dose of 100 mg/kg of body weight per day, administered by gavage for 10 days, protected 100% of mice against death due to infection with five different strains of T. gondii, including the highly virulent RH strain. A dose of 50 mg/kg/day protected at least 80% of mice infected with the same inoculum, and a dose as low as 9.3 mg/kg/day protected 40 to 60% of mice. Treatment with 50 mg/kg/day for 30 days completely eradicated parasites from mice infected with four of five strains of T. gondii. 566C80 was active in vitro against the cyst stage of T. gondii at concentrations of 50 to 100 micrograms/ml. In vivo activity against this form of T. gondii was examined in mice infected for 6 weeks with strain ME49 and then treated orally with 100 mg of 566C80 per kg per day for 8 weeks. Treated mice sacrificed at 2-week intervals revealed a steady decline in the numbers of cysts in their brains compared with untreated controls. In addition, mortality as well as clinical signs of brain infection was absent from treated mice, whereas control mice had a high mortality rate and showed clinical signs of central nervous system infection. These results reveal remarkable in vitro and in vivo activities of 566C80 against T. gondii.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo F. G., Guptill D. R., Remington J. S. Azithromycin, a macrolide antibiotic with potent activity against Toxoplasma gondii. Antimicrob Agents Chemother. 1988 May;32(5):755–757. doi: 10.1128/aac.32.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F. G., Remington J. S. Effect of clindamycin on acute and chronic toxoplasmosis in mice. Antimicrob Agents Chemother. 1974 Jun;5(6):647–651. doi: 10.1128/aac.5.6.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., Luft B. J. Activity of roxithromycin (RU 28965), a macrolide, against Toxoplasma gondii infection in mice. Antimicrob Agents Chemother. 1986 Aug;30(2):323–324. doi: 10.1128/aac.30.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannemann B. R., Morris V. A., Araujo F. G., Remington J. S. Assessment of human natural killer and lymphokine-activated killer cell cytotoxicity against Toxoplasma gondii trophozoites and brain cysts. J Immunol. 1989 Oct 15;143(8):2684–2691. [PubMed] [Google Scholar]
- Frenkel J. K., Escajadillo A. Cyst rupture as a pathogenic mechanism of toxoplasmic encephalitis. Am J Trop Med Hyg. 1987 May;36(3):517–522. doi: 10.4269/ajtmh.1987.36.517. [DOI] [PubMed] [Google Scholar]
- Frenkel J. K., Nelson B. M., Arias-Stella J. Immunosuppression and toxoplasmic encephalitis: clinical and experimental aspects. Hum Pathol. 1975 Jan;6(1):97–111. doi: 10.1016/s0046-8177(75)80111-0. [DOI] [PubMed] [Google Scholar]
- Gill H. S., Prakash O. Chemotherapy and chemoprophylaxis of experimental toxoplasmosis. Indian J Med Res. 1972 Jul;60(7):1022–1027. [PubMed] [Google Scholar]
- Gutteridge W. E. Antimalarial drugs currently in development. J R Soc Med. 1989;82 (Suppl 17):63–68. [PMC free article] [PubMed] [Google Scholar]
- Haverkos H. W. Assessment of therapy for toxoplasma encephalitis. The TE Study Group. Am J Med. 1987 May;82(5):907–914. doi: 10.1016/0002-9343(87)90151-3. [DOI] [PubMed] [Google Scholar]
- Hofflin J. M., Conley F. K., Remington J. S. Murine model of intracerebral toxoplasmosis. J Infect Dis. 1987 Mar;155(3):550–557. doi: 10.1093/infdis/155.3.550. [DOI] [PubMed] [Google Scholar]
- Hudson A. T., Randall A. W., Fry M., Ginger C. D., Hill B., Latter V. S., McHardy N., Williams R. B. Novel anti-malarial hydroxynaphthoquinones with potent broad spectrum anti-protozoal activity. Parasitology. 1985 Feb;90(Pt 1):45–55. doi: 10.1017/s0031182000049003. [DOI] [PubMed] [Google Scholar]
- Israelski D. M., Araujo F. G., Wachtel J. S., Heinrichs L., Remington J. S. Differences in microbicidal activities of human macrophages against Toxoplasma gondii and Trypanosoma cruzi. Infect Immun. 1990 Jan;58(1):263–265. doi: 10.1128/iai.58.1.263-265.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelski D. M., Remington J. S. Toxoplasmic encephalitis in patients with AIDS. Infect Dis Clin North Am. 1988 Jun;2(2):429–445. [PubMed] [Google Scholar]
- Luft B. J., Brooks R. G., Conley F. K., McCabe R. E., Remington J. S. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA. 1984 Aug 17;252(7):913–917. [PubMed] [Google Scholar]
- Luft B. J., Remington J. S. AIDS commentary. Toxoplasmic encephalitis. J Infect Dis. 1988 Jan;157(1):1–6. doi: 10.1093/infdis/157.1.1. [DOI] [PubMed] [Google Scholar]
- McLeod R., Remington J. S. A method to evaluate the capacity of monocytes and macrophages to inhibit multiplication of an intracellular pathogen. J Immunol Methods. 1979 May 10;27(1):19–29. doi: 10.1016/0022-1759(79)90235-7. [DOI] [PubMed] [Google Scholar]
- Mills J. Pneumocystis carinii and Toxoplasma gondii infections in patients with AIDS. Rev Infect Dis. 1986 Nov-Dec;8(6):1001–1011. doi: 10.1093/clinids/8.6.1001. [DOI] [PubMed] [Google Scholar]
- Sabin A. B., Feldman H. A. Dyes as Microchemical Indicators of a New Immunity Phenomenon Affecting a Protozoon Parasite (Toxoplasma). Science. 1948 Dec 10;108(2815):660–663. doi: 10.1126/science.108.2815.660. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Conley F. K., Remington J. S. Differences in virulence and development of encephalitis during chronic infection vary with the strain of Toxoplasma gondii. J Infect Dis. 1989 Apr;159(4):790–794. doi: 10.1093/infdis/159.4.790. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Remington J. S. A method for obtaining large numbers of trophozoites of avirulent strains of Toxoplasma gondii using an antibody to interferon-gamma. J Parasitol. 1989 Feb;75(1):174–176. [PubMed] [Google Scholar]
- Werner H., Masihi K. N., Tischer I., Adusu E. The effect of chemo-immunotherapy with SDDS, pyrimethamine and anti-toxoplasma serum on toxoplasma gondii cysts in latent infected NMRI mice. Tropenmed Parasitol. 1977 Dec;28(4):528–532. [PubMed] [Google Scholar]
- Werner H., Matuschka F. R., Brandenburg I. Structural changes of Toxoplasma gondii bradyzoites and cysts following therapy with sulfamethoxypyrazine-pyrimethamine: studies by light and electron microscopy. Consequences for chemotherapy. Zentralbl Bakteriol Orig A. 1979 Oct;245(1-2):240–253. [PubMed] [Google Scholar]



