Abstract
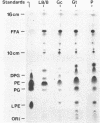
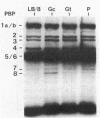
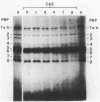
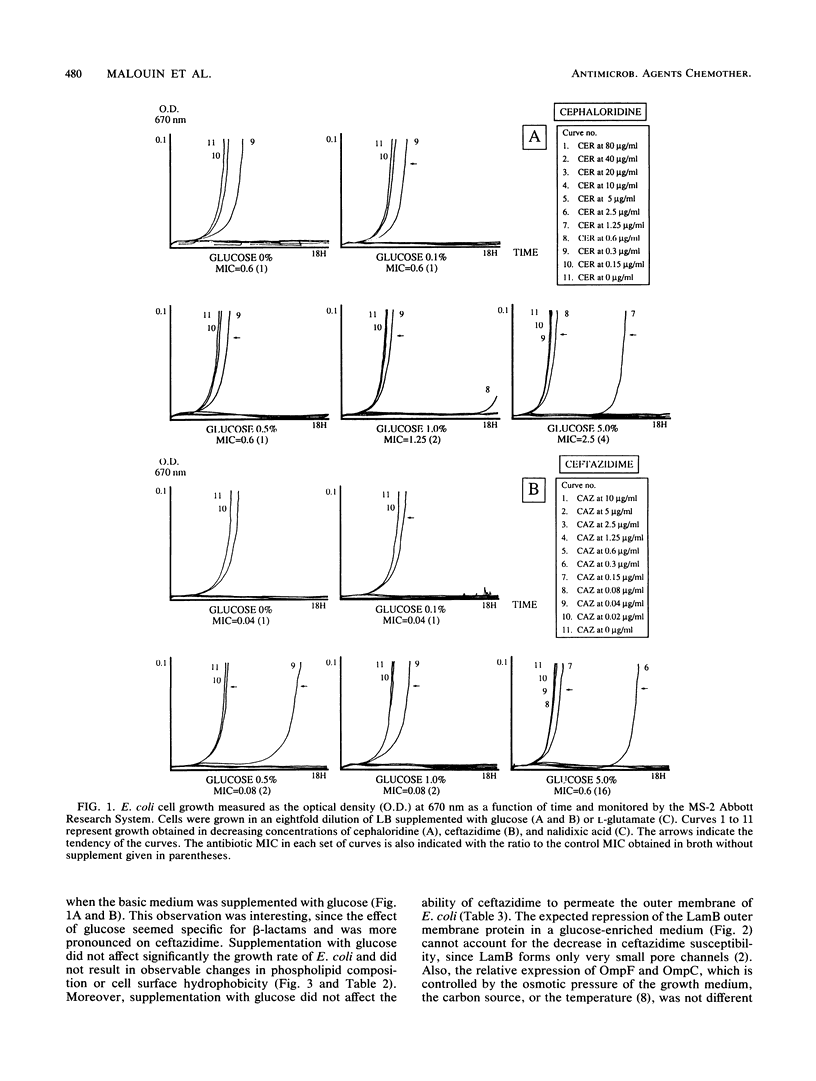
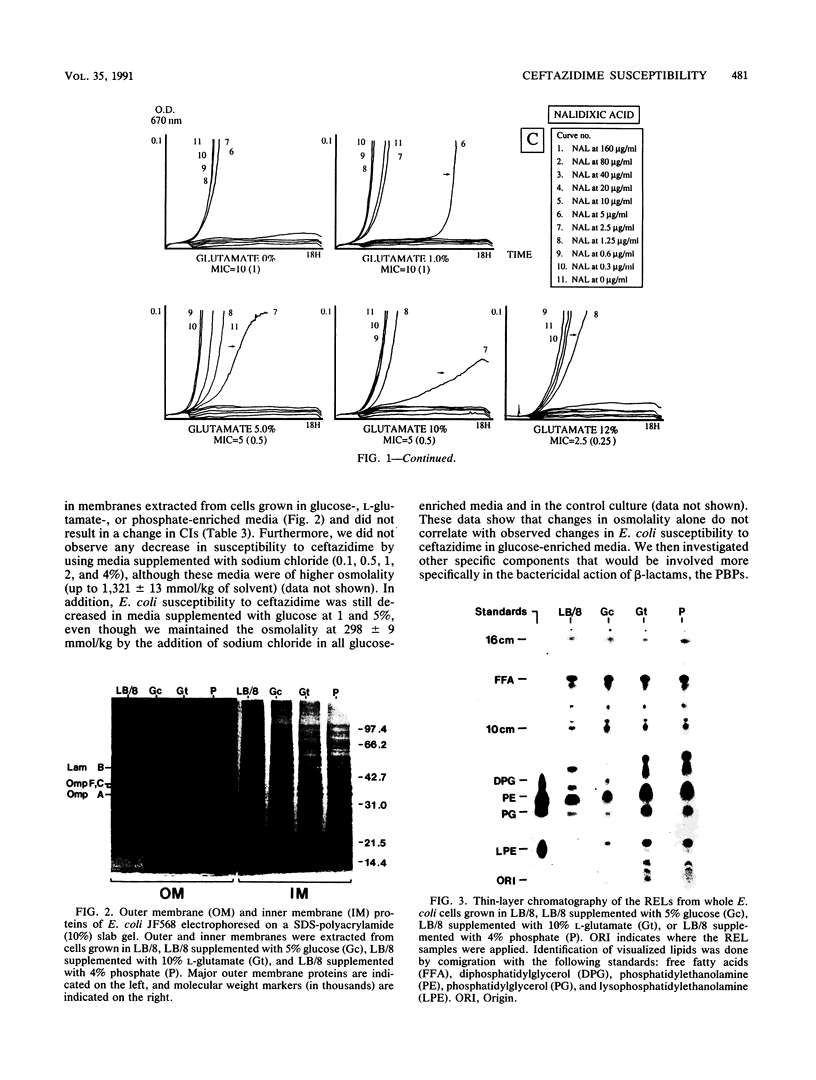
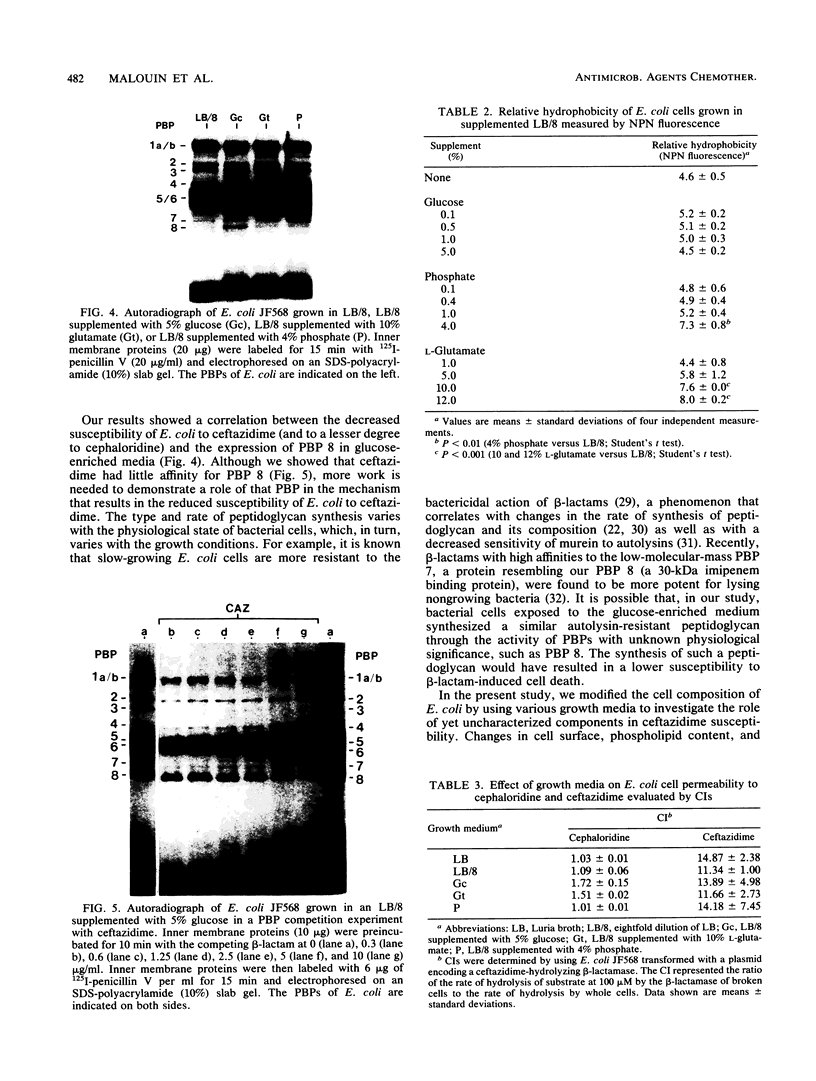
Cell composition and surface properties of Escherichia coli were modified by using various growth media to investigate the role of yet uncharacterized components in ceftazidime susceptibility. An eightfold dilution of Luria broth was used as the basic growth medium and was supplemented with up to 4% phosphate, 5% glucose, or 12% L-glutamate. Decreases in cephaloridine and ceftazidime susceptibility, of two- and eightfold, respectively, were observed only in the glucose-enriched medium. The outer membrane permeability to ceftazidime and cephaloridine was evaluated by crypticity indices. Indices were unchanged under all growth conditions. Fluorometry of whole cells with 1-N-phenylnaphthylamine showed that glucose does not affect the interaction of this hydrophobic probe with the membranes but showed that elevated concentrations of phosphate or glutamate cause a marked increase in cell hydrophobicity, which, in turn, correlates with an increase in the susceptibility of E. coli to nalidixic acid. Growth in phosphate- or glutamate-enriched media caused an augmentation in major phospholipid species and may explain the increased hydrophobicity and susceptibility of E. coli to nalidixic acid. These data showed that E. coli susceptibility to ceftazidime is not influenced by cell surface hydrophobicity and suggested that the contribution of a nonspecific lipophilic diffusion route for entry of ceftazidime into cells is not likely to occur or is distinct from that of more hydrophobic molecules such as nalidixic acid. Finally, the penicillin-binding proteins of the E. coli cells