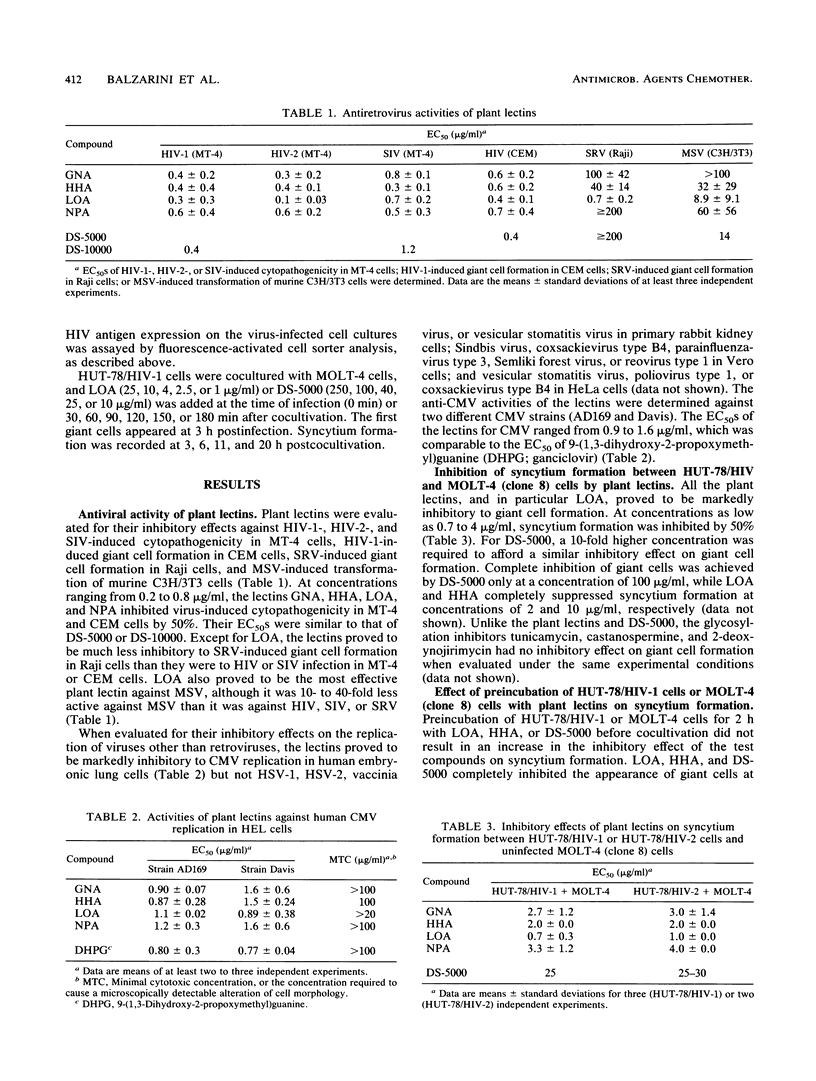
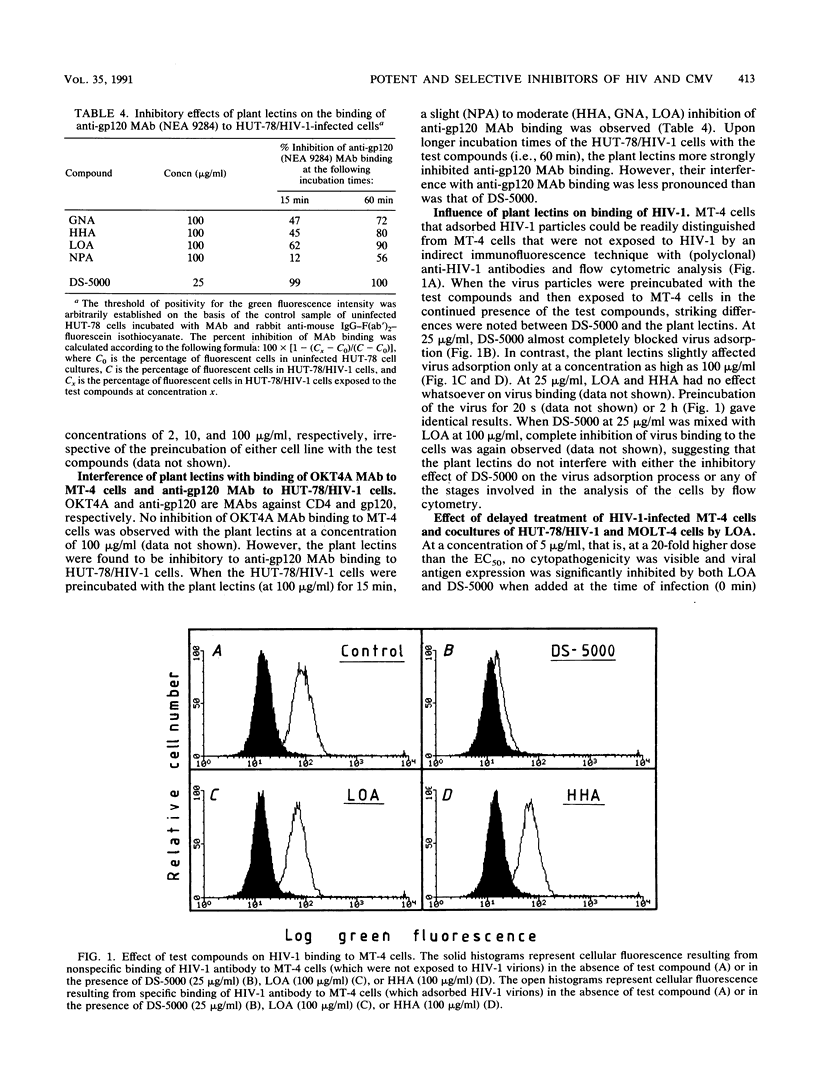
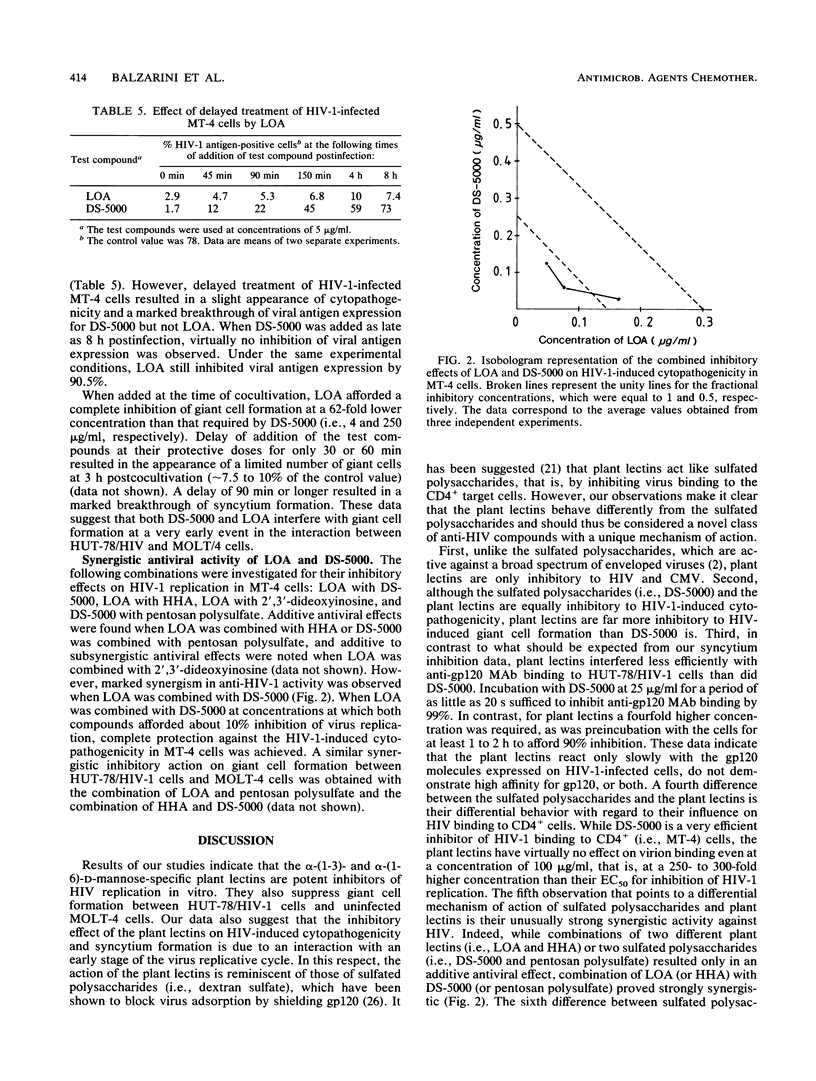
Abstract
The alpha-(1-3)-D-mannose- and alpha-(1-6)-D-mannose-specific agglutinins (lectins) from Galanthus nivalis, Hippeastrum hybrid, Narcissus pseudonarcissus, and Listera ovata inhibited infection of MT-4 cells by human immunodeficiency virus types 1 and 2 (HIV-1 and HIV-2) and simian immunodeficiency virus at concentrations comparable to the concentrations at which dextran sulfate (molecular weight, 5,000 [DS-5000]) inhibits these viruses (50% effective concentration, 0.2 to 0.6 microgram/ml). Unlike DS-5000, however, the plant lectins did not inhibit the replication of other enveloped viruses, except for human cytomegalovirus (50% effective concentration, 0.9 to 1.6 microgram/ml). The plant lectins suppressed syncytium formation between persistently HIV-1- or HIV-2-infected HUT-78 cells and uninfected MOLT-4 (clone 8) cells at concentrations that were 5- to 10-fold lower than that required for DS-5000. Unlike DS-5000, however, the plant lectins did not inhibit HIV-1 binding to CD4+ cells. Combination of the plant lectins with DS-5000 led to a potent synergistic inhibition of HIV-1-induced cytopathogenicity in MT-4 cells and syncytium formation between HIV-infected HUT-78 cells and MOLT-4 cells. Our data suggest that alpha-(1-3)-D- and alpha-(1-6)-D-mannose-specific plant lectins interfere with an event in the HIV replicative cycle that is subsequent to the attachment of the virions to the cells (i.e., the fusion process).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba M., Pauwels R., Balzarini J., Arnout J., Desmyter J., De Clercq E. Mechanism of inhibitory effect of dextran sulfate and heparin on replication of human immunodeficiency virus in vitro. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6132–6136. doi: 10.1073/pnas.85.16.