Abstract
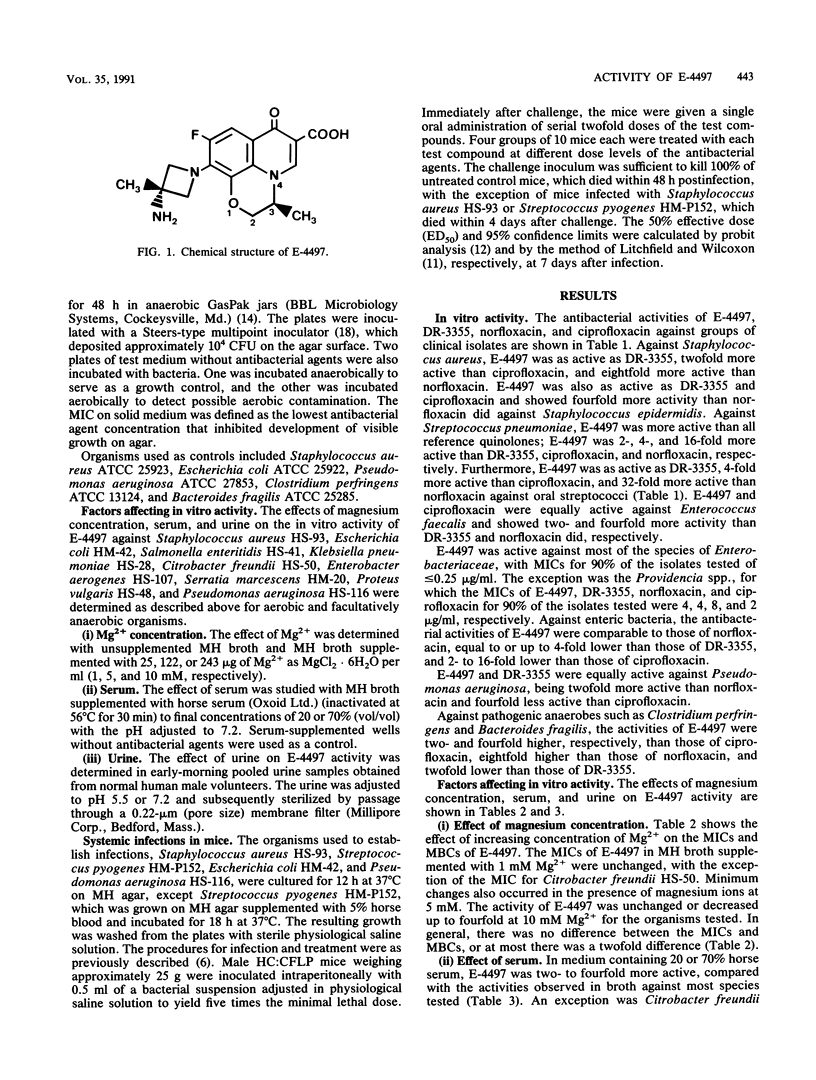
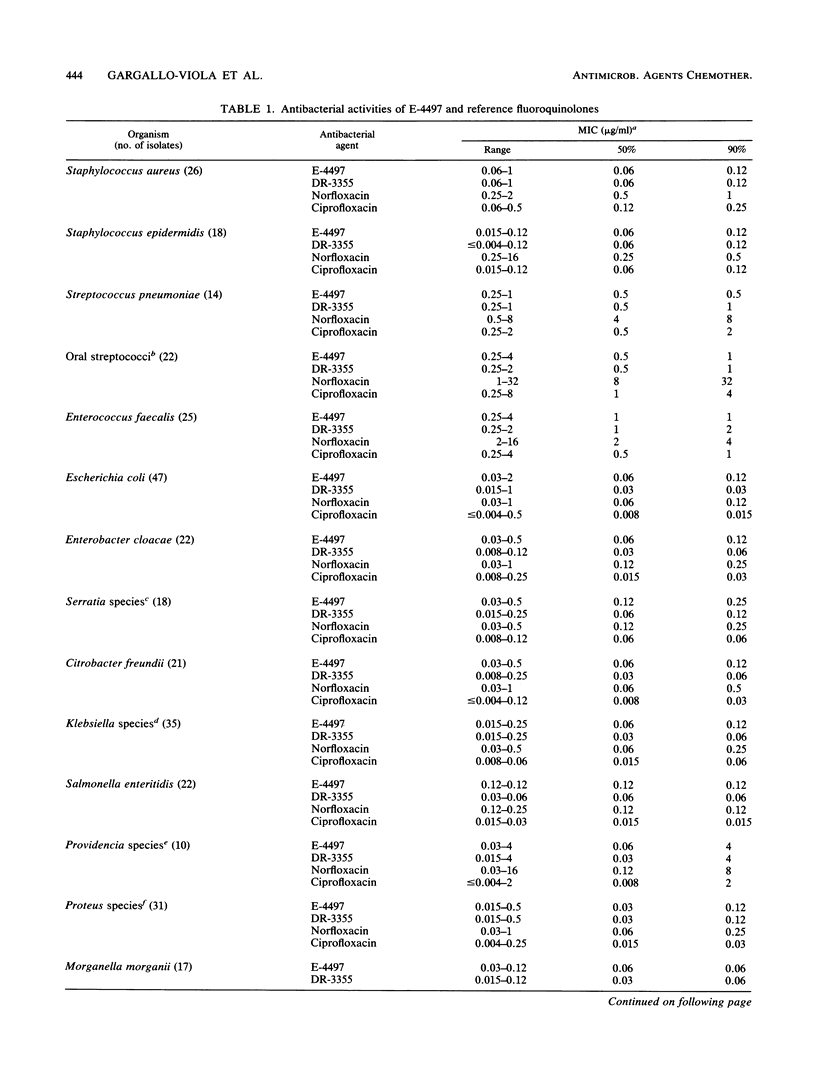
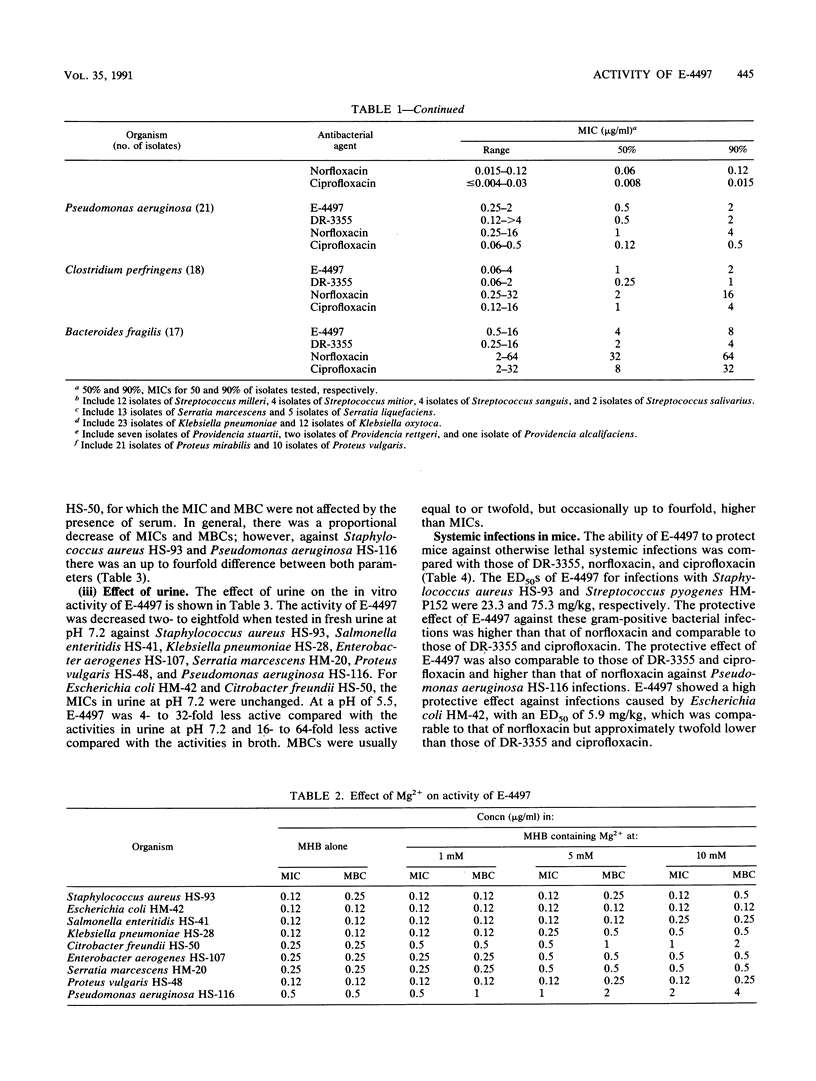
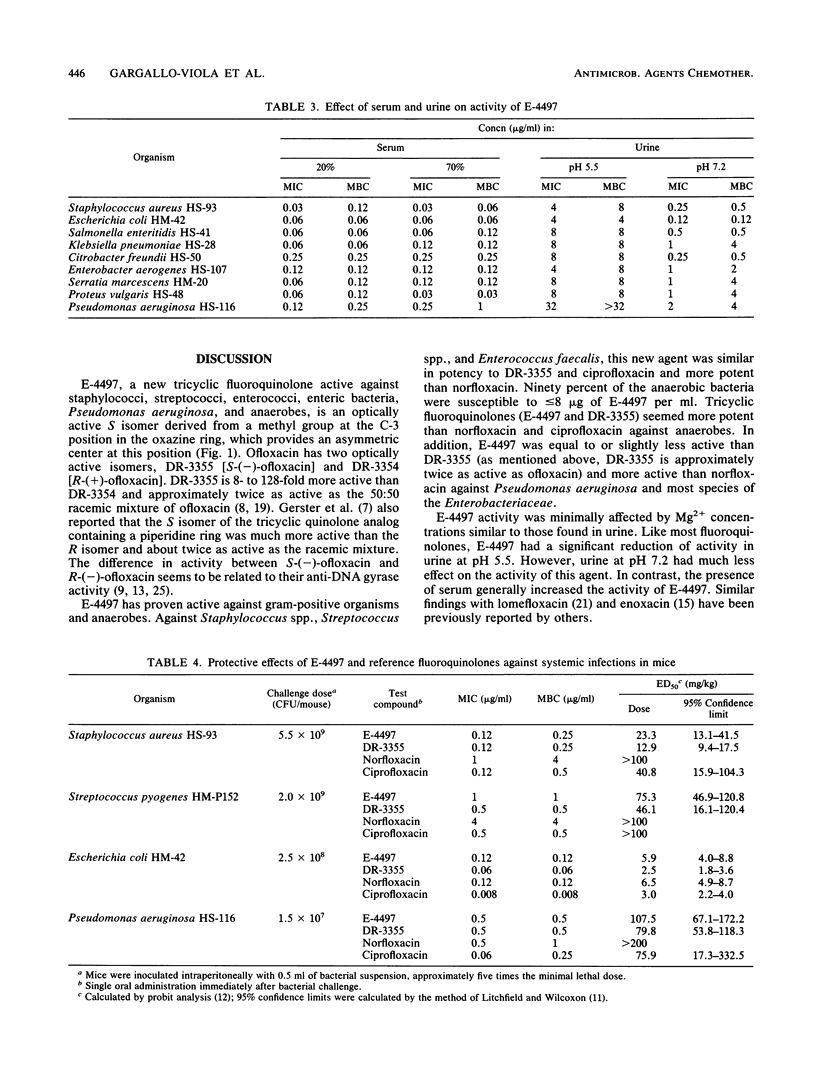
The in vitro and in vivo antibacterial activities of a new tricyclic fluoroquinolone, E-4497 [S(-)-9-fluoro-3-methyl-10-(3-amine-3-methyl-azetidin-1-yl)-7-oxo- 2,3-dihydro- 7H-pyrido-(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid], were evaluated in comparison with those of DR-3355 [S-(-)-ofloxacin], norfloxacin, and ciprofloxacin. E-4497 was more potent than norfloxacin and as potent as or more potent than DR-3355 and ciprofloxacin against Staphylococcus spp., Streptococcus spp., and Enterococcus faecalis. With the exception of Providencia spp., E-4497 inhibited 90% of the Enterobacteriaceae at less than or equal to 0.25 micrograms/ml. Against enteric bacteria, E-4497 was similar in potency to norfloxacin but less potent than DR-3355 and ciprofloxacin. For Pseudomonas aeruginosa, the MICs of E-4497, DR-3355, norfloxacin, and ciprofloxacin for 90% of strains were 2, 2, 4, and 0.5 micrograms/ml, respectively. Against Clostridium perfringens and Bacteroides fragilis, E-4497 (MICs for 90% of strains, 2 and 8 micrograms/ml, respectively) was two- to fourfold more active than norfloxacin and ciprofloxacin. E-4497 activity decreased moderately in the presence of 10 mM Mg2+. Urine at pH 5.5 caused a significant decrease in activity compared with urine at pH 7.2. However, the presence of serum either had no effect or increased the activity of E-4497. In general, E-4497 was bactericidal at the MIC. In systemic infections with Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa in mice, the protective effect of E-4497 was generally greater than that of norfloxacin and comparable to those of DR-3355 and ciprofloxacin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu D. T., Fernandes P. B. Structure-activity relationships of the fluoroquinolones. Antimicrob Agents Chemother. 