Abstract
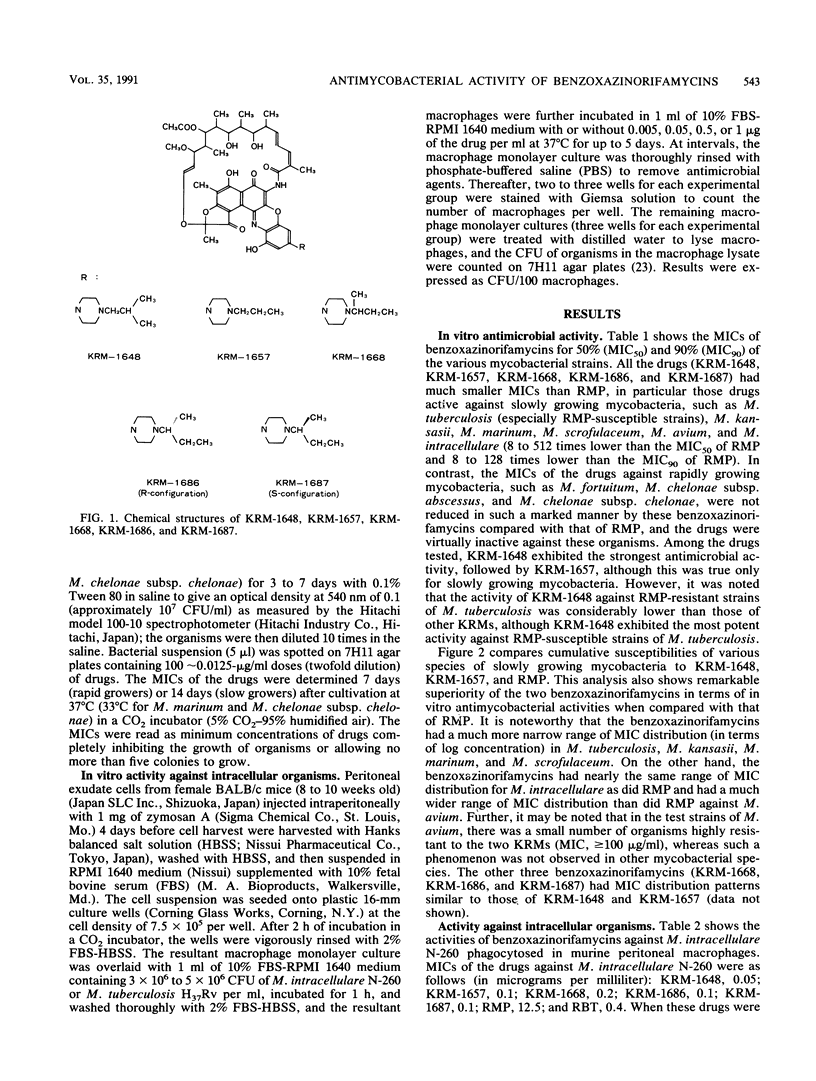
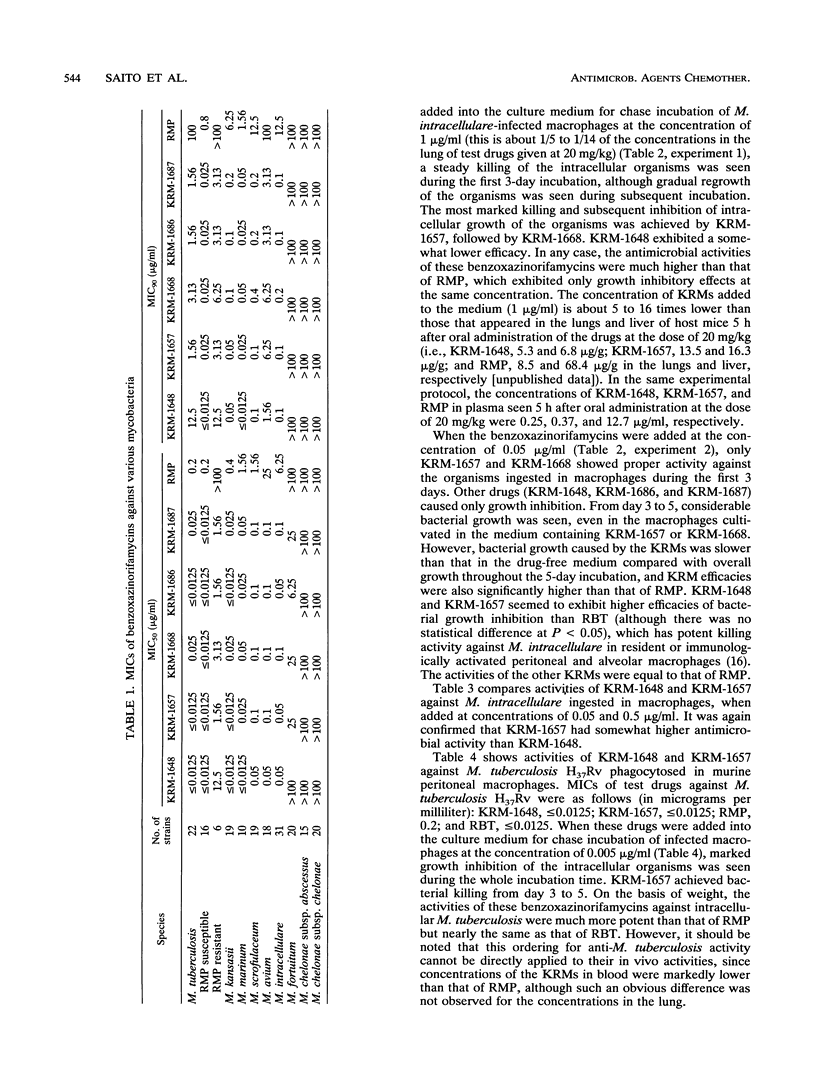
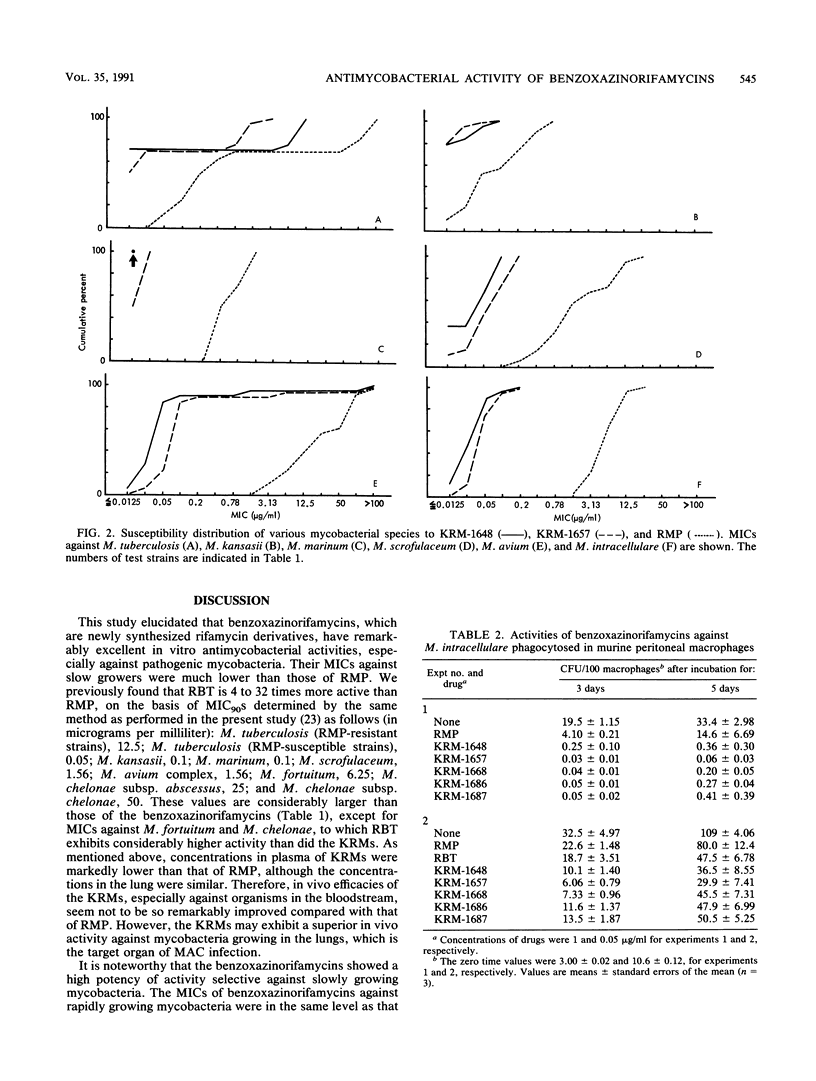
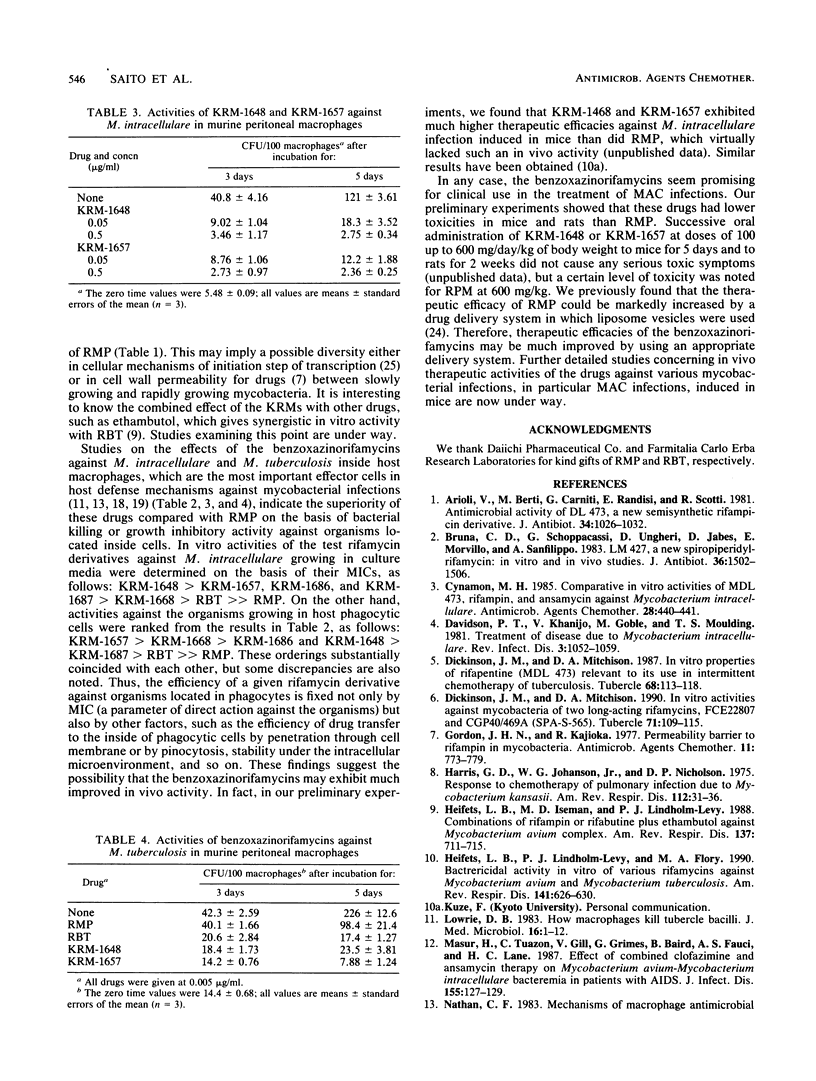
Newly synthesized rifamycin derivatives, KRM-1648, KRM-1657, KRM-1668, KRM-1686, and KRM-1687, having the chemical structures of 3'-hydroxy-5'-(4-alkylpiperazinyl)-benzoxazinorifamycins (alkyl residues: isobutyl, propyl, sec-butyl, sec-butyl [R configuration], and sec-butyl [S configuration], respectively), were studied for their in vitro antimycobacterial activities. Representative (KRM-1648) MICs for 90% of the strains tested, determined by the agar dilution method on 7H11 medium, of various pathogenic mycobacteria (9 species, 174 strains) were as follows (in micrograms per milliliter): Mycobacterium tuberculosis (rifampin [RMP]-susceptible strains), less than or equal to 0.0125; M. tuberculosis (RMP-resistant strains), 12.5; M. kansasii, 0.05; M. marinum, less than or equal to 0.0125; M. scrofulaceum, 0.1; M. avium, 1.56; M. intracellulare, 0.1; M. fortuitum, greater than 100; and M. chelonae subsp. abscessus and M. chelonae subsp. chelonae, greater than 100. These values are more than 64 times lower than those of RMP, except for the values against RMP-resistant M. tuberculosis (8 times lower) and those against rapid growers, including M. fortuitum and M. chelonae (the same as those of RMP). The other derivatives had similar levels of in vitro activity against these mycobacteria. When murine peritoneal macrophages in which M. intracellulare was phagocytosed in vitro were cultured in the presence of the benzoxazinorifamycins (1 microgram/ml), much more rapid killing of the organisms ingested in the macrophages was seen