Abstract
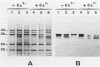
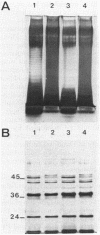
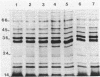
Mechanisms of resistance to pefloxacin were investigated in four isogenic Pseudomonas aeruginosa strains: S (parent isolate; MIC, 2 micrograms/ml), PT1 and PT2 (posttherapy isolates obtained in animals; MICs, 32 and 128 micrograms/ml, respectively), and PT2-r (posttherapy isolate obtained after six in vitro subpassages of PT2; MIC, 32 micrograms/ml). [2-3H]adenine incorporation (indirect evidence of DNA gyrase activity) in EDTA-permeabilized cells was less affected by pefloxacin in PT2 and PT2-r (50% inhibitory concentration, 0.27 and 0.26 microgram/ml, respectively) than it was in S and PT1 (50% inhibitory concentration, 0.04 and 0.05 microgram/ml, respectively). Reduced [14C]pefloxacin labeling of intact cells in strains PT1 and PT2 correlated with more susceptibility to EDTA and the presence of more calcium (P less than 0.05) and phosphorus in the outer membrane fractions. Outer membrane protein analysis showed reduced expression of protein D2 (47 kDa) in strains PT1 and PT2. Other proteins were apparently similar in all strains. The addition of calcium chloride (2 mM) to the sodium dodecyl sulfate-solubilized samples of outer membrane proteins, before heating and Western blotting, probed with monoclonal antibody anti-OmpF showed electrophoretic mobility changes of OmpF in strains PT1 and PT2 which were not seen in strain S. Calcium-induced changes were reversed with ethyleneglycoltetraacetate. Decreased [14C]pefloxacin labeling was further correlated with an altered lipopolysaccharide pattern and increased 3-deoxy-D-mannooctulosonic acid concentration (P less than 0.01). These findings suggested that resistance to pefloxacin is associated with altered DNA gyrase in strain PT2-r, with altered permeability in PT1, and with both mechanisms in PT2. The decreased expression of protein D2 and the higher calcium and lipopolysaccharide contents of the outer membrane could be responsible for the permeability deficiency in P. aeruginosa.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedard J., Chamberland S., Wong S., Schollaardt T., Bryan L. E. Contribution of permeability and sensitivity to inhibition of DNA synthesis in determining susceptibilities of Escherichia coli, Pseudomonas aeruginosa, and Alcaligenes faecalis to ciprofloxacin. Antimicrob Agents Chemother. 1989 Sep;33(9):1457–1464. doi: 10.1128/aac.33.9.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido F., Veuthey C., Blaser J., Bauernfeind A., Pechère J. C. Novel resistance to imipenem associated with an altered PBP-4 in a Pseudomonas aeruginosa clinical isolate. J Antimicrob Chemother. 1990 Jan;25(1):57–68. doi: 10.1093/jac/25.1.57. [DOI] [PubMed] [Google Scholar]
- Benbrook D. M., Miller R. V. Effects of norfloxacin on DNA metabolism in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1986 Jan;29(1):1–6. doi: 10.1128/aac.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser J., Dudley M. N., Gilbert D., Zinner S. H. Influence of medium and method on the in vitro susceptibility of Pseudomonas aeruginosa and other bacteria to ciprofloxacin and enoxacin. Antimicrob Agents Chemother. 1986 May;29(5):927–929. doi: 10.1128/aac.29.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser J., Lüthy R. Comparative study on antagonistic effects of low pH and cation supplementation on in-vitro activity of quinolones and aminoglycosides against Pseudomonas aeruginosa. J Antimicrob Chemother. 1988 Jul;22(1):15–22. doi: 10.1093/jac/22.1.15. [DOI] [PubMed] [Google Scholar]
