Abstract
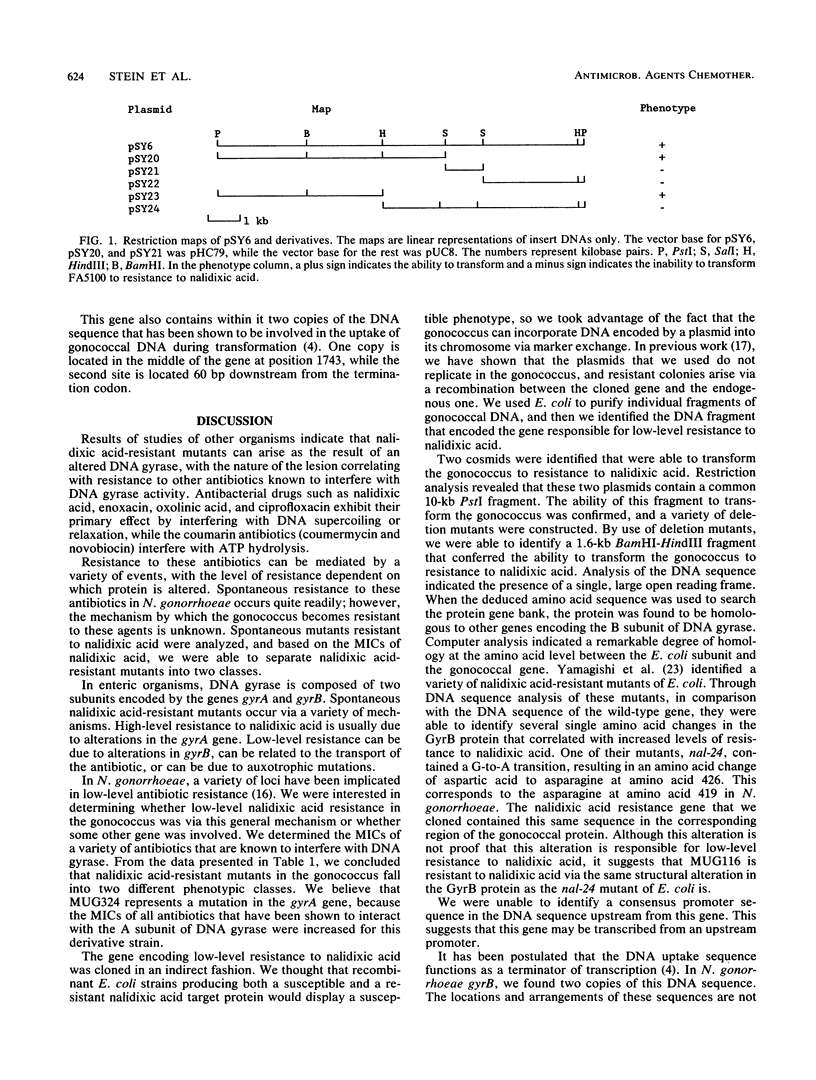
Nalidixic acid-resistant derivatives of Neisseria gonorrhoeae WR302 were identified and categorized into two classes on the basis of their susceptibilities to this antimicrobial agent. The MIC of nalidixic acid for the derivative strain MUG116 was fourfold greater than that for its isogenic parental strain WR302 (2 versus 0.5 micrograms/ml, respectively). MUG324 was significantly more resistant to nalidixic acid (greater than 64 micrograms/ml). The MICs of other antimicrobial agents known to interact with either the gyrA or gyrB gene products were determined. Although the nalidixic acid MIC for MUG116 increased, no significant increases in the MICs of other agents that interact with the gyrA gene product were seen. The MICs of all agents that interact with the gyrA gene product were significantly increased for MUG324. The gene that imparts low-level nalidixic acid resistance was cloned from strain MUG116. The DNA sequence of this gene was determined, and by comparing the deduced amino acid sequence with sequences of proteins in data bases, this protein was found to be approximately 70% homologous with the gyrB gene product of Escherichia coli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon G. J., Levitt M., Sternglanz R. Studies on the mechanism of action of nalidixic acid. Antimicrob Agents Chemother. 1973 Oct;4(4):479–486. doi: 10.1128/aac.4.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. D., Scocca J. J. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6982–6986. doi: 10.1073/pnas.85.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling R. B., Adams B. S. Nalidixic acid-resistant auxotrophs of Escherichia coli. J Bacteriol. 1970 Nov;104(2):1027–1029. doi: 10.1128/jb.104.2.1027-1029.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Hrebenda J., Heleszko H., Brzostek K., Bielecki J. Mutation affecting resistance of Escherichia coli K12 to nalidixic acid. J Gen Microbiol. 1985 Sep;131(9):2285–2292. doi: 10.1099/00221287-131-9-2285. [DOI] [PubMed] [Google Scholar]
- Moriya S., Ogasawara N., Yoshikawa H. Structure and function of the region of the replication origin of the Bacillus subtilis chromosome. III. Nucleotide sequence of some 10,000 base pairs in the origin region. Nucleic Acids Res. 1985 Apr 11;13(7):2251–2265. doi: 10.1093/nar/13.7.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F., Sarubbi F. A., Jr, Blackman E. Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J Bacteriol. 1975 Nov;124(2):740–749. doi: 10.1128/jb.124.2.740-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. C., Petricoin E. F., Griffiss J. M., Schneider H. Use of transformation to construct Neisseria gonorrhoeae strains with altered lipooligosaccharides. Infect Immun. 1988 Apr;56(4):762–765. doi: 10.1128/iai.56.4.762-765.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. C., Silver L. E., Clark V. L., Young F. E. Construction and characterization of a new shuttle vector, pLES2, capable of functioning in Escherichia coli and Neisseria gonorrhoeae. Gene. 1983 Nov;25(2-3):241–247. doi: 10.1016/0378-1119(83)90228-7. [DOI] [PubMed] [Google Scholar]
- Stein D. C., Young F. E., Tenover F. C., Clark V. L. Characterization of a chimeric beta-lactamase plasmid of Neisseria gonorrhoeae which can function in Escherichia coli. Mol Gen Genet. 1983;189(1):77–84. doi: 10.1007/BF00326058. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wagenvoort J. H., van der Willigen A. H., van Vliet H. J., Michel M. F., Van Klingeren B. Resistance of Neisseria gonorrhoeae to enoxacin. J Antimicrob Chemother. 1986 Sep;18(3):429–429. doi: 10.1093/jac/18.3.429. [DOI] [PubMed] [Google Scholar]
- Yamagishi J., Yoshida H., Yamayoshi M., Nakamura S. Nalidixic acid-resistant mutations of the gyrB gene of Escherichia coli. Mol Gen Genet. 1986 Sep;204(3):367–373. doi: 10.1007/BF00331012. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yeung K. H., Dillon J. R. Norfloxacin resistant Neisseria gonorrhoeae in North America. Lancet. 1990 Sep 22;336(8717):759–759. doi: 10.1016/0140-6736(90)92261-f. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Kojima T., Yamagishi J., Nakamura S. Quinolone-resistant mutations of the gyrA gene of Escherichia coli. Mol Gen Genet. 1988 Jan;211(1):1–7. doi: 10.1007/BF00338386. [DOI] [PubMed] [Google Scholar]


