Abstract
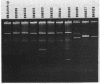
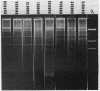
Escherichia coli and Salmonella strains resistant to gentamicin and apramycin were isolated from cattle in France and Belgium and from patients in hospitals. Homology between plasmids of both human and animal origins encoding aminoglycoside 3-N-acetyltransferase was revealed by digestion with several restriction endonucleases and confirmed by hybridization with different replicon-specific probes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bräu B., Pilz U., Piepersberg W. Genes for gentamicin-(3)-N-acetyltransferases III and IV: I. Nucleotide sequence of the AAC(3)-IV gene and possible involvement of an IS140 element in its expression. Mol Gen Genet. 1984;193(1):179–187. doi: 10.1007/BF00327434. [DOI] [PubMed] [Google Scholar]
- Campbell I. G., Mee B. J. Mapping of trimethoprim resistance genes from epidemiologically related plasmids. Antimicrob Agents Chemother. 1987 Sep;31(9):1440–1441. doi: 10.1128/aac.31.9.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaslus-Dancla E., Gerbaud G., Martel J. L., Lagorce M., Lafont J. P., Courvalin P. Probable transmission between animals of a plasmid encoding aminoglycoside 3-N-acetyltransferase IV and dihydrofolate reductase I. Vet Microbiol. 1987 Oct;15(1-2):97–104. doi: 10.1016/0378-1135(87)90134-9. [DOI] [PubMed] [Google Scholar]
- Chaslus-Dancla E., Glupcznski Y., Gerbaud G., Lagorce M., Lafont J. P., Courvalin P. Detection of apramycin resistant Enterobacteriaceae in hospital isolates. FEMS Microbiol Lett. 1989 Oct 15;52(3):261–265. doi: 10.1016/0378-1097(89)90208-5. [DOI] [PubMed] [Google Scholar]
- Chaslus-Dancla E., Lafont J. P. Resistance to gentamicin and apramycin in Escherichia coli from calves in France. Vet Rec. 1985 Jul 27;117(4):90–91. doi: 10.1136/vr.117.4.90. [DOI] [PubMed] [Google Scholar]
- Chaslus-Dancla E., Martel J. L., Carlier C., Lafont J. P., Courvalin P. Emergence of aminoglycoside 3-N-acetyltransferase IV in Escherichia coli and Salmonella typhimurium isolated from animals in France. Antimicrob Agents Chemother. 1986 Feb;29(2):239–243. doi: 10.1128/aac.29.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Carlier C. Resistance towards aminoglycoside-aminocyclitol antibiotics in bacteria. J Antimicrob Chemother. 1981 Jul;8 (Suppl A):57–69. doi: 10.1093/jac/8.suppl_a.57. [DOI] [PubMed] [Google Scholar]
- Couturier M., Bex F., Bergquist P. L., Maas W. K. Identification and classification of bacterial plasmids. Microbiol Rev. 1988 Sep;52(3):375–395. doi: 10.1128/mr.52.3.375-395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein M., Rownd T. H. A rapid method for extracting DNA from agarose gels. Plasmid. 1978 Sep;1(4):557–562. doi: 10.1016/0147-619x(78)90012-4. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering A. M., White L. O., Reeves D. S. AAC(1): a new aminoglycoside-acetylating enzyme modifying the Cl aminogroup of apramycin. J Antimicrob Chemother. 1987 Dec;20(6):803–813. doi: 10.1093/jac/20.6.803. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Mee B. J., Nikoletti S. M. Plasmids encoding trimethoprim resistance in bacterial isolates from man and pigs. J Appl Bacteriol. 1983 Apr;54(2):225–235. doi: 10.1111/j.1365-2672.1983.tb02611.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon P., Popoff M. Y., Coynault C., Marly J., Miras I. Virulence-associated plasmids of Salmonella serotype Typhimurium in experimental murine infection. Ann Inst Pasteur Microbiol. 1986 Jul-Aug;137B(1):47–60. doi: 10.1016/s0769-2609(86)80093-x. [DOI] [PubMed] [Google Scholar]
- Phillips I., Shannon K. Aminoglycoside resistance. Br Med Bull. 1984 Jan;40(1):28–35. doi: 10.1093/oxfordjournals.bmb.a071943. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984 Oct;20(4):608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall E. J., Rowe B., Ferguson J. L., Ward L. R. Characterization of plasmids conferring resistance to gentamicin and apramycin in strains of Salmonella typhimurium phage type 204c isolated in Britain. J Hyg (Lond) 1986 Dec;97(3):419–426. doi: 10.1017/s0022172400063609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner K. J., Wise P. J., Lewis M. J. Molecular relationships between trimethoprim R plasmids obtained from human and animal sources. J Appl Bacteriol. 1986 Dec;61(6):535–540. doi: 10.1111/j.1365-2672.1986.tb01726.x. [DOI] [PubMed] [Google Scholar]
- Wray C., Hedges R. W., Shannon K. P., Bradley D. E. Apramycin and gentamicin resistance in Escherichia coli and salmonellas isolated from farm animals. J Hyg (Lond) 1986 Dec;97(3):445–456. doi: 10.1017/s0022172400063622. [DOI] [PMC free article] [PubMed] [Google Scholar]