were also investigated. Penicillin-binding protein 8 was only markedly labeled with 125I-penicillin V in inner membranes extracted from cells grown with glucose. Results of this study suggest that the unexpected change in penicillin-binding protein 8 observed in the presence of glucose may be responsible for the increase in MICs of cephaloridine and ceftazidime.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Benz R., Schmid A., Nakae T., Vos-Scheperkeuter G. H. Pore formation by LamB of Escherichia coli in lipid bilayer membranes. J Bacteriol. 1986 Mar;165(3):978–986. doi: 10.1128/jb.165.3.978-986.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai T. J., Foulds J. Escherichia coli K-12 tolF mutants: alterations in protein composition of the outer membrane. J Bacteriol. 1977 May;130(2):781–786. doi: 10.1128/jb.130.2.781-786.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S., Bayer A. S., Schollaardt T., Wong S. A., Bryan L. E. Characterization of mechanisms of quinolone resistance in Pseudomonas aeruginosa strains isolated in vitro and in vivo during experimental endocarditis. Antimicrob Agents Chemother. 1989 May;33(5):624–634. doi: 10.1128/aac.33.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S., Malouin F., Rabin H. R., Schollaardt T., Parr T. R., Jr, Bryan L. E. Persistence of Pseudomonas aeruginosa during ciprofloxacin therapy of a cystic fibrosis patient: transient resistance to quinolones and protein F-deficiency. J Antimicrob Chemother. 1990 Jun;25(6):995–1010. doi: 10.1093/jac/25.6.995. [DOI] [PubMed] [Google Scholar]
