Abstract
Background and purpose:
The contribution of endothelin-1 (ET-1) to vascular hyper-reactivity associated with chronic ethanol intake, a major risk factor in several cardiovascular diseases, remains to be investigated.
Experimental approach:
The biphasic haemodynamic responses to ET-1 (0.01–0.1 nmol kg−1, i.v.) or to the selective ETB agonist, IRL1620 (0.001–1.0 nmol kg−1, i.v.), with or without ETA or ETB antagonists (BQ123 (c(DTrp-Dasp-Pro-Dval-Leu)) at 1 and 2.5 mg kg−1 and BQ788 (N-cis-2,6-dimethyl-piperidinocarbonyl-L-γ-methylleucyl1-D-1methoxycarbonyltryptophanyl-D-norleucine) at 0.25 mg kg−1, respectively) were tested in anaesthetized rats, after 2 weeks' chronic ethanol treatment. Hepatic parameters and ET receptor protein levels were also determined.
Key results:
The initial hypotensive responses to ET-1 or IRL1620 were unaffected by chronic ethanol intake, whereas the subsequent pressor effects induced by ET-1, but not by IRL1620, were potentiated. BQ123 at 2.5 but not 1 mg kg−1 reduced the pressor responses to ET-1 in ethanol-treated rats. Conversely, BQ788 (0.25 mg kg−1) potentiated ET-1-induced increases in mean arterial blood pressure in control as well as in ethanol-treated rats. Interestingly, in the latter group, increases in heart rate, induced by ET-1 at a dose of 0.025 mg kg−1 were enhanced following ETB receptor blockade. Finally, we observed higher levels of ETA receptor in the heart and mesenteric artery and a reduction of ETB receptor protein levels in the aorta and kidney from rats chronically treated with ethanol.
Conclusions and implications:
Increased vascular reactivity to ET-1 and altered protein levels of ETA and ETB receptors could play a role in the pathogenesis of cardiovascular complications associated with chronic ethanol consumption.
Keywords: chronic ethanol consumption, blood pressure, ET-1, ETA receptors
Introduction
Chronic ethanol intake constitutes an important cardiovascular risk factor in the general population (Kurihara et al., 2004). However, the mechanisms involved in these deleterious properties of chronic ethanol consumption have yet to be fully investigated (Strogatz et al., 1991; Kurihara et al., 2004). Enhanced secretion of hormones and neurotransmitters, stimulation of the sympathetic nervous system (Chan et al., 1985), as well as a myogenic mechanism involving alteration of contractile properties of vascular smooth muscle (Chan and Sutter, 1982), are triggered in conditions of chronic ethanol intake. Furthermore, other reports investigating the chronic effects of ethanol on the cardiovascular system suggest that exacerbation of vascular responsiveness to constrictor agents (Strickland and Wooles, 1988; Hatton et al., 1992) or impairment of vascular relaxation (Utkan et al., 2001) contribute to the enhanced blood pressure associated with chronic ethanol consumption. We have recently provided evidence that chronic ethanol intake enhances endothelin-1 (ET-1)-induced contraction in the isolated rat carotid artery, a consequence of a reduced expression of vasodilator endothelial ETB receptors (Tirapelli et al., 2006b). Thus, based on the above-mentioned study, we hypothesized that chronic ethanol consumption could alter the ET-1 pathway, also in vivo.
ET-1 is a 21 amino-acid peptide, belonging to a family of potent vasoconstrictors (Yanagisawa et al., 1988). This property of ETs involves the activation of two receptor types, ETA and ETB (Alexander et al., 2007). The ETA receptor is restricted to vascular smooth muscle and activation of this G-protein-coupled receptor triggers vasoconstriction (Haynes and Webb, 1993). ETB receptors were initially described on vascular endothelium, mediating relaxation through production of nitric oxide (NO) (Hirata et al., 1993) or prostacyclin (PGI2) (Filep et al., 1991; Schilling et al., 1995; Matsuda et al., 1999) or causing contraction via the same receptor population localized in vascular smooth muscle (Ihara et al., 1991; Tirapelli et al., 2005).
ET-1 has potent vasoconstrictor, mitogenic and pro-inflammatory properties, which may contribute to the progression of several cardiovascular disorders (D'Orléans-Juste et al., 2002; Tostes and Muscara, 2005). Interestingly, some reports have described that ethanol consumption induces an increase in ET-1 production. Tsuji et al. (1992) observed that ethanol increased the production of ET-1 and ET-2 in human cultured umbilical vein. Furthermore, Nanji et al. (1994) reported increased plasma ET-1 levels in rats treated with ethanol, suggesting that chronic ethanol consumption alters the ET-1 pathway.
In the present study, we therefore aimed to determine whether and how chronic ethanol consumption affects the ET system in rats. We compared the effect of ethanol intake for 2 weeks on the haemodynamic responses to ET-1 in anaesthetized rats and assessed putative alterations in the expression of both, ETA and ETB receptors in the heart, mesenteric artery, aorta, liver and kidney. Our results highlight a possible correlation between ET-1-induced increase in blood pressure and enhanced levels of ET receptors in resistance vessels after chronic treatment with ethanol, suggesting a biochemical modification underlying the development of the pathological ethanol-induced vascular hyper-responsiveness.
Methods
Ethanol treatment
Experiments were performed in accordance with the principles and guidelines of the Canadian Council on Animal Care, using male Wistar rats obtained from Charles River (St Constant, QC, Canada). The rats, initially weighing 300–350 g (90–100 days old), were randomly divided into two groups: control and ethanol-treated animals. Control rats received tap water ad libitum, whereas rats from the ethanol group received 20% (v/v) ethanol in their drinking water (Tirapelli et al., 2006a, 2006b). All animals had free access to Purina Lab ChowR. To avoid a considerable loss of animals, the ethanol-treated group was submitted to a brief and gradual adaptation period. The animals received 5% ethanol in their drinking water during the first week, 10% in the second and 20% in the third week. At the end of the third week, the experimental stage was initiated and lasted for 2 weeks.