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M., Snoeck R., Pauwels R., de Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother. 1988 Nov;32(11):1742–1745. doi: 10.1128/aac.32.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J., Mitsuya H., De Clercq E., Broder S. Aurintricarboxylic acid and Evans Blue represent two different classes of anionic compounds which selectively inhibit the cytopathogenicity of human T-cell lymphotropic virus type III/lymphadenopathy-associated virus. Biochem Biophys Res Commun. 1986 Apr 14;136(1):64–71. doi: 10.1016/0006-291x(86)90877-6. [DOI] [PubMed] [Google Scholar]
- Balzarini J., Mitsuya H., De Clercq E., Broder S. Comparative inhibitory effects of suramin and other selected compounds on the infectivity and replication of human T-cell lymphotropic virus (HTLV-III)/lymphadenopathy-associated virus (LAV). Int J Cancer. 1986 Mar 15;37(3):451–457. doi: 10.1002/ijc.2910370318. [DOI] [PubMed] [Google Scholar]
- Balzarini J., Naesens L., Herdewijn P., Rosenberg I., Holy A., Pauwels R., Baba M., Johns D. G., De Clercq E. Marked in vivo antiretrovirus activity of 9-(2-phosphonylmethoxyethyl)adenine, a selective anti-human immunodeficiency virus agent. Proc Natl Acad Sci U S A. 1989 Jan;86(1):332–336. doi: 10.1073/pnas.86.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel F., Guétard D., Brun-Vézinet F., Chamaret S., Rey M. A., Santos-Ferreira M. O., Laurent A. G., Dauguet C., Katlama C., Rouzioux C. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986 Jul 18;233(4761):343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Descamps J., Verhelst G., Walker R. T., Jones A. S., Torrence P. F., Shugar D. Comparative efficacy of antiherpes drugs against different strains of herpes simplex virus. J Infect Dis. 1980 May;141(5):563–574. doi: 10.1093/infdis/141.5.563. [DOI] [PubMed] [Google Scholar]