- Conrad R. S., Wulf R. G., Clay D. L. Effects of carbon sources on antibiotic resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1979 Jan;15(1):59–66. doi: 10.1128/aac.15.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz G. R., Mandell G. L. Drug therapy. Beta-lactam antibiotics (2). N Engl J Med. 1988 Feb 25;318(8):490–500. doi: 10.1056/NEJM198802253180806. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malouin F., Campbell G. D., Halpenny M., Becker G. W., Parr T. R., Jr Outer membrane and porin characteristics of Serratia marcescens grown in vitro and in rat intraperitoneal diffusion chambers. Infect Immun. 1990 May;58(5):1247–1253. doi: 10.1128/iai.58.5.1247-1253.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouin F., Parr T. R., Jr, Bryan L. E. Identification of a group of Haemophilus influenzae penicillin-binding proteins that may have complementary physiological roles. Antimicrob Agents Chemother. 1990 Feb;34(2):363–365. doi: 10.1128/aac.34.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. The new beta-lactamase-stable cephalosporins. Ann Intern Med. 1982 Sep;97(3):408–419. doi: 10.7326/0003-4819-97-3-408. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Normark S. Sensitivity of Escherichia coli to various beta-lactams is determined by the interplay of outer membrane permeability and degradation by periplasmic beta-lactamases: a quantitative predictive treatment. Mol Microbiol. 1987 Jul;1(1):29–36. doi: 10.1111/j.1365-2958.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Pisabarro A. G., de Pedro M. A., Vázquez D. Structural modifications in the peptidoglycan of Escherichia coli associated with changes in the state of growth of the culture. J Bacteriol. 1985 Jan;161(1):238–242. doi: 10.1128/jb.161.1.238-242.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston D. A., Wu C. Y., Blaszczak L. C., Seitz D. E., Halligan N. G. Biological characterization of a new radioactive labeling reagent for bacterial penicillin-binding proteins. Antimicrob Agents Chemother. 1990 May;34(5):718–721. doi: 10.1128/aac.34.