- Bourguignon G. J., Levitt M., Sternglanz R. Studies on the mechanism of action of nalidixic acid. Antimicrob Agents Chemother. 1973 Oct;4(4):479–486. doi: 10.1128/aac.4.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celesk R. A., Robillard N. J. Factors influencing the accumulation of ciprofloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1989 Nov;33(11):1921–1926. doi: 10.1128/aac.33.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S., Bayer A. S., Schollaardt T., Wong S. A., Bryan L. E. Characterization of mechanisms of quinolone resistance in Pseudomonas aeruginosa strains isolated in vitro and in vivo during experimental endocarditis. Antimicrob Agents Chemother. 1989 May;33(5):624–634. doi: 10.1128/aac.33.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. S., Georgopapadakou N. H. Routes of quinolone permeation in Escherichia coli. Antimicrob Agents Chemother. 1988 Apr;32(4):438–442. doi: 10.1128/aac.32.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. X., Clynes N., Neu H. C. Resistance to ciprofloxacin appearing during therapy. Am J Med. 1989 Nov 30;87(5A):28S–31S. doi: 10.1016/0002-9343(89)90015-6. [DOI] [PubMed] [Google Scholar]
- Coughlin R. T., Tonsager S., McGroarty E. J. Quantitation of metal cations bound to membranes and extracted lipopolysaccharide of Escherichia coli. Biochemistry. 1983 Apr 12;22(8):2002–2007. doi: 10.1021/bi00277a041. [DOI] [PubMed] [Google Scholar]
- Daikos G. L., Lolans V. T., Jackson G. G. Alterations in outer membrane proteins of Pseudomonas aeruginosa associated with selective resistance to quinolones. Antimicrob Agents Chemother. 1988 May;32(5):785–787. doi: 10.1128/aac.32.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh N., Wakebe H., Yoshihara E., Nakae T., Nishino T. Role of protein F in maintaining structural integrity of the Pseudomonas aeruginosa outer membrane. J Bacteriol. 1989 Feb;171(2):983–990. doi: 10.1128/jb.171.2.983-990.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Bell A. Antibiotic uptake into gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 1988 Dec;7(6):713–720. doi: 10.1007/BF01975036. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Wong P. G. Compounds which increase the permeability of the Pseudomonas aeruginosa outer membrane. Antimicrob Agents Chemother. 1984 Jul;26(1):48–52. doi: 10.1128/aac.26.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987 Apr;31(4):582–586. doi: 10.1128/aac.31.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y., Sato K., Fujii T., Hirai K., Inoue M., Iyobe S., Mitsuhashi S. Some properties of subunits of DNA gyrase from Pseudomonas aeruginosa PAO1 and its nalidixic acid-resistant mutant. J Bacteriol. 1987 May;169(5):2322–2325. doi: 10.1128/jb.169.5.2322-2325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Kuzio J., Angus B. L., Hancock R. E. Chemical and chromatographic analysis of lipopolysaccharide from an antibiotic-supersusceptible mutant of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1982 Feb;21(2):310–319. doi: 10.1128/aac.21.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legakis N. J., Tzouvelekis L. S., Makris A., Kotsifaki H. Outer membrane alterations in multiresistant mutants of Pseudomonas aeruginosa selected by ciprofloxacin. Antimicrob Agents Chemother. 1989 Jan;33(1):124–127. doi: 10.1128/aac.33.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucain C., Regamey P., Bellido F., Pechére J. C. Resistance emerging after pefloxacin therapy of experimental Enterobacter cloacae peritonitis. Antimicrob Agents Chemother. 1989 Jun;33(6):937–943. doi: 10.1128/aac.33.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M. J., Drusano G. L., Mobley H. L. Emergence of resistance to imipenem in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987 Dec;31(12):1892–1896. doi: 10.1128/aac.31.12.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchou B., Bellido F., Charnas R., Lucain C., Pechère J. C. Contribution of beta-lactamase hydrolysis and outer membrane permeability to ceftriaxone resistance in Enterobacter cloacae. Antimicrob Agents Chemother. 1987 Oct;31(10):1589–1595. doi: 10.1128/aac.31.10.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michéa-Hamzehpour M., Auckenthaler R., Regamey P., Pechère J. C. Resistance occurring after fluoroquinolone therapy of experimental Pseudomonas aeruginosa peritonitis. Antimicrob Agents Chemother. 1987 Nov;31(11):1803–1808. doi: 10.1128/aac.31.11.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michéa-Hamzehpour M., Pechère J. C., Marchou B., Auckenthaler R. Combination therapy: a way to limit emergence of resistance? Am J Med. 1986 Jun 30;80(6B):138–142. doi: 10.1016/0002-9343(86)90491-2. [DOI] [PubMed] [Google Scholar]