1989 Feb;33(2):131–135. doi: 10.1128/aac.33.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll R., Esteve M., Moros M., Xicota M. A., Parés J. In vitro antibacterial activity of irloxacin (E-3432) on clinical isolates. Drugs Exp Clin Res. 1987;13(2):75–77. [PubMed] [Google Scholar]
- Espinoza A. M., Chin N. X., Novelli A., Neu H. C. Comparative in vitro activity of a new fluorinated 4-quinolone, T-3262 (A-60969). Antimicrob Agents Chemother. 1988 May;32(5):663–670. doi: 10.1128/aac.32.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes P. B., Chu D. T., Swanson R. N., Ramer N. R., Hanson C. W., Bower R. R., Stamm J. M., Hardy D. J. A-61827 (A-60969), a new fluoronaphthyridine with activity against both aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1988 Jan;32(1):27–32. doi: 10.1128/aac.32.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargallo-Viola D., Esteve M., Moros M., Coll R., Xicota M. A., de Andres C., Roser R., Guinea J. Comparative in vitro and in vivo activities of six new monofluoroquinolone and difluoroquinolone 3-carboxylic acids with a 7-azetidin ring substituent. Antimicrob Agents Chemother. 1990 Dec;34(12):2318–2326. doi: 10.1128/aac.34.12.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargallo D., Moros M., Coll R., Esteve M., Parés J., Xicota M. A., Guinea J. Activity of E-3846, a new fluoroquinolone, in vitro and in experimental cystitis and pyelonephritis in rats. Antimicrob Agents Chemother. 1988 May;32(5):636–641. doi: 10.1128/aac.32.