Blood ethanol and serum measurements of glucose, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, total proteins and bilirubin
Blood samples were collected for analysis of blood ethanol content at the end of the second week of ethanol feeding. All samples were collected during the morning period, from the carotid artery of anaesthetized rats using heparinized syringes. The samples were analysed as previously described (Tirapelli et al., 2006a) using a CG-17A gas chromatography (Shimadzu, Kyoto, Japan) equipped with a flame-ionization detector and an HSS-4A headspace sampler (Shimadzu). Injections were made in the split mode onto a Supelcowax 10 (Supelco, Bellefonte, PA, USA) column (30 m × 25 mm i.d. and 25 μm film thickness). Calibration standards were prepared in the same headspace vials (0.10–3.16 mg mL−1) and results were expressed as milligrams of ethanol per millilitre of blood.
For glucose, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, total proteins and bilirubin measurements, blood was collected from the carotid artery of anaesthetized rats. The samples were centrifuged at 8000–10 000 g for 10 min at room temperature. The serum was assayed using commercial kits (Labtest Diagnóstica, São Paulo, São Paulo, Brazil) with the auto-analyzer ABBOTT (model ABAA VP) and results were expressed as units per litre, grams per litre or milligrams per litre.
In vivo experimental procedures
The experimental approaches used in this study are in accordance with Gobeil et al. (1996) and Gendron et al. (2005) with slight modifications. Briefly, rats were anaesthetized with ketamine/xylazine (87/13 mg kg−1) injected intramuscularly. A polyethylene catheter (PE-50) filled with heparin/saline solution was inserted into the common right carotid artery to monitor the mean arterial blood pressure (MAP) and heart rate (HR) with a transducer linked to a blood pressure analyzer (Micro-Med, Louisville, KY, USA). Another catheter (PE-50) was inserted into the left jugular vein to administer pharmacological agents (agonists and antagonists). Dose–response curves to ET-1 (0.01–0.1 nmol kg−1), IRL1620 ({succinyl-[Glu9,Ala11,15]-ET-1(8-210}) (0.001–1 nmol kg−1), a selective endothelin ETB receptor agonist, phenylephrine (1.5–48 μg kg−1) or acetylcholine (1.5–48 μg kg−1) were performed in control and ethanol-treated rats. On the basis of results obtained in our dose–response curves, doses of 0.01 and 0.025 nmol kg−1of ET-1 were used to investigate the mechanisms underlying the increase in blood pressure induced by chronic ethanol consumption. Because there was a loss of responsiveness to ET-1 after repeated administrations, parallel assays were needed in which the following antagonists were injected i.v. 5 min before ET-1: the endothelin ETA receptor antagonist c(DTrp-Dasp-Pro-Dval-Leu) (BQ123) (1 or 2.5 mg kg−1) and the endothelin ETB receptor antagonist BQ788 (N-cis-2,6-dimethyl-piperidinocarbonyl-L-γ-methylleucyl1-D-1methoxycarbonyltryptophanyl-D-norleucine) (0.25 mg kg−1). The doses of antagonists were based on previous reports (McMurdo et al., 1993; Honore et al., 2002).
Western immunoblotting
Total protein was extracted from heart, kidney, arterial mesenteric bed, aorta and liver. The Bradford assay was used to determine protein concentration. The assay was performed as previously described (Tirapelli et al., 2005). In brief, proteins were denatured in 2 × Laemmli sample buffer by heating to 95 °C for 5 min, cooled on ice and followed by centrifugation (12 000 g; 5 min; 15 °C). Total protein (30–50 μg) was separated by electrophoresis on 10% SDS polyacrylamide gel and transferred to methanol-activated polyvinylidene fluoride membrane (Amersham, Little Chalfont, Buckinghamshire, UK) in Tris-glycine buffer containing 20% of methanol. Membranes were blocked on Tris-buffered saline Tween-20 with 8% non-fat dry milk and incubated with rabbit polyclonal antiserum (1:200) raised against rat ETB (AER-002) or (1:200) rat ETA receptors (AER-001) (Alomone Labs, Jerusalem, Israel). GAPDH was used as an internal control and detected with rabbit polyclonal antiserum (1/400), (sc-25778) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). As second antibody, the goat anti-rabbit IgG coupled to horseradish peroxidase (sc-2004) (Santa Cruz Biotechnology Inc.) was used. Visualization of protein bands was carried out with the enhanced chemiluminescence's ECL detection system (Amersham). Densitometric analysis was performed with a densitometer (Gel Doc; Bio-Rad, Piscataway, New Jersey, USA) to determine level of protein expression. As a final specificity control, antibodies were mixed with antigenic peptides used to raise either the ETA or ETB antiserum, prior to incubation with total protein-containing membranes, as recommended by Alomone Labs.
Statistical analysis
The results (mean±s.e.mean) were compared using Student's t-test or one-way ANOVA (followed by Bonferroni's multiple comparison test) as indicated in the text and legends. Results of statistical tests with P<0.05 were considered as significant.
Drugs
ET-1 and IRL1620 were purchased from Peptide International (Louisville, Kentucky, USA), phenylephrine and acetylcholine from Sigma Co (Oakville, Ontario, Canada). BQ123 and BQ788 were synthesized by Dr Witold Neugebauer (Department of Pharmacology, University of Sherbrooke). Drugs were dissolved in phosphate-buffered saline (pH 7.4) except for BQ123 and BQ788, which were diluted in 10% dimethylsulphoxide and subsequently in phosphate-buffered saline. The concentration of dimethylsulphoxide in the final solution had no effects per se on basal cardiovascular parameters or on the pharmacological effects of agonists or antagonists used in the present study.
Results
Body weight, blood ethanol measurements and serum levels of glucose, AST, ALT, alkaline phosphatase, total proteins and bilirubin
Results for body weight, blood ethanol measurements and serum levels of glucose, AST, ALT, alkaline phosphatase, total proteins and bilirubin are summarized in Table 1. As expected, ethanol plasma levels were increased only in animals given ethanol in drinking water. Furthermore, there was a reduction in body weight in the ethanol-treated rats compared with that in control animals. On the other hand, the values of the other metabolic variables measured did not differ between the groups.
