Abstract
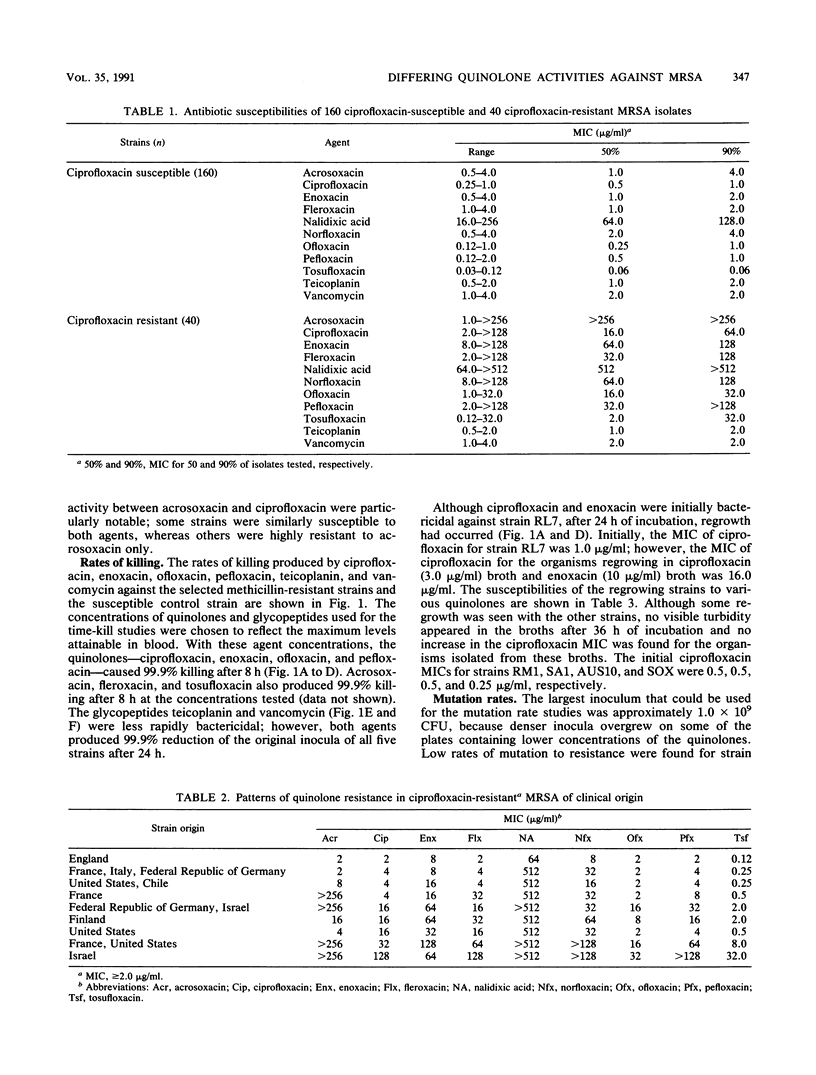
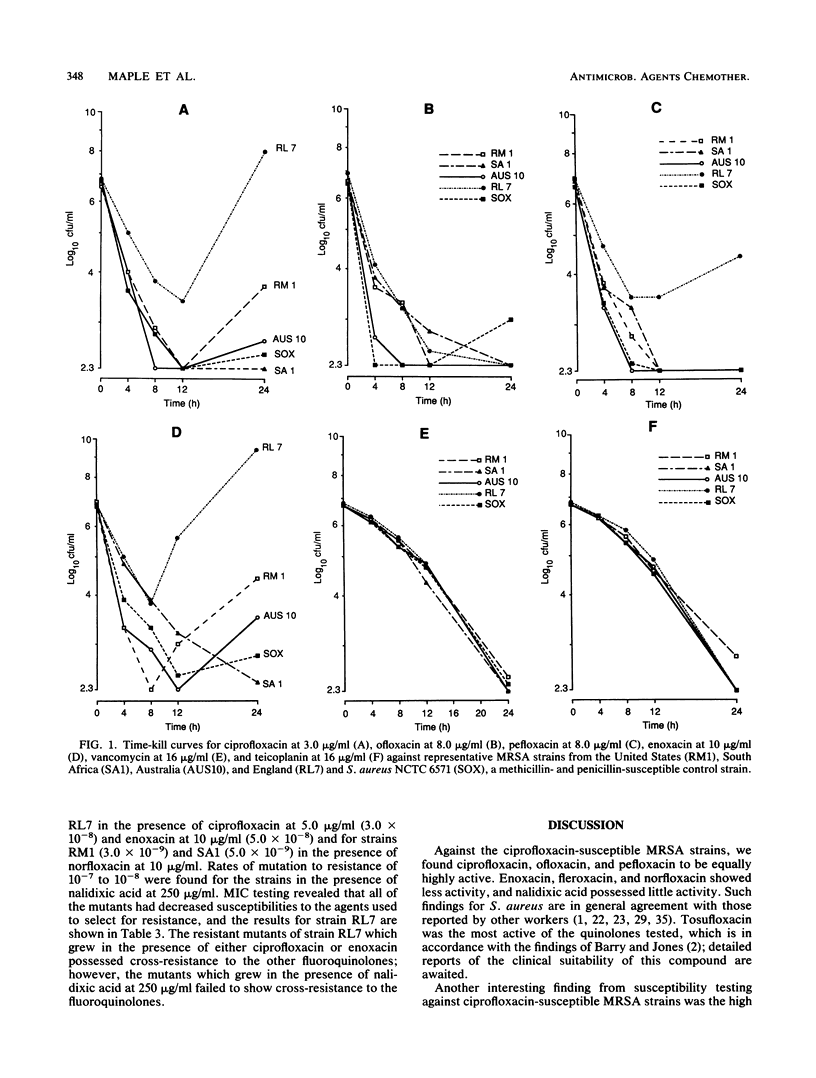
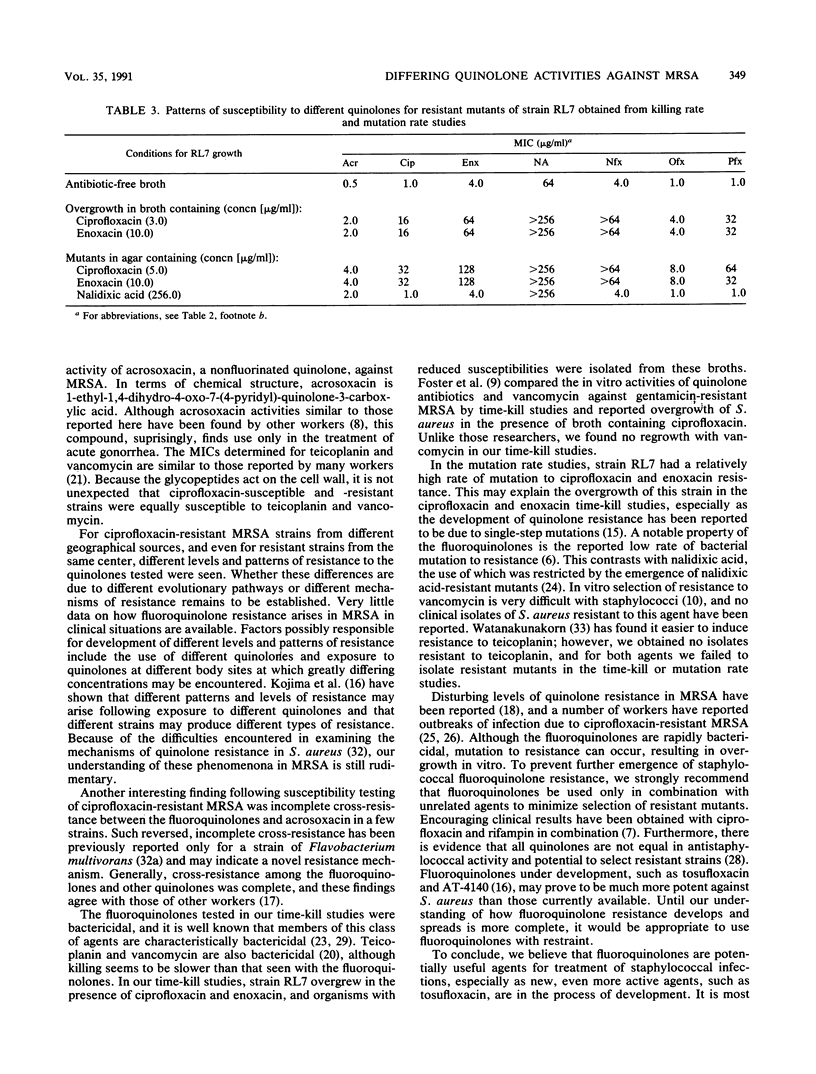
The in vitro activities of nine quinolones (seven fluoroquinolones, nalidixic acid, and acrosoxacin) against methicillin-resistant Staphylococcus aureus (MRSA) were compared with those of the glycopeptides teicoplanin and vancomycin. MICs against 160 strains of ciprofloxacin-susceptible (MIC, less than 2.0 micrograms/ml) MRSA and 40 strains of ciprofloxacin-resistant (MIC, greater than or equal to 2.0 micrograms/ml) MRSA were determined. The following MICs for 50% of the strains tested (in micrograms per milliliter) were obtained for ciprofloxacin-susceptible and -resistant strains, respectively: tosufloxacin, 0.06 and 2.0; ofloxacin, 0.25 and 16; ciprofloxacin, 0.5 and 16; pefloxacin, 0.5 and 32; acrosoxacin, 1.0 and greater than 256; enoxacin, 1.0 and 64; fleroxacin, 1.0 and 32; norfloxacin, 2.0 and 64; nalidixic acid, 64 and 512; teicoplanin, 1.0 and 1.0; vancomycin, 2.0 and 2.0. In mutation rate studies using a range of antibiotic concentrations to reflect those achievable in vivo, resistant mutants grew only on plates containing nalidixic acid (rate of mutation to resistance, 10(-7) to 10(-8) and on plates containing low concentrations of ciprofloxacin, enoxacin, and norfloxacin (rate of mutation to resistance, 10(-8) to 10(-9). In time-kill studies, 99.9% killing was found within 8 h for all of the quinolones tested (norfloxacin and nalidixic acid were not tested). Teicoplanin and vancomycin were less rapidly bactericidal. For the clinical isolates of ciprofloxacin-resistant MRSA, different levels and patterns of quinolone resistance were found. Generally, cross-resistance among the fluoroquinolones was complete; however, incomplete cross-resistance did occur with the nonfluorinated quinolone acrosoxacin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auckenthaler R., Michéa-Hamzehpour M., Pechère J. C. In-vitro activity of newer quinolones against aerobic bacteria. J Antimicrob Chemother. 1986 Apr;17 (Suppl B):29–39. doi: 10.1093/jac/17.suppl_b.29. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N. In-vitro activities of temafloxacin, tosufloxacin (A-61827) and five other fluoroquinolone agents. J Antimicrob Chemother. 1989 Apr;23(4):527–535. doi: 10.1093/jac/23.4.527. [DOI] [PubMed] [Google Scholar]