- Hammar L., Eriksson S., Morein B. Human immunodeficiency virus glycoproteins: lectin binding properties. AIDS Res Hum Retroviruses. 1989 Oct;5(5):495–506. doi: 10.1089/aid.1989.5.495. [DOI] [PubMed] [Google Scholar]
- Hansen J. E., Nielsen C. M., Nielsen C., Heegaard P., Mathiesen L. R., Nielsen J. O. Correlation between carbohydrate structures on the envelope glycoprotein gp120 of HIV-1 and HIV-2 and syncytium inhibition with lectins. AIDS. 1989 Oct;3(10):635–641. doi: 10.1097/00002030-198910000-00003. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Feinberg M. B., Reyes G. R., Rabin L., Banapour B., Chakrabarti S., Moss B., Wong-Staal F., Steimer K. S., Engleman E. G. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986 Oct 23;323(6090):725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- Lifson J., Coutré S., Huang E., Engleman E. Role of envelope glycoprotein carbohydrate in human immunodeficiency virus (HIV) infectivity and virus-induced cell fusion. J Exp Med. 1986 Dec 1;164(6):2101–2106. doi: 10.1084/jem.164.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews T. J., Weinhold K. J., Lyerly H. K., Langlois A. J., Wigzell H., Bolognesi D. P. Interaction between the human T-cell lymphotropic virus type IIIB envelope glycoprotein gp120 and the surface antigen CD4: role of carbohydrate in binding and cell fusion. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5424–5428. doi: 10.1073/pnas.84.15.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Looney D. J., Kuno S., Ueno R., Wong-Staal F., Broder S. Dextran sulfate suppression of viruses in the HIV family: inhibition of virion binding to CD4+ cells. Science. 1988 Apr 29;240(4852):646–649. doi: 10.1126/science.2452480. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Popovic M., Yarchoan R., Matsushita S., Gallo R. C., Broder S. Suramin protection of T cells in vitro against infectivity and cytopathic effect of HTLV-III. Science. 