Abstract
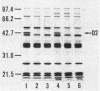
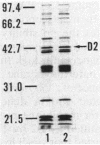
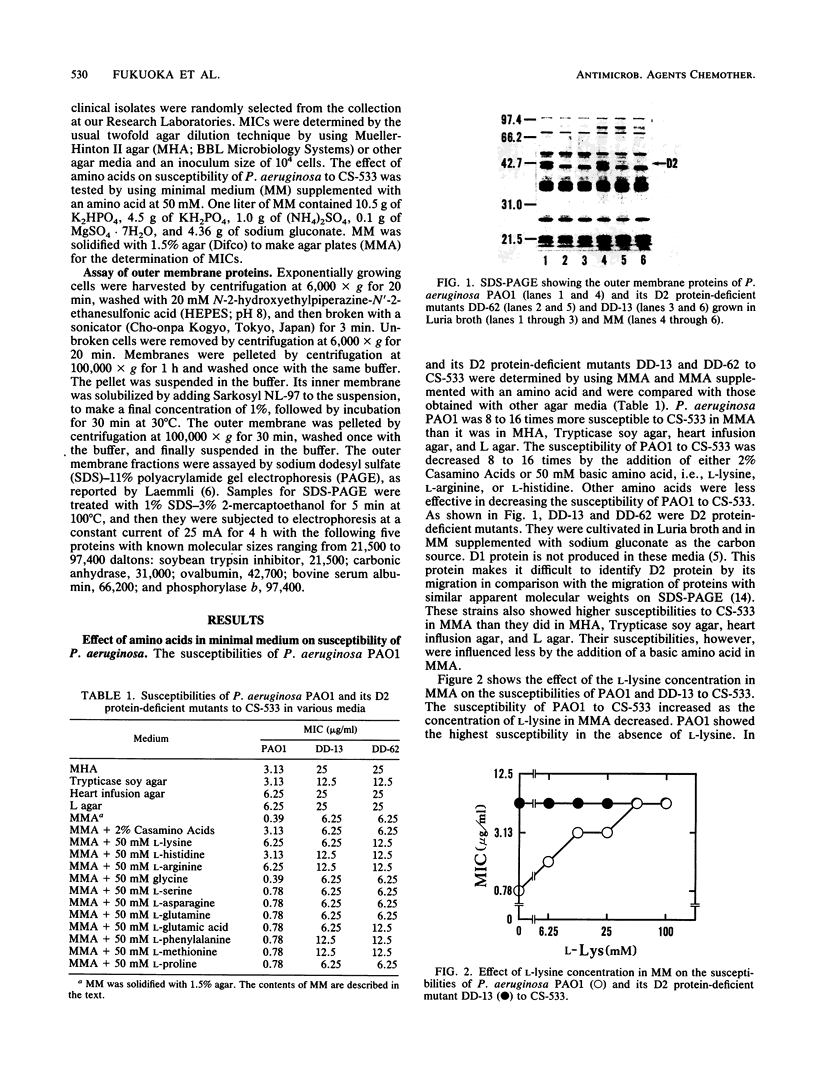
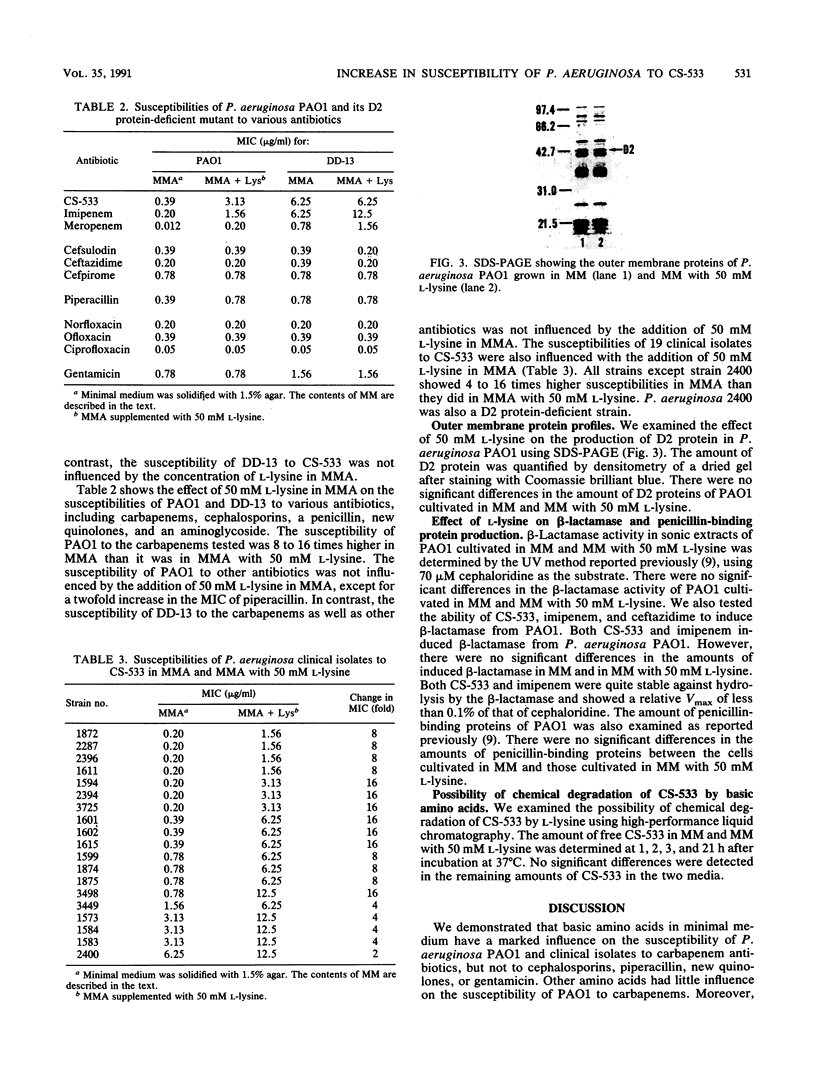
The in vitro susceptibility of Pseudomonas aeruginosa PAO1 to carbapenem antibiotics, such as CS-533, was influenced by various concentrations of basic amino acids, i.e., L-lysine, L-histidine, and L-arginine, in agar media. P. aeruginosa PAO1 showed higher susceptibility to carbapenems in minimal medium than it did in rich media such as Mueller-Hinton II agar. The susceptibility was decreased by the addition of a basic amino acid to the minimal medium, whereas it was influenced less by other amino acids. The susceptibility of PAO1 to cephalosporins, piperacillin, quinolones, and gentamicin was not influenced by the addition of a basic amino acid to the minimal medium. A significant change in susceptibility to carbapenems by the addition of a basic amino acid was not observed with D2 protein-deficient mutants of PAO1. Clinical isolates of P. aeruginosa also showed an increase in susceptibility in minimal medium. L-Lysine in minimal medium did not have any influence on the production of D2 protein, beta-lactamases, or penicillin-binding proteins of PAO1 or on the chemical degradation of CS-533. These results strongly indicate that the increase in susceptibility of P. aeruginosa to carbapenems relates to less competition with basic amino acids for permeation through the D2 protein channel of P. aeruginosa.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Büscher K. H., Cullmann W., Dick W., Opferkuch W. Imipenem resistance in Pseudomonas aeruginosa resulting from diminished expression of an outer membrane protein. Antimicrob Agents Chemother. 1987 May;31(5):703–708. doi: 10.1128/aac.31.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büscher K. H., Cullmann W., Dick W., Wendt S., Opferkuch W. Imipenem resistance in Pseudomonas aeruginosa is due to diminished expression of outer membrane proteins. J Infect Dis. 1987 Oct;156(4):681–684. doi: 10.1093/infdis/156.4.681. [DOI] [PubMed] [Google Scholar]
- Gitlitz P. H., Sunderman F. W., Jr, Hohnadel D. C. Ion-exchange chromatography of amino acids in sweat collected from healthy subjects during sauna bathing. Clin Chem. 1974 Oct;20(10):1305–1312. [PubMed] [Google Scholar]