Table 1.
Body weight, blood ethanol levels and serum levels of glucose, AST, ALT, total proteins, alkaline phosphatase, total bilirubin and direct bilirubin obtained from control and ethanol-treated rats
| Control | Ethanol | n | |
|---|---|---|---|
| Body weight (g) | 466±7 | 415±7 | 32 |
| Blood ethanol (mg mL−1) | ND | 1.9±0.2* | 11 |
| Glucose (mg L−1) | 1064±77 | 1090±59 | 12 |
| AST (U L−1) | 128±12 | 98±7 | 15 |
| ALT (U L−1) | 68±3 | 62±8 | 15 |
| Total proteins (g L−1) | 73±2 | 74±2 | 14 |
| Alkaline phosphatase (U L−1) | 186±17 | 177±15 | 16 |
| Total bilirubin (mg L−1) | 4±0.2 | 4±0.2 | 13 |
| Direct bilirubin (mg L−1) | 2±0.1 | 2±0.2 | 14 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; ND, non-detectable.
*Significantly different compared with control group (P<0.05, Student's t-test).
Numbers in the last column indicate the number of replicates. Values shown are means±s.e.mean.
Effect of chronic ethanol consumption on basal MAP and HR
The baseline MAP of ethanol-treated rats (105±1.6 mm Hg, n=48) was higher that that of the control group (85±0.7 mm Hg, n=50) (P<0.05; Student's t-test). Likewise, the basal values of the systolic blood pressure and diastolic blood pressure were higher in the ethanol group (118±2 mm Hg and 92±1.6 mm Hg, respectively) when compared with control group (103±1.1 mm Hg and 74±0.7 mm Hg, respectively) (P<0.05; Student's t-test). On the other hand, no HR changes were observed between control (253±3.4 b.p.m., n=50) and ethanol-treated rats (247±3.7 b.p.m., n=48).
Effect of chronic ethanol consumption on the depressor or pressor response induced by ET-1, IRL1620, phenylephrine and acetylcholine
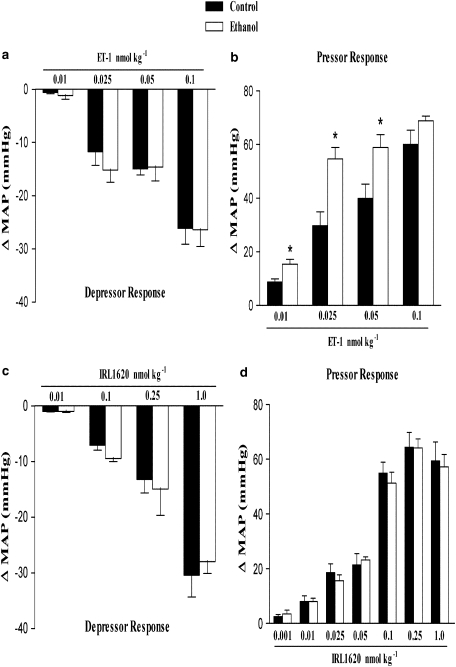
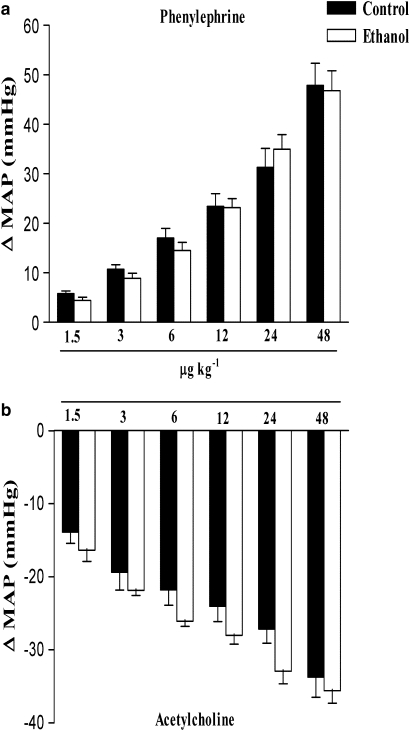
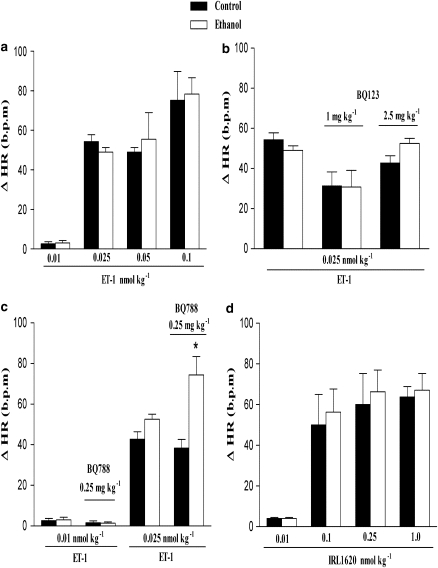
In anaesthetized rats, bolus intravenous injection of ET-1 or IRL1620 produced a transient fall in blood pressure followed by a sustained pressor response. Figure 1 shows the maximal decrease and increase in MAP induced by ET-1 (0.01–0.1 nmol kg−1) or IRL1620, a selective endothelin ETB receptor agonist (0.001–1.0 nmol kg−1). We observed that the decrease in MAP induced by ET-1 and IRL1620 did not differ between groups. However, after treatment for 2 weeks with ethanol, rats presented a higher increase in MAP induced by ET-1, at all doses except the highest (0.1 nmol kg−1). On the other hand, IRL1620-induced increase in MAP did not differ between treatment groups. Note that as shown in Figure 2, chronic ethanol consumption did not alter either the increase in MAP induced by phenylephrine, or the decrease in MAP induced by acetylcholine (Figure 2). In addition, no differences were observed in the ET-1 or IRL1620-dependent increases in HR between control or ethanol-treated groups (Figures 5a and d).
Figure 1.