compared with when the same amount of RMP was added to the medium. The addition of benzoxazinorifamycins at the concentration of 0.05 micrograms/ml caused more marked suppression of intracellular growth of the organisms compared with addition of RMP. KRM-1648 and KRM-1657 inhibited intracellular growth of M. tuberculosis, and their efficacies were much greater than that of RMP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arioli V., Berti M., Carniti G., Randisi E., Rossi E., Scotti R. Antibacterial activity of DL 473, a new semisynthetic rifamycin derivative. J Antibiot (Tokyo) 1981 Aug;34(8):1026–1032. doi: 10.7164/antibiotics.34.1026. [DOI] [PubMed] [Google Scholar]
- Cynamon M. H. Comparative in vitro activities of MDL 473, rifampin, and ansamycin against Mycobacterium intracellulare. Antimicrob Agents Chemother. 1985 Sep;28(3):440–441. doi: 10.1128/aac.28.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson P. T., Khanijo V., Goble M., Moulding T. S. Treatment of disease due to Mycobacterium intracellulare. Rev Infect Dis. 1981 Sep-Oct;3(5):1052–1059. doi: 10.1093/clinids/3.5.1052. [DOI] [PubMed] [Google Scholar]
- Della Bruna C., Schioppacassi G., Ungheri D., Jabès D., Morvillo E., Sanfilippo A. LM 427, a new spiropiperidylrifamycin: in vitro and in vivo studies. J Antibiot (Tokyo) 1983 Nov;36(11):1502–1506. doi: 10.7164/antibiotics.36.1502. [DOI] [PubMed] [Google Scholar]
- Dickinson J. M., Mitchison D. A. In vitro activities against mycobacteria of two long-acting rifamycins, FCE22807 and CGP40/469A (SPA-S-565). Tubercle. 1990 Jun;71(2):109–115. doi: 10.1016/0041-3879(90)90005-s. [DOI] [PubMed] [Google Scholar]