- Calain P., Waldvogel F. Clinical efficacy of teicoplanin. Eur J Clin Microbiol Infect Dis. 1990 Feb;9(2):127–129. doi: 10.1007/BF01963637. [DOI] [PubMed] [Google Scholar]
- Chu D. T., Fernandes P. B. Structure-activity relationships of the fluoroquinolones. Antimicrob Agents Chemother. 1989 Feb;33(2):131–135. doi: 10.1128/aac.33.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullmann W., Stieglitz M., Baars B., Opferkuch W. Comparative evaluation of recently developed quinolone compounds--with a note on the frequency of resistant mutants. Chemotherapy. 1985;31(1):19–28. doi: 10.1159/000238309. [DOI] [PubMed] [Google Scholar]
- Dworkin R. J., Lee B. L., Sande M. A., Chambers H. F. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989 Nov 4;2(8671):1071–1073. doi: 10.1016/s0140-6736(89)91083-0. [DOI] [PubMed] [Google Scholar]
- Felmingham D., O'Hare M. D., Robbins M. J., Wall R. A., Williams A. H., Cremer A. W., Ridgway G. L., Grüneberg R. N. Comparative in vitro studies with 4-quinolone antimicrobials. Drugs Exp Clin Res. 1985;11(5):317–329. [PubMed] [Google Scholar]
- Foster J. K., Lentino J. R., Strodtman R., DiVincenzo C. Comparison of in vitro activity of quinolone antibiotics and vancomycin against gentamicin- and methicillin-resistant Staphylococcus aureus by time-kill kinetic studies. Antimicrob Agents Chemother. 1986 Dec;30(6):823–827. doi: 10.1128/aac.30.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackbarth C. J., Chambers H. F. Methicillin-resistant staphylococci: detection methods and treatment of infections. Antimicrob Agents Chemother. 1989 Jul;33(7):995–999. doi: 10.1128/aac.33.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackbarth C. J., Chambers H. F. Methicillin-resistant staphylococci: genetics and mechanisms of resistance. Antimicrob Agents Chemother. 1989 Jul;33(7):991–994. doi: 10.1128/aac.33.7.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. The fluoroquinolones: pharmacology, clinical uses, and toxicities in humans. Antimicrob Agents Chemother. 1985 Nov;28(5):716–721. doi: 10.1128/aac.28.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen O. B. The demise of the 'old' methicillin-resistant Staphylococcus aureus. J Hosp Infect. 1986 Mar;7 (Suppl A):13–17. doi: 10.1016/0195-6701(86)90003-4. [DOI] [PubMed] [Google Scholar]