Effect of chronic ethanol consumption on the maximal variation in mean arterial blood pressure (MAP) induced by i.v. injection of ET-1 (0.01, 0.025, 0.05 or 0.1 nmol kg−1) or IRL1620 (0.001, 0.01, 0.025, 0.05, 0.1, 0.25 or 1.0 nmol kg−1) in the anaesthetized rat. Each point represents the mean±s.e.mean of 6–11 independent experiments for the maximal depressor (a, c) and pressor (b, d) response induced by ET-1 or IRL1620. No depressor responses to IRL1620 were found in the dose range of 0.001–0.05 nmol kg−1 (only the doses of 0.01 nmol kg−1 and higher of IRL1620 are shown) (*P<0.05 compared with control group; Student's t-test).
Figure 2.
Effect of chronic ethanol consumption on the maximal variation in mean arterial blood pressure (MAP) induced by i.v injection of phenylephrine (1.5–48 μg kg−1) or acetylcholine (1.5–48 μg kg−1) in the anaesthetized rat. Each point represents the mean±s.e.mean of 6–7 independent experiments for the maximal pressor (a) and depressor (b) response induced by phenylephrine or acetylcholine, respectively.
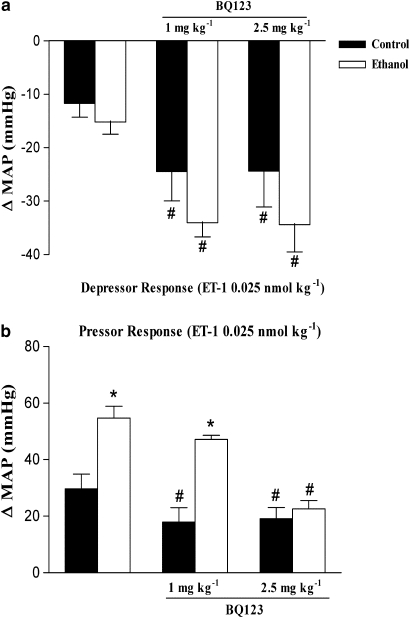
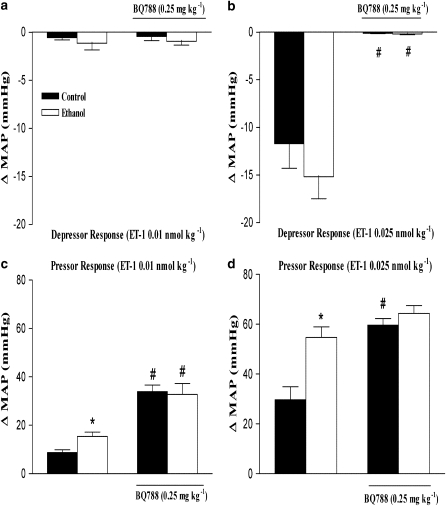
Effect of BQ123 and BQ788 on the pressor or depressor response and changes in HR induced by ET-1 in control and ethanol-treated rats
To investigate further the role of ET receptors in cardiovascular responses to ET-1 after ethanol treatment, we performed experiments with selective ETA and ETB receptor antagonists. The effects of BQ123 and BQ788 on the depressor and pressor responses to ET-1 are represented in Figures 3 and 4. At 1 mg kg−1, BQ123, a selective ETA antagonist, significantly reduced the increase in MAP induced by ET-1 (0.025 nmol kg−1) in control rats, but failed to do so in ethanol-treated rats (Figure 3b). On the other hand, when administered at 2.5 mg kg−1, BQ123 reduced ET-1-induced increase in MAP in both control and ethanol-treated rats (Figure 3b). The selective ETA antagonist also potentiated the initial ET-1-induced hypotensive responses in control or ethanol-treated rats (Figure 3a). At the concentrations used in the present study, BQ123 did not alter basal MAP as previously observed (Honore et al., 2002). On the other hand, BQ788, a selective ETB antagonist, abolished the ET-1-induced hypotensive responses (at 0.025 nmol kg−1) in control and ethanol-treated rats (Figure 4b). Conversely, the increase in MAP induced by ET-1 (0.01 nmol kg−1) was potentiated by BQ788 (0.25 mg kg−1) in control and ethanol-treated rats (Figure 4c), whereas the pressor response to a higher dose of the same agonist (0.025 nmol kg−1) was significantly enhanced by the ETB antagonist only in control animals (Figures 4c and d). Administration of BQ788 produced the same increases in basal MAP in control (6.2±1.0 mm Hg, n=16) as in ethanol-treated rats (8.5±2.0 mm Hg, n=16).
Figure 3.
Effect of BQ123 (1 or 2.5 mg kg−1) on the maximal variation in mean arterial blood pressure (MAP) induced by i.v. injection of endothelin (ET)-1 (0.025 nmol kg−1) in anaesthetized control and ethanol-treated rats. Each point represents the mean±s.e.mean of 5–6 independent experiments for the maximal depressor (a) and pressor (b) response induced by ET-1 (*P<0.05 compared with control group; # compared with the respective group in the absence of BQ123; ANOVA followed by Bonferroni's comparison test).
Figure 4.
Effect of BQ788 (0.25 mg kg−1) on the maximal variation in mean arterial blood pressure (MAP) induced by i.v. injection of endothelin (ET)-1 (0.01 or 0.025 nmol kg−1) in anaesthetized control and ethanol-treated rats. Each point represents the mean±s.e.mean of 6–11 independent experiments for the maximal depressor (a, b) and pressor (c, d) response induced by ET-1 (*P<0.05 compared with control group; # compared with the respective group in the absence of BQ788; ANOVA followed by Bonferroni's comparison test).
Finally, the increase in HR induced by ET-1 (0.025 nmolkg−1) was unaltered by BQ123 (1 or 2.5 nmol kg−1) in control or ethanol-treated rats (Figure 5b). In contrast, increases in HR induced by ET-1, at 0.025 but not 0.01 nmol kg−1, were potentiated by BQ788 (0.25 mg kg−1) in ethanol-treated but not in control rats (Figure 5c).
Figure 5.
