Abstract
Background and purpose:
Cannabidiol is a Cannabis-derived non-psychotropic compound that exerts a plethora of pharmacological actions, including anti-inflammatory, neuroprotective and antitumour effects, with potential therapeutic interest. However, the actions of cannabidiol in the digestive tract are largely unexplored. In the present study, we investigated the effect of cannabidiol on intestinal motility in normal (control) mice and in mice with intestinal inflammation.
Experimental approach:
Motility in vivo was measured by evaluating the distribution of an orally administered fluorescent marker along the small intestine; intestinal inflammation was induced by the irritant croton oil; contractility in vitro was evaluated by stimulating the isolated ileum, in an organ bath, with ACh.
Key results:
In vivo, cannabidiol did not affect motility in control mice, but normalized croton oil-induced hypermotility. The inhibitory effect of cannabidiol was counteracted by the cannabinoid CB1 receptor antagonist rimonabant, but not by the cannabinoid CB2 receptor antagonist SR144528 (N-[-1S-endo-1,3,3-trimethyl bicyclo [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide), by the opioid receptor antagonist naloxone or by the α2-adrenergic antagonist yohimbine. Cannabidiol did not reduce motility in animals treated with the fatty acid amide hydrolase (FAAH) inhibitor N-arachidonoyl-5-hydroxytryptamine, whereas loperamide was still effective. In vitro, cannabidiol inhibited ACh-induced contractions in the isolated ileum from both control and croton oil-treated mice.
Conclusions and implications:
Cannabidiol selectively reduces croton oil-induced hypermotility in mice in vivo and this effect involves cannabinoid CB1 receptors and FAAH. In view of its low toxicity in humans, cannabidiol may represent a good candidate to normalize motility in patients with inflammatory bowel disease.
Keywords: cannabidiol, cannabinoid receptors, fatty acid amide hydrolase, inflammatory bowel disease, intestinal transit, intestinal motility, intestine, phytocannabinoids
Introduction
The plant Cannabis sativa contains more than 60 terpenophenolic compounds, named phytocannabinoids. The best-studied phytocannabinoid is Δ9-tetrahydrocannabinol, which binds specific G-protein-coupled receptors, named cannabinoid (CB1 and CB2) receptors (Mechoulam et al., 2002; Russo and Guy, 2006; Pertwee, 2007; Alexander et al., 2008). The well-known psychotropic effects of Δ9-tetrahydrocannabinol, which are largely mediated by activation of brain cannabinoid CB1 receptors, have always raised a number of clinical and ethical problems. Therefore, a valid therapeutic alternative may be the use of non-psychotropic phytocannabinoids, including cannabidiol (CBD). CBD, unlike Δ9-tetrahydrocannabinol, has very low affinity for both cannabinoid CB1 and CB2 receptors (McPartland et al., 2007), although it has been proposed that CBD may modulate endocannabinoid function through its ability to inhibit the hydrolysis of anandamide and to act as a transient receptor potential vanilloid 1 agonist (Watanabe et al., 1998; Bisogno et al., 2001). CBD is a major component of Sativex, a preparation of cannabinoids, which has been approved by Health Canada for the treatment of neuropathic pain in multiple sclerosis.
The pharmacological profile of CBD has been recently reviewed (Mechoulam et al., 2007). Briefly stated, CBD has been shown to exert (1) antioxidant (Hampson et al., 1998), neuroprotective (Iuvone et al., 2004; Esposito et al., 2006) and antiproliferative actions (Ligresti et al., 2006; Massi et al., 2008) in cultured cells and (2) anti-anxiety (Guimarães et al., 1990; Moreira et al., 2006), hypnotic (Carlini and Cunha, 1981), anticonvulsant (Carlini and Cunha, 1981), neuroprotective (Dirikoc et al., 2007; Esposito et al., 2007; Hayakawa et al., 2007), antinausea (Rock et al., 2008), anti-ischaemic (Durst et al., 2007), anticancer (Massi et al., 2008) and notably anti-inflammatory effects in rodents in vivo. The anti-inflammatory effects of CBD have been demonstrated in both acute (Costa et al., 2004) and chronic (Malfait et al., 2000; Costa et al., 2007) experimental models of inflammation, that is, paw oedema and arthritis.
Although oxidative stress plays an important role in the pathogenesis of a number of gastrointestinal diseases (Rezaie et al., 2007) and also may alter intestinal motility (Van der Vliet et al., 1989; Peluso et al., 2002), the effects of CBD (which is a well-known antioxidant compound) (Hampson et al., 1998) in the digestive tract are largely unexplored. Early studies showed that CBD did not modify gastric emptying and small intestinal transit in mice and rats (Chesher et al., 1973; Shook and Burks, 1989). These results are in agreement with more recent studies showing the lack of effect of CBD on defecation in mice (Fride et al., 2005). In the present study, we have evaluated the effect (and the mode of action) of CBD on intestinal hypermotility induced by the irritant croton oil. Croton oil-induced ileitis is characterized by increased intestinal expression of CB1 receptors and fatty acid amide hydrolase (FAAH) activity (Izzo et al., 2001b).