1984 Oct 12;226(4671):172–174. doi: 10.1126/science.6091268. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Robinson W. E., Jr, Montefiori D. C., Mitchell W. M. Evidence that mannosyl residues are involved in human immunodeficiency virus type 1 (HIV-1) pathogenesis. AIDS Res Hum Retroviruses. 1987 Fall;3(3):265–282. doi: 10.1089/aid.1987.3.265. [DOI] [PubMed] [Google Scholar]
- Schols D., Baba M., Pauwels R., De Clercq E. Flow cytometric method to demonstrate whether anti-HIV-1 agents inhibit virion binding to T4+ cells. J Acquir Immune Defic Syndr. 1989;2(1):10–15. [PubMed] [Google Scholar]
- Schols D., Baba M., Pauwels R., Desmyter J., De Clercq E. Specific interaction of aurintricarboxylic acid with the human immunodeficiency virus/CD4 cell receptor. Proc Natl Acad Sci U S A. 1989 May;86(9):3322–3326. doi: 10.1073/pnas.86.9.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schols D., Pauwels R., Desmyter J., De Clercq E. Dextran sulfate and other polyanionic anti-HIV compounds specifically interact with the viral gp120 glycoprotein expressed by T-cells persistently infected with HIV-1. Virology. 1990 Apr;175(2):556–561. doi: 10.1016/0042-6822(90)90440-3. [DOI] [PubMed] [Google Scholar]
- Shibuya N., Goldstein I. J., Van Damme E. J., Peumans W. J. Binding properties of a mannose-specific lectin from the snowdrop (Galanthus nivalis) bulb. J Biol Chem. 1988 Jan 15;263(2):728–734. [PubMed] [Google Scholar]
- Shibuya N., Goldstein I. J., Van Damme E. J., Peumans W. J. Binding properties of a mannose-specific lectin from the snowdrop (Galanthus nivalis) bulb. J Biol Chem. 1988 Jan 15;263(2):728–734. [PubMed] [Google Scholar]
- Van Damme E. J., Allen A. K., Peumans W. J. Leaves of the Orchid Twayblade (Listera ovata) Contain a Mannose-Specific Lectin. Plant Physiol. 1987 Oct;85(2):566–569. doi: 10.1104/pp.85.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]