Maximal decrease in heart rate (HR) induced by i.v. injection of endothelin (ET)-1 (0.01, 0.025, 0.05 or 0.1 nmol kg−1) (a), ET-1 (0.025 nmol kg−1) in the presence of BQ123 (1 or 2.5 mg kg−1) (b), ET-1 (0.01 or 0.025 nmol kg−1) in the presence of or BQ788 (0.25 mg kg−1)(c), IRL1620 (0.01, 0.1, 0.25 or 1.0 nmol kg−1) (d) in the anaesthetized rat. Each point represents the mean±s.e.mean of 5–6 independent experiments (*P<0.05 compared with control group; Student's t-test).
Effect of chronic ethanol consumption on protein levels of ETA and ETB receptors in the heart, mesenteric artery, kidney, aorta and liver
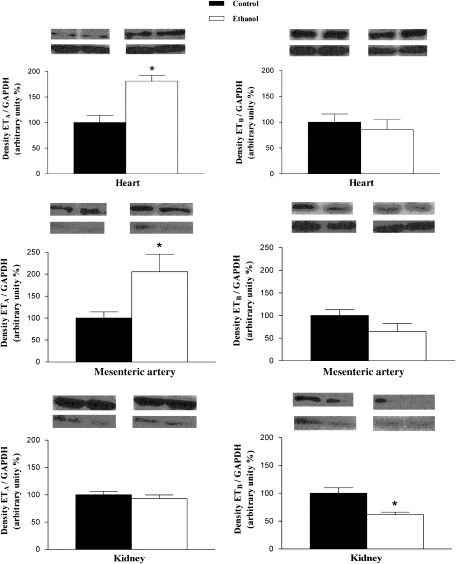
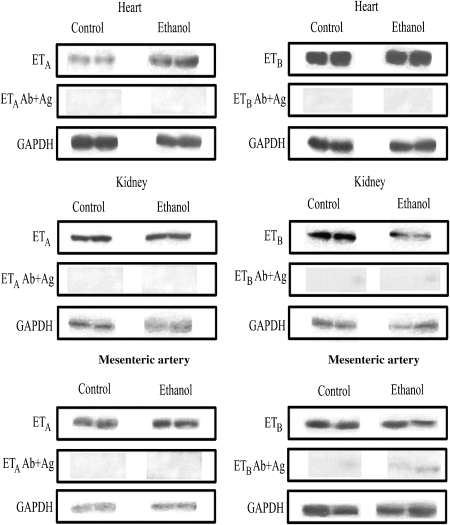
Western blot assays showed that protein levels of ETA receptors were increased in the heart and mesenteric artery from ethanol-treated rats when compared with control (Figure 6). On the other hand, no alterations in protein levels of ETA receptors were found in the kidney (Figure 6), aorta (control: 100.0±7.7%, n=4; ethanol: 65.2±16.0%, n=4) and liver (control: 100.0±4.2%, n=4; ethanol: 157±45.7%, n=4). The protein levels of ETB receptors were decreased in the kidney (Figure 6) and aorta (control: 100.0±9.2%, n=4; ethanol: 40.7±6.7%, n=4) (P<0.05; Student's t-test), but not altered in the heart, mesenteric artery (Figure 6) and liver (control: 100±26%, n=4; ethanol: 77±18%, n=4) from ethanol chronically treated rats. As a final control, Figure 7 shows the specificity of the antibodies used in the present study in tissue homogenates derived from both control or ethanol-treated animals, as pretreatment of total protein containing membranes of the heart, kidney or mesentery artery incubated with the ETA or ETB antibodies pre-incubated with their respective specific antigenic peptide, markedly reduced the detection of ETA and ETB receptors. Similar results were obtained with antigenic peptides for ETA and ETB receptors incubated with tissue homogenates of aorta and liver (results not shown).
Figure 6.
Representative western immunoblots of 50 μg total protein extracted from heart, mesenteric artery and kidney from control or ethanol-treated rats. The bar graphs show the relative absorbance values of ETA and ETB receptor bands. Values were normalized by the corresponding GAPDH bands (used as an internal standard). Results are reported as means±s.e.mean and are representative of 4–5 experiments (*P<0.05 compared with control group; Student's t-test).
Figure 7.
Representative western immunoblots of 50 μg total protein extracted from heart, kidney and mesenteric artery from control or ethanol-treated rats with ETA or ETB antibodies in absence (upper panels) or presence (mid-panels) of their respective antigenic peptides. Ab: antibody, Ag: antigenic peptide.
Discussion
We have shown in the present study that rats chronically treated with ethanol exhibit an enhanced, ETA-dependent, pressor response to ET-1. In addition, a positive correlation was found between this enhanced response to the 21 amino-acid peptide and an increased ETA receptor protein level in cardiac tissue and in a resistance vessel, whereas the ETB receptor was reduced in aortic and renal tissue homogenates.
Our data show that chronic ethanol consumption increases ET-1-induced pressor response but does not alter the initial hypotensive response to the peptide. Furthermore, chronic ethanol consumption did not alter the biphasic pressor response elicited by IRL1620, a selective ETB receptor agonist. Thus, enhanced ET-1-induced pressor response may not be due to decreased endothelial-dependent dilatation or vasoconstriction following activation of ETB receptors.
We also found that chronic ethanol consumption did not alter acetylcholine-induced hypotension or the pressor response induced by phenylephrine, a selective α1-adrenoreceptor agonist. Thus, the two above-mentioned series of experiments further strengthen our hypothesis that ethanol treatment specifically affects the ET-1 pathway. It is of interest that Resstel et al. (2006) recently reported an enhanced pressor response to phenylephrine in non-anaesthetized (telemetry instrumented) rats, whereas we were unable, in the present study, to observe the same potentiation of the hypertensive effect of the α1-adrenoreceptor agonist in anaesthetized animals. Worthy of mention, Resstel et al. (2006) also applied a continuous infusion protocol rather than the bolus injections of phenylephrine used in the present study.