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J. P., Miyashiro D., Sahm D., Flamm R., Bush K. Novel plasmid-mediated beta-lactamase (TEM-10) conferring selective resistance to ceftazidime and aztreonam in clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother. 1989 Sep;33(9):1451–1456. doi: 10.1128/aac.33.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984 Oct;20(4):608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Cozens R. Changes in peptidoglycan composition and penicillin-binding proteins in slowly growing Escherichia coli. J Bacteriol. 1987 Nov;169(11):5308–5310. doi: 10.1128/jb.169.11.5308-5310.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Markiewicz Z., Tomasz A. Autolysis-resistant peptidoglycan of anomalous composition in amino-acid-starved Escherichia coli. J Bacteriol. 1988 Mar;170(3):1373–1376. doi: 10.1128/jb.170.3.1373-1376.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E. Phenotypic tolerance: the search for beta-lactam antibiotics that kill nongrowing bacteria. Rev Infect Dis. 1986 Jul-Aug;8 (Suppl 3):S279–S291. doi: 10.1093/clinids/8.supplement_3.s279. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Schwartz J. Penicillin-binding protein 7 and its relationship to lysis of nongrowing Escherichia coli. J Bacteriol. 1987 Nov;169(11):4912–4915. doi: 10.1128/jb.169.11.4912-4915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu H., Nikaido H. Role of beta-lactam hydrolysis in the mechanism of resistance of a beta-lactamase-constitutive Enterobacter cloacae strain to expanded-spectrum beta-lactams. Antimicrob Agents Chemother. 1985 Mar;27(3):393–398. doi: 10.1128/aac.27.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Diffusion of beta-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob Agents Chemother. 1985 Jan;27(1):84–92. doi: 10.1128/aac.27.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]