- Dickinson J. M., Mitchison D. A. In vitro properties of rifapentine (MDL473) relevant to its use in intermittent chemotherapy of tuberculosis. Tubercle. 1987 Jun;68(2):113–118. doi: 10.1016/0041-3879(87)90026-2. [DOI] [PubMed] [Google Scholar]
- Harris G. D., Johanson W. G., Nicholson D. P. Response to chemotherapy of pulmonary infection due to Mycobacterium kansasii. Am Rev Respir Dis. 1975 Jul;112(1):31–36. doi: 10.1164/arrd.1975.112.1.31. [DOI] [PubMed] [Google Scholar]
- Heifets L. B., Iseman M. D., Lindholm-Levy P. J. Combinations of rifampin or rifabutine plus ethambutol against Mycobacterium avium complex. Bactericidal synergistic, and bacteriostatic additive or synergistic effects. Am Rev Respir Dis. 1988 Mar;137(3):711–715. doi: 10.1164/ajrccm/137.3.711. [DOI] [PubMed] [Google Scholar]
- Heifets L. B., Lindholm-Levy P. J., Flory M. A. Bactericidal activity in vitro of various rifamycins against Mycobacterium avium and Mycobacterium tuberculosis. Am Rev Respir Dis. 1990 Mar;141(3):626–630. doi: 10.1164/ajrccm/141.3.626. [DOI] [PubMed] [Google Scholar]
- Hui J., Gordon N., Kajioka R. Permeability barrier to rifampin in mycobacteria. Antimicrob Agents Chemother. 1977 May;11(5):773–779. doi: 10.1128/aac.11.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killing intracellular mycobacteria: dogmas and realities. 5th Forum in Microbiology. Proceedings. Res Microbiol. 1990 Feb;141(2):191–270. doi: 10.1016/0923-2508(90)90029-p. [DOI] [PubMed] [Google Scholar]
- Lowrie D. B. How macrophages kill tubercle bacilli. J Med Microbiol. 1983 Feb;16(1):1–12. doi: 10.1099/00222615-16-1-1. [DOI] [PubMed] [Google Scholar]