Abdel-Rahman et al. (1985) suggested that anaesthetics depress the cardiovascular system to a greater extent in ethanol-fed than in control rats. In the present study however, no significant alterations in basal haemodynamic parameters (or HR) were noted in ethanol-treated rats under anaesthesia when compared with non-anaesthetized animals. These findings are in accordance with previous results in conscious rats using the same ethanol treatment protocol (Resstel et al., 2006).
Interestingly, changes in MAP induced by ET-1 at 0.01 nmol kg−1 were higher in ethanol-treated rats after administration of BQ788. This result suggests that the enhancement of ET-1-induced pressor responses after ethanol treatment involves an increased response to the peptide mediated by ETA but not ETB receptors. However, it is noteworthy that the ETA antagonist, BQ123 failed to reduce the hypertensive state induced by chronic ethanol in the present rat model. We attribute this lack of effect of BQ123 to the acute (that is, 5 min before injection of ET-1), rather than a chronic, administration protocol in the present study. It remains to be seen if a 4- to 5-day regimen with BQ123 i.v. or an orally available ETA antagonist would correct the ethanol-induced hypertensive state in this rat model.
Patients with cirrhosis and experimental animal models of this disease show elevated serum and hepatic levels of ET-1 (Moore et al., 1992; Gandhi et al., 1996; Ikura et al., 2004). Metabolic parameters were also analysed in our study to assess whether alterations in hepatic function following ethanol consumption could be involved in the exacerbated cardiovascular responses to ET-1 in our model. Glucose, AST, ALT, alkaline phosphatase, total proteins and bilirubin, which are markers for hepatic dysfunction in cirrhotic stages, were unchanged by the 2-week ethanol treatment.
Chronic ethanol intake has been associated with increases in blood pressure attributed to several mechanisms, including the secretion of hormones and neurotransmitters, stimulation of the sympathetic nervous system, alteration of baroreceptor activity and volume overload (Altura and Altura, 1982; Chan and Sutter, 1982). The present results support the concept that enhanced vascular responsiveness to ET-1 may also be involved in the increases in systemic resistance induced by chronic ingestion of ethanol.
The results obtained with ET antagonists suggest that the enhancement of ET-1-induced pressor responses after ethanol treatment might involve an increased response to the peptide mediated by ETA but not ETB receptors. However, ethanol consumption for 2 weeks did not affect resting HR, suggesting that alterations in chronotropic cardiac parameters do not mediate the elevation of basal blood pressure observed in ethanol-treated rats. This postulate is in accordance with previous studies reporting a lack of alteration in resting HR after long-term ethanol consumption (Beilin et al., 1992; Resstel et al., 2006). Furthermore, increases in HR triggered by ET-1 and IRL1620 were not altered by chronic ethanol consumption. Moreover, the administration of BQ123 did not alter the changes in HR induced by ET-1. Interestingly, changes in HR induced by ET-1 were significantly higher in ethanol-treated rats after administration of BQ788. Taken together, these results suggest that chronic ethanol consumption alters the pattern of response mediated by cardiac ETA receptors, which exerts positive chronotropic effect in the heart (Robu et al., 2003).
By western blots, we also demonstrated that ETA protein levels in the heart and mesenteric artery were increased in rats chronically treated with ethanol. Thus, we suggest that changes in HR elicited by ET-1 in the ethanol-treated rats, under BQ788 treatment, were caused by increased cardiac ETA receptor protein levels, reported to be involved in ET-1-induced positive chronotropic effects (Robu et al., 2003). Alternatively, based on our experiments with BQ788, we suggest that ETB receptors play a protective role in ethanol-treated rats by counteracting ETA-mediated positive chronotropic effects. Additionally, long-term ethanol consumption may be associated with remodelling as ETA receptor activation mediates cardiac hypertrophy in rats (Hafiz et al., 2004). Furthermore, the overexpression of ETA receptors, which mediate ET-1-induced contraction in the rat arterial mesenteric bed (D'Orleans-Juste et al., 1993), could also be involved in the increased pressor response induced by ET-1 in ethanol-treated rats. Finally, the reduced expression of ETB protein levels in the aorta could also contribute to the increased responsiveness to ET-1 after treatment with ethanol, as these receptors are known to produce relaxation (Sudjarwo et al., 1992) in this tissue as previously suggested by Tirapelli et al. (2006b) using rat carotid arteries under the same experimental conditions.
Previously, renal ETB receptor deficiency has been associated with increased blood pressure (Tazawa et al., 2004), suggesting that the reduced expression of this receptor type observed in the present investigation in kidney homogenates could also contribute to the increased responsiveness to ET-1 as well as the overall hypertensive state of rats chronically treated with ethanol. Whether the altered expression of ET receptors is a cause or consequence of ethanol-induced enhancement in blood pressure remains to be determined.
Alterations in the ET pathway have been reported in some vascular diseases such as cerebral ischaemia (Salom et al., 2000), subarachnoid haemorrhage (Alabadi et al., 1997) and hypertension (Cardillo et al., 1999). The present study demonstrates for the first time that chronic ethanol potentiates pressor responses to ET-1 and alters ETA and ETB receptor proteins in the heart, kidney, aorta and mesenteric artery confirming that chronic ethanol consumption alters the ET-1 pathway. Treatment for 2 weeks with ethanol induced a twofold increase in plasma ET-1 levels in rats that presented blood ethanol levels within the range that we found in our experiments (Nanji et al., 1994). On the basis of previous findings from the literature as well as from the present study it is suggested that the ET pathway could play a role in the pathogenesis of cardiovascular complications associated with chronic ethanol consumption (Alabadi et al., 1997; Salom et al., 2000).
Acknowledgments
We thank Eduardo Tozatto, Sonia AC Dreossi and Antonio Zanardo Filho for excellent technical support. This project was supported by FAPESP and CAPES (Brazil) (process number: 3191-04-4) and the Canadian Institutes of Health Research (MOP-57764) (Canada).
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BQ123
c(DTrp-Dasp-Pro-Dval-Leu)
- BQ788
N-cis-2,6-dimethyl-piperidinocarbonyl-L-γ-methylleucyl1-D-1methoxycarbonyltryptophanyl-D-norleucine
- ET-1
endothelin-1
- IRL1620
{succinyl-[Glu9,Ala11,15]-ET-1(8-210}
Conflict of interest
The authors state no conflict of interest.