- Kayser F. H., Novak J. In vitro activity of ciprofloxacin against gram-positive bacteria. An overview. Am J Med. 1987 Apr 27;82(4A):33–39. [PubMed] [Google Scholar]
- Kojima T., Inoue M., Mitsuhashi S. In vitro activity of AT-4140 against quinolone- and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1990 Jun;34(6):1123–1127. doi: 10.1128/aac.34.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limb D. I., Dabbs D. J., Spencer R. C. In-vitro selection of bacteria resistant to the 4-quinolone agents. J Antimicrob Chemother. 1987 Jan;19(1):65–71. doi: 10.1093/jac/19.1.65. [DOI] [PubMed] [Google Scholar]
- Maple P. A., Hamilton-Miller J. M., Brumfitt W. Comparative in-vitro activity of vancomycin, teicoplanin, ramoplanin (formerly A16686), paldimycin, DuP 721 and DuP 105 against methicillin and gentamicin resistant Staphylococcus aureus. J Antimicrob Chemother. 1989 Apr;23(4):517–525. doi: 10.1093/jac/23.4.517. [DOI] [PubMed] [Google Scholar]
- Maple P. A., Hamilton-Miller J. M., Brumfitt W. World-wide antibiotic resistance in methicillin-resistant Staphylococcus aureus. Lancet. 1989 Mar 11;1(8637):537–540. doi: 10.1016/s0140-6736(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Maple P., Hamilton-Miller J., Brumfitt W. Ciprofloxacin resistance in methicillin- and gentamicin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1989 Jul;8(7):622–624. doi: 10.1007/BF01968141. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. In vitro activity of teichomycin compared with those of other antibiotics. Antimicrob Agents Chemother. 1983 Sep;24(3):425–428. doi: 10.1128/aac.24.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlod D. J., Saravolatz L. D., Somerville M. M. In-vitro susceptibility of staphylococci to fleroxacin in comparison with six other quinolones. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):35–41. doi: 10.1093/jac/22.supplement_d.35. [DOI] [PubMed] [Google Scholar]