- Miller R. V., Ku C. M. Characterization of Pseudomonas aeruginosa mutants deficient in the establishment of lysogeny. J Bacteriol. 1978 Jun;134(3):875–883. doi: 10.1128/jb.134.3.875-883.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J Biochem. 1978 Jul;84(1):179–191. doi: 10.1093/oxfordjournals.jbchem.a132106. [DOI] [PubMed] [Google Scholar]
- Mutharia L. M., Hancock R. E. Characterization of two surface-localized antigenic sites on porin protein F of Pseudomonas aeruginosa. Can J Microbiol. 1985 Apr;31(4):381–386. doi: 10.1139/m85-073. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Peterson A. A., Haug A., McGroarty E. J. Physical properties of short- and long-O-antigen-containing fractions of lipopolysaccharide from Escherichia coli 0111:B4. J Bacteriol. 1986 Jan;165(1):116–122. doi: 10.1128/jb.165.1.116-122.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J. P., Dudek E. J., DiVincenzo C. A., Lucks D. A., Lerner S. A. Emergence of resistance to imipenem during therapy for Pseudomonas aeruginosa infections. J Infect Dis. 1986 Aug;154(2):289–294. doi: 10.1093/infdis/154.2.289. [DOI] [PubMed] [Google Scholar]
- Quinn J. P., Studemeister A. E., DiVincenzo C. A., Lerner S. A. Resistance to imipenem in Pseudomonas aeruginosa: clinical experience and biochemical mechanisms. Rev Infect Dis. 1988 Jul-Aug;10(4):892–898. doi: 10.1093/clinids/10.4.892. [DOI] [PubMed] [Google Scholar]
- Rella M., Haas D. Resistance of Pseudomonas aeruginosa PAO to nalidixic acid and low levels of beta-lactam antibiotics: mapping of chromosomal genes. Antimicrob Agents Chemother. 1982 Aug;22(2):242–249. doi: 10.1128/aac.22.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M., Bryan L. E., Hancock R. E., McGroarty E. J. Heterogeneity of lipopolysaccharides from Pseudomonas aeruginosa: analysis of lipopolysaccharide chain length. J Bacteriol. 1988 Feb;170(2):512–521. doi: 10.1128/jb.170.2.512-521.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard N. J., Scarpa A. L. Genetic and physiological characterization of ciprofloxacin resistance in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1988 Apr;32(4):535–539. doi: 10.1128/aac.32.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Osborn M. J. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry. 1979 Oct 2;18(20):4425–4430. doi: 10.1021/bi00587a024. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Stanton M. G. Colorimetric determination of inorganic phosphate in the presence of biological material and adenosine triphosphate. Anal Biochem. 1968 Jan;22(1):27–34. doi: 10.1016/0003-2697(68)90255-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trias J., Nikaido H. Outer membrane protein D2 catalyzes facilitated diffusion of carbapenems and penems through the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Jan;34(1):52–57. doi: 10.1128/aac.34.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev. 1989 Oct;2(4):378–424. doi: 10.1128/cmr.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff W. A., Hancock R. E. Construction and characterization of Pseudomonas aeruginosa protein F-deficient mutants after in vitro and in vivo insertion mutagenesis of the cloned gene. J Bacteriol. 1988 Jun;170(6):2592–2598. doi: 10.1128/jb.170.6.2592-2598.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff W. A., Hancock R. E. Pseudomonas aeruginosa outer membrane protein F: structural role and relationship to the Escherichia coli OmpA protein. J Bacteriol. 1989 Jun;171(6):3304–3309. doi: 10.1128/jb.171.6.3304-3309.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Auwera P., de Moor G., Lacroix G., Mambour A., Rossion N., Schuyteneer F. In vitro activity of enoxacin compared with norfloxacin and amikacin. Eur J Clin Microbiol. 1985 Feb;4(1):55–58. doi: 10.1007/BF02148662. [DOI] [PubMed] [Google Scholar]