References
- Abdel-Rahman AR, Dar MS, Wooles WR. Effect of chronic ethanol administration on arterial baroreceptor function and pressor and depressor responsiveness in rats. J Pharmacol Exp Ther. 1985;232:194–201. [PubMed] [Google Scholar]
- Alabadi JA, Torregrosa G, Miranda FJ, Salom JB, Centeno JM, Alborch E. Impairment of the modulatory role of nitric oxide on the endothelin-1-elicited contraction of cerebral arteries: a pathogenetic factor in cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1997;41:245–253. doi: 10.1097/00006123-199707000-00039. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 2nd edn (2007 revision) Br J Pharmacol. 2007;150 Suppl 1:S1–S168. doi: 10.1038/sj.bjp.0707199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altura BM, Altura BT. Microvascular and vascular smooth muscle actions of ethanol, acetaldehyde, and acetate. Fed Proc. 1982;41:2447–2451. [PubMed] [Google Scholar]
- Beilin LJ, Hoffmann P, Nilsson H, Skarphedinsson J, Folkow B. Effect of chronic ethanol consumption upon cardiovascular reactivity, heart rate and blood pressure in spontaneously hypertensive and Wistar–Kyoto rats. J Hypertens. 1992;10:645–650. [PubMed] [Google Scholar]
- Cardillo C, Kilcoyne CM, Waclawiw M, Cannon RO, III, Panza JA. Role of endothelin in the increased vascular tone of patients with essential hypertension. Hypertension. 1999;33:753–758. doi: 10.1161/01.hyp.33.2.753. [DOI] [PubMed] [Google Scholar]
- Chan TC, Sutter MC. The effects of chronic ethanol consumption on cardiac function in rats. Can J Physiol Pharmacol. 1982;60:777–782. doi: 10.1139/y82-108. [DOI] [PubMed] [Google Scholar]
- Chan TC, Wall RA, Sutter MC. Chronic ethanol consumption, stress, and hypertension. Hypertension. 1985;7:519–524. doi: 10.1161/01.hyp.7.4.519. [DOI] [PubMed] [Google Scholar]
- D'Orleans-Juste P, Claing A, Warner TD, Yano M, Telemaque S. Characterization of receptors for endothelins in the perfused arterial and venous mesenteric vasculature of the rat. Br J Pharmacol. 1993;110:687–692. doi: 10.1111/j.1476-5381.1993.tb13866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Orléans-Juste P, Labonté J, Bkaily G, Choufani S, Plante M, Honoré JC. Function of the endothelin B receptor in cardiovascular physiology and pathophysiology. Pharmacol Ther. 2002;95:221–238. doi: 10.1016/s0163-7258(02)00235-8. [DOI] [PubMed] [Google Scholar]
- Filep J, Battistini B, Coté YP, Beaudoin AR, Sirois P. Endothelin-1-induced prostacyclin release from bovine aortic endothelial cells. Biochem Biophys Res Commun. 1991;177:171–176. doi: 10.1016/0006-291x(91)91964-e. [DOI] [PubMed] [Google Scholar]
- Gandhi CR, Sproat LA, Subbotin VM. Increased hepatic endothelin-1 levels and endothelin receptor density in cirrhotic rats. Life Sci. 1996;58:55–62. doi: 10.1016/0024-3205(95)02255-4. [DOI] [PubMed] [Google Scholar]
- Gendron G, Gobeil F, Jr, Belanger S, Gagnon S, Regoli D, D'Orleans-Juste P. Urotensin II-induced hypotensive responses in Wistar–Kyoto (Wky) and spontaneously hypertensive (Shr) rats. Peptides. 2005;26:1468–1474. doi: 10.1016/j.peptides.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Gobeil F, Jr, Filteau C, Pheng LH, Jukic D, Nguyen-Le XK, Regoli D. In vitro and in vivo characterization of bradykinin B2 receptors in the rabbit and in the guinea pig. Can J Physiol Pharmacol. 1996;74:137–144. [PubMed] [Google Scholar]
- Hafiz S, Wharton J, Chester AH, Yacoub MH. Profibrotic effect of endothelin-1 via the ETA receptor in cultured human cardiac fibroblasts. Cell Physiol Biochem. 2004;14:285–294. doi: 10.1159/000080338. [DOI] [PubMed] [Google Scholar]
- Hatton DC, Bukoski RD, Edgar S, McCarron DA. Chronic alcohol consumption lowers blood pressure but enhances vascular contractility in Wistar rats. J Hypertens. 1992;10:529–537. doi: 10.1097/00004872-199206000-00005. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Webb DJ. The endothelin family of peptides: local hormones with diverse roles in health and disease. Clin Sci. 1993;84:485–500. doi: 10.1042/cs0840485. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohata K, et al. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J Clin Invest. 1993;91:1367–1373. doi: 10.1172/JCI116338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore JC, Plante M, Bkaily G, Rae GA, D'Orleans-Juste P. Pressor and pulmonary responses to ET-1(1-31) in guinea-pigs. Br J Pharmacol. 2002;136:819–828. doi: 10.1038/sj.bjp.0704782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M, Saeki T, Funabashi K, Nakamichi K, Yano M, Fukuroda T, et al. Two endothelin receptor subtypes in porcine arteries. J Cardiovasc Pharmacol. 1991;17 Suppl 7:S119–S121. doi: 10.1097/00005344-199100177-00031. [DOI] [PubMed] [Google Scholar]
- Ikura Y, Ohsawa W, Naruko T, Muraguchi T, Hirayama M, Suekane T, et al. Expression of hepatic endothelin system in human cirrhotic livers. J Pathol. 2004;204:301–310. doi: 10.1002/path.1644. [DOI] [PubMed] [Google Scholar]