- Ronald A. R., Turck M., Petersdorf R. G. A critical evaluation of nalidixic acid in urinary-tract infections. N Engl J Med. 1966 Nov 17;275(20):1081–1089. doi: 10.1056/NEJM196611172752001. [DOI] [PubMed] [Google Scholar]
- Schaefler S. Methicillin-resistant strains of Staphylococcus aureus resistant to quinolones. J Clin Microbiol. 1989 Feb;27(2):335–336. doi: 10.1128/jcm.27.2.335-336.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalit I., Berger S. A., Gorea A., Frimerman H. Widespread quinolone resistance among methicillin-resistant Staphylococcus aureus isolates in a general hospital. Antimicrob Agents Chemother. 1989 Apr;33(4):593–594. doi: 10.1128/aac.33.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M. In vitro comparison of A-56619, A-56620, amifloxacin, ciprofloxacin, enoxacin, norfloxacin, and ofloxacin against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Feb;29(2):325–326. doi: 10.1128/aac.29.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. L., Cabezudo I., Wenzel R. P. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):309–317. doi: 10.7326/0003-4819-97-3-309. [DOI] [PubMed] [Google Scholar]
- Townsend D. E., Ashdown N., Bolton S., Bradley J., Duckworth G., Moorhouse E. C., Grubb W. B. The international spread of methicillin-resistant Staphylococcus aureus. J Hosp Infect. 1987 Jan;9(1):60–71. doi: 10.1016/0195-6701(87)90097-1. [DOI] [PubMed] [Google Scholar]
- Ubukata K., Itoh-Yamashita N., Konno M. Cloning and expression of the norA gene for fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1989 Sep;33(9):1535–1539. doi: 10.1128/aac.33.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanakunakorn C. In-vitro selection of resistance of Staphylococcus aureus to teicoplanin and vancomycin. J Antimicrob Chemother. 1990 Jan;25(1):69–72. doi: 10.1093/jac/25.1.69. [DOI] [PubMed] [Google Scholar]
- Williams A. H., Grüneberg R. N. Teicoplanin. J Antimicrob Chemother. 1984 Nov;14(5):441–445. [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev. 1989 Oct;2(4):378–424. doi: 10.1128/cmr.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Caekenberghe D. L., Pattyn S. R. Reversed incomplete cross-resistance among the older and newer quinolone antibiotics. J Antimicrob Chemother. 1987 Mar;19(3):404–404. doi: 10.1093/jac/19.3.404. [DOI] [PubMed] [Google Scholar]