- Masur H., Tuazon C., Gill V., Grimes G., Baird B., Fauci A. S., Lane H. C. Effect of combined clofazimine and ansamycin therapy on Mycobacterium avium-Mycobacterium intracellulare bacteremia in patients with AIDS. J Infect Dis. 1987 Jan;155(1):127–129. doi: 10.1093/infdis/155.1.127. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Mechanisms of macrophage antimicrobial activity. Trans R Soc Trop Med Hyg. 1983;77(5):620–630. doi: 10.1016/0035-9203(83)90190-6. [DOI] [PubMed] [Google Scholar]
- O'Brien R. J., Lyle M. A., Snider D. E., Jr Rifabutin (ansamycin LM 427): a new rifamycin-S derivative for the treatment of mycobacterial diseases. Rev Infect Dis. 1987 May-Jun;9(3):519–530. doi: 10.1093/clinids/9.3.519. [DOI] [PubMed] [Google Scholar]
- Perumal V. K., Gangadharam P. R., Iseman M. D. Effect of rifabutin on the phagocytosis and intracellular growth of Mycobacterium intracellulare in mouse resident and activated peritoneal and alveolar macrophages. Am Rev Respir Dis. 1987 Aug;136(2):334–337. doi: 10.1164/ajrccm/136.2.334. [DOI] [PubMed] [Google Scholar]
- Pezzia W., Raleigh J. W., Bailey M. C., Toth E. A., Silverblatt J. Treatment of pulmonary disease due to Mycobacterium kansasii: recent experience with rifampin. Rev Infect Dis. 1981 Sep-Oct;3(5):1035–1039. doi: 10.1093/clinids/3.5.1035. [DOI] [PubMed] [Google Scholar]
- Rastogi N., David H. L. Mechanisms of pathogenicity in mycobacteria. Biochimie. 1988 Aug;70(8):1101–1120. doi: 10.1016/0300-9084(88)90272-6. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Frehel C., Ryter A., Ohayon H., Lesourd M., David H. L. Multiple drug resistance in Mycobacterium avium: is the wall architecture responsible for exclusion of antimicrobial agents? Antimicrob Agents Chemother. 1981 Nov;20(5):666–677. doi: 10.1128/aac.20.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynearson T. K., Shronts J. S., Wolinsky E. Rifampin: in vitro effect on atypical mycobacteria. Am Rev Respir Dis. 1971 Aug;104(2):272–274. doi: 10.1164/arrd.1971.104.2.272. [DOI] [PubMed] [Google Scholar]