- Kurihara T, Tomiyama H, Hashimoto H, Yamamoto Y, Yano E, Yamashina A. Excessive alcohol intake increases the risk of arterial stiffening in men with normal blood pressure. Hypertens Res. 2004;27:669–673. doi: 10.1291/hypres.27.669. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Beppu S, Ohmori F, Yamada M, Miyatake K. Involvement of cyclo-oxygenase-generated vasodilating eicosanoid(s) in addition to nitric oxide in endothelin-1 induced endothelium-dependent vasorelaxation in guinea pig aorta. Heart Vessels. 1999;8:121–127. doi: 10.1007/BF01744796. [DOI] [PubMed] [Google Scholar]
- McMurdo L, Corder R, Thiemermann C, Vane JR. Incomplete inhibition of the pressor effects of endothelin-1 and related peptides in the anaesthetized rat with BQ123 provides evidence for more than one vasoconstrictor receptor. Br J Pharmacol. 1993;108:557–561. doi: 10.1111/j.1476-5381.1993.tb12840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K, Wendon J, Frazer M, Karani J, Willians R, Badr K. Plasma endothelin immunoreactivity in liver disease and the hepatorenal syndrome. N Engl J Med. 1992;327:1774–1778. doi: 10.1056/NEJM199212173272502. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Khwaja S, Khettry U, Sadrzadeh SMH. Plasma endothelin levels in chronic ethanol fed rats: relationship to pathologic liver injury. Life Sci. 1994;54:423–428. doi: 10.1016/0024-3205(94)00700-4. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Tirapelli CR, Lanchote VL, Uyemura SA, de Oliveira AM, Correa FM. Chronic ethanol consumption alters cardiovascular functions in conscious rats. Life Sci. 2006;78:2179–2187. doi: 10.1016/j.lfs.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Robu VG, Pfeiffer ES, Robia SL, Balijepalli RC, Pi YQ, Kamp TJ, et al. Localization of functional endothelin receptor signalling complexes in cardiac transverse tubules. J Biol Chem. 2003;278:48154–48161. doi: 10.1074/jbc.M304396200. [DOI] [PubMed] [Google Scholar]
- Salom JB, Centeno JM, Torregrosa G, Ortí M, Barberá MD, Alborch E. Vasoconstriction to endothelin-1 in the goat middle cerebral artery after transient global cerebral ischemia. J Stroke Cerebrovasc Dis. 2000;9:16–21. [Google Scholar]
- Schilling L, Feger GI, Ehrenreich H, Whal M. Endothelin-3-induced relaxation of isolated rat basilar artery is mediated by an endothelial ETB-type endothelin receptor. J Cereb Blood Flow Met. 1995;15:699–705. doi: 10.1038/jcbfm.1995.86. [DOI] [PubMed] [Google Scholar]
- Strickland JA, Wooles WR. Effect of acute and chronic ethanol on the agonist responses of vascular smooth muscle. Eur J Pharmacol. 1988;152:83–91. doi: 10.1016/0014-2999(88)90838-2. [DOI] [PubMed] [Google Scholar]
- Strogatz DS, James SA, Haines PS, Elmer PJ, Gerber AM, Browning SR, et al. Alcohol consumption and blood pressure in black adults: the Pitt County Study. Am J Epidemiol. 1991;133:442–450. doi: 10.1093/oxfordjournals.aje.a115911. [DOI] [PubMed] [Google Scholar]
- Sudjarwo SA, Hori M, Karaki H. Effect of endothelin-3 on cytosolic calcium level in vascular endothelium and on smooth muscle contraction. Eur J Pharmacol. 1992;229:137–142. doi: 10.1016/0014-2999(92)90547-h. [DOI] [PubMed] [Google Scholar]
- Tazawa N, Okada Y, Nakata M, Izumoto H, Takasu M, Takaoka M, et al. Exaggerated vascular and renal pathology in endothelin-B-receptor deficient rats with subtotal nephrectomy. J Cardiovasc Pharmacol. 2004;44:S467–S470. doi: 10.1097/01.fjc.0000166316.45882.94. [DOI] [PubMed] [Google Scholar]
- Tirapelli CR, Al-Khoury J, Bkaily G, D'Orleans-Juste P, Lanchote VL, Uyemura SA, et al. Chronic ethanol consumption enhances phenylephrine-induced contraction in the isolated rat aorta. J Pharmacol Exp Ther. 2006a;316:233–241. doi: 10.1124/jpet.105.092999. [DOI] [PubMed] [Google Scholar]
- Tirapelli CR, Casolari DA, Montezano AC, Yogi A, Tostes RC, Legros E, et al. Ethanol consumption enhances endothelin-1-induced contraction in the isolated rat carotid. J Pharmacol Exp Ther. 2006b;318:819–827. doi: 10.1124/jpet.106.103010. [DOI] [PubMed] [Google Scholar]
- Tirapelli CR, Casolari DA, Yogi A, Montezano AC, Tostes RC, Legros E, et al. Functional characterization and expression of endothelin receptors in rat carotid artery: involvement of nitric oxide, a vasodilator prostanoid and the opening of K+ channels in ETB-induced relaxation. Br J Pharmacol. 2005;146:903–912. doi: 10.1038/sj.bjp.0706388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tostes RC, Muscara MN. Endothelin receptor antagonists: another potential alternative for cardiovascular diseases. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:287–301. doi: 10.2174/1568006054553390. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Kawano S, Michida T, Masuda E, Nagano K, Takei Y, et al. Ethanol stimulates immunoreactive endothelin-1 and -2 release from cultured human umbilical vein endothelial cells. Alcohol Clin Exp Res. 1992;16:347–349. doi: 10.1111/j.1530-0277.1992.tb01389.x. [DOI] [PubMed] [Google Scholar]
- Utkan T, Yildiz F, Ilbay G, Ozdemirci S, Erden BF, Gacar N, et al. Blood pressure and vascular reactivity to endothelin-1, phenylephrine, serotonin, KCl and acetylcholine following chronic alcohol consumption in vitro. Fundam Clin Pharmacol. 2001;15:157–165. doi: 10.1046/j.1472-8206.2001.00024.x. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara K, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]