- Gotoh N., Nishino T. Decreases of the susceptibility to low molecular weight beta-lactam antibiotics in imipenem-resistant Pseudomonas aeruginosa mutants: role of outer membrane protein D2 in their diffusion. J Antimicrob Chemother. 1990 Feb;25(2):191–198. doi: 10.1093/jac/25.2.191. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Chin N. X., Saha G., Labthavikul P. In vitro activity against aerobic and anaerobic gram-positive and gram-negative bacteria and beta-lactamase stability of RS-533, a novel carbapenem. Antimicrob Agents Chemother. 1986 Dec;30(6):828–834. doi: 10.1128/aac.30.6.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. Comparative in vitro activity of N-formimidoyl thienamycin against gram-positive and gram-negative aerobic and anaerobic species and its beta-lactamase stability. Antimicrob Agents Chemother. 1982 Jan;21(1):180–187. doi: 10.1128/aac.21.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya S., Yamazaki M., Sugawara S. Effect of 7 alpha substitution of cephems on their beta-lactamase stability and affinity for penicillin-binding proteins in Morganella morganii. Antimicrob Agents Chemother. 1983 Apr;23(4):522–525. doi: 10.1128/aac.23.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J. P., Dudek E. J., DiVincenzo C. A., Lucks D. A., Lerner S. A. Emergence of resistance to imipenem during therapy for Pseudomonas aeruginosa infections. J Infect Dis. 1986 Aug;154(2):289–294. doi: 10.1093/infdis/154.2.289. [DOI] [PubMed] [Google Scholar]
- Satake S., Yoshihara E., Nakae T. Diffusion of beta-lactam antibiotics through liposome membranes reconstituted from purified porins of the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 May;34(5):685–690. doi: 10.1128/aac.34.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studemeister A. E., Quinn J. P. Selective imipenem resistance in Pseudomonas aeruginosa associated with diminished outer membrane permeability. Antimicrob Agents Chemother. 1988 Aug;32(8):1267–1268. doi: 10.1128/aac.32.8.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trias J., Dufresne J., Levesque R. C., Nikaido H. Decreased outer membrane permeability in imipenem-resistant mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1989 Aug;33(8):1202–1206. doi: 10.1128/aac.33.8.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trias J., Nikaido H. Outer membrane protein D2 catalyzes facilitated diffusion of carbapenems and penems through the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Jan;34(1):52–57. doi: 10.1128/aac.34.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trias J., Nikaido H. Protein D2 channel of the Pseudomonas aeruginosa outer membrane has a binding site for basic amino acids and peptides. J Biol Chem. 1990 Sep 15;265(26):15680–15684. [PubMed] [Google Scholar]
- Tuomanen E. Phenotypic tolerance: the search for beta-lactam antibiotics that kill nongrowing bacteria. Rev Infect Dis. 1986 Jul-Aug;8 (Suppl 3):S279–S291. doi: 10.1093/clinids/8.supplement_3.s279. [DOI] [PubMed] [Google Scholar]