Methods
Animals
All animal procedures and experiments complied with the Principles of Laboratory Animal Care (NIH publication no. 86-23, revised 1985) and the Italian DL no. 116 of 27 January 1992 and associated guidelines in the European Communities Council Directive of 24 November 1986 (86/609/ECC). Male ICR mice (Harlan Italy, S Pietro al Natisone, UD, Italy) (24–26 g) were used after 1 week of acclimation. Food was withheld 6 h before transit measurement and 18 h before the induction of intestinal inflammation.
Intestinal inflammation
Inflammation was induced as previously described (Puig and Pol, 1998; Borrelli et al., 2006). Mice received orally two doses of croton oil (20 μl per mouse) in two consecutive days. Motility was measured 4 days after the first administration of croton oil. This time was selected on the basis of a previous work (Puig and Pol, 1998; Izzo et al., 2001b), in which maximal inflammatory response occurred 4 days after the first treatment.
In vivo transit
Transit was measured by evaluating the intestinal location of rhodamine-B-labeled dextran (Capasso et al., 2005, 2008). Animals were given fluorescent-labeled dextran (100 μL of 25 mg mL−1 stock solution) via a gastric tube into the stomach. At 20 min after administration, the animals were killed by asphyxiation with CO2 and the entire small intestine with its content was divided into 10 equal parts. The intestinal contents of each bowel segment were vigorously mixed with 2 mL of saline solution to obtain a supernatant containing the rhodamine. The supernatant was centrifuged at 35 g to precipitate the intestinal chyme. The fluorescence in duplicate aliquots of the cleared supernatant was read in a multi-well fluorescence plate reader (LS55 Luminescence spectrometer, Perkin Elmer Instruments, Waltham, MA, USA; excitation 530±5 nm and emission 590±10 nm) for quantification of the fluorescent signal in each intestinal segment. From the distribution of the fluorescent marker along the intestine, we calculated the geometric centre (GC) of small intestinal transit as follows:
GC=Σ (fraction of fluorescence per segment × segment number)
GC ranged from 1 (minimal motility) to 10 (maximal motility). This procedure has yielded an accurate, non-radioactive measurement of intestinal transit (Capasso et al., 2005).
In vivo drug administration
CBD (1–10 mg kg−1), JWH 015 (2-methyl-1-propyl-1H indol-3-yl)-1-naphthalenymethanone) (10 mg kg−1), loperamide (0.075 mg kg−1), clonidine (0.075 mg kg−1), N-arachidonoyl-5-hydroxytryptamine (AA-5-HT, 7.5 mg kg−1) or vehicles were given intraperitoneally 30 min before rhodamine administration to mice with inflammation. In some experiments, naloxone (2 mg kg−1, to block opioid receptors), rimonabant (0.1 mg kg−1, to block cannabinoid CB1 receptors), SR144528 (1 mg kg−1, to block cannabinoid CB2 receptors) or yohimbine (1 mg kg−1, to block α2-adrenoceptors) were given 10 min before CBD (5 mg kg−1) or before the corresponding receptor agonists, that is, loperamide 0.075 mg kg−1, clonidine 0.075 mg kg−1 or JWH 015 10 mg kg−1. In preliminary experiments, CBD (5 and 10 mg kg−1) was given 30 min before rhodamine administration to control mice (that is, mice not treated with croton oil). The doses of antagonists used in the present study (that is, rimonabant, SR144528, naloxone, yohimbine) were selected on the basis of previous work (Capasso et al., 2001, 2008); the doses of loperamide and clonidine were selected on the basis of preliminary experiments that showed that these agonists, both at the 0.075 mg kg−1 dose, had an inhibitory effect on motility which was similar to that produced by CBD 5 mg kg−1.
In vitro experiments
Segments (1–1.5 cm) of the terminal ileum from both control and croton oil-treated mice (killed by asphyxiation with CO2) were removed, flushed free of luminal contents and placed in Krebs' solution (composition in mM: NaCl 119, KCl 4.75, KH2PO4 1.2, NaHCO3 25, MgSO4 1.5, CaCl2 2.5 and glucose 11). The isolated organ was set up to record contractions from the longitudinal axis in an organ bath filled with warm (37 °C) aerated (95% O2/5% CO2) Krebs' solution (Capasso et al., 2006). The tissues were connected to an isotonic transducer (load 0.5 g) connected to a ‘Gemini' recording apparatus (Ugo Basile, Comerio, Italy). At the beginning of each experiment, the ileum was stimulated with ACh (1 mM) to obtain a maximal contraction (100% contraction). After at least 1 h for equilibration, the tissues were stimulated with ACh (1 μM) (Borrelli et al., 2006). ACh was added to the bath and left in contact with the tissue for 30 s and then washed out. The interval between each stimulation was 20 min. After at least three stable control contractions, the contractile responses were repeated in the presence of increasing (non-cumulative) concentrations of CBD (0.01–100 μM) added 20 min before ACh (that is, after washing the tissue). Preliminary experiments showed that a 20 min contraction time was sufficient for CBD to achieve the maximal inhibitory response. In some experiments, control tissues were stimulated with prostaglandin F2α (0.2 μM, added to the bath and left in contact with the tissue for 60 s and then washed out) and the effect of