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerster J. F., Rohlfing S. R., Pecore S. E., Winandy R. M., Stern R. M., Landmesser J. E., Olsen R. A., Gleason W. B. Synthesis, absolute configuration, and antibacterial activity of 6,7-dihydro-5,8-dimethyl-9-fluoro-1-oxo-1H,5H- benzo[ij]quinolizine-2-carboxylic acid. J Med Chem. 1987 May;30(5):839–843. doi: 10.1021/jm00388a016. [DOI] [PubMed] [Google Scholar]
- Hayakawa I., Atarashi S., Yokohama S., Imamura M., Sakano K., Furukawa M. Synthesis and antibacterial activities of optically active ofloxacin. Antimicrob Agents Chemother. 1986 Jan;29(1):163–164. doi: 10.1128/aac.29.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura M., Shibamura S., Hayakawa I., Osada Y. Inhibition of DNA gyrase by optically active ofloxacin. Antimicrob Agents Chemother. 1987 Feb;31(2):325–327. doi: 10.1128/aac.31.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz G. W., Seo S. M. WIN 57273, a new fluoroquinolone with enhanced in vitro activity versus gram-positive pathogens. Antimicrob Agents Chemother. 1990 Jul;34(7):1376–1380. doi: 10.1128/aac.34.7.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitscher L. A., Sharma P. N., Chu D. T., Shen L. L., Pernet A. G. Chiral DNA gyrase inhibitors. 2. Asymmetric synthesis and biological activity of the enantiomers of 9-fluoro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-2,3-dihydro-7H- pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid (ofloxacin). J Med Chem. 1987 Dec;30(12):2283–2286. doi: 10.1021/jm00395a017. [DOI] [PubMed] [Google Scholar]
- Reeves D. S., Bywater M. J., Holt H. A. The activity of enoxacin against clinical bacterial isolates in comparison with that of five other agents, and factors affecting that activity. J Antimicrob Chemother. 1984 Sep;14 (Suppl 100):7–17. doi: 10.1093/jac/14.suppl_c.7. [DOI] [PubMed] [Google Scholar]
- Smith R. P., Baltch A. L., Hammer M. C., Conroy J. V. In vitro activities of PD 117,596 and reference antibiotics against 448 clinical bacterial strains. Antimicrob Agents Chemother. 1988 Sep;32(9):1450–1455. doi: 10.1128/aac.32.9.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une T., Fujimoto T., Sato K., Osada Y. In vitro activity of DR-3355, an optically active ofloxacin. Antimicrob Agents Chemother. 1988 Sep;32(9):1336–1340. doi: 10.1128/aac.32.9.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. C., Wright A. J. Symposium on antimicrobial agents. The quinolones. Mayo Clin Proc. 1987 Nov;62(11):1007–1012. doi: 10.1016/s0025-6196(12)65073-3. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Ashby J. P., Matthews R. S. In vitro activity of lomefloxacin, a new quinolone antimicrobial agent, in comparison with those of other agents. Antimicrob Agents Chemother. 1988 May;32(5):617–622. doi: 10.1128/aac.32.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Ashby J. P., Andrews J. M. In vitro activity of PD 127,391, an enhanced-spectrum quinolone. Antimicrob Agents Chemother. 1988 Aug;32(8):1251–1256. doi: 10.1128/aac.32.8.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev. 1989 Oct;2(4):378–424. doi: 10.1128/cmr.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C., Ng E. Y., Souza K. S., McHugh G. L., Swartz M. N. Antagonism of wild-type and resistant Escherichia coli and its DNA gyrase by the tricyclic 4-quinolone analogs ofloxacin and S-25930 stereoisomers. Antimicrob Agents Chemother. 1987 Nov;31(11):1861–1863. doi: 10.1128/aac.31.11.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xicota M. A., Coll R., Esteve M., Moros M., Parés J. In vitro antibacterial activity of E-3604, a new 6-fluoroquinolone, on clinical isolates. Drugs Exp Clin Res. 1987;13(3):133–136. [PubMed] [Google Scholar]