- Saito H., Sato K., Tomioka H. Comparative in vitro and in vivo activity of rifabutin and rifampicin against Mycobacterium avium complex. Tubercle. 1988 Sep;69(3):187–192. doi: 10.1016/0041-3879(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Saito H., Tomioka H. Therapeutic efficacy of liposome-entrapped rifampin against Mycobacterium avium complex infection induced in mice. Antimicrob Agents Chemother. 1989 Apr;33(4):429–433. doi: 10.1128/aac.33.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Steinberg J. L., Nardell E. A., Kass E. H. Antibiotic prophylaxis after exposure to antibiotic-resistant Mycobacterium tuberculosis. Rev Infect Dis. 1988 Nov-Dec;10(6):1208–1219. doi: 10.1093/clinids/10.6.1208. [DOI] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]
- Woodley C. L., Kilburn J. O. In vitro susceptibility of Mycobacterium avium complex and Mycobacterium tuberculosis strains to a spiro-piperidyl rifamycin. Am Rev Respir Dis. 1982 Sep;126(3):586–587. doi: 10.1164/arrd.1982.126.3.586. [DOI] [PubMed] [Google Scholar]
- Yajko D. M., Nassos P. S., Hadley W. K. Therapeutic implications of inhibition versus killing of Mycobacterium avium complex by antimicrobial agents. Antimicrob Agents Chemother. 1987 Jan;31(1):117–120. doi: 10.1128/aac.31.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Inderlied C. B., Berlin O. G., Gottlieb M. S. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev Infect Dis. 1986 Nov-Dec;8(6):1024–1033. doi: 10.1093/clinids/8.6.1024. [DOI] [PubMed] [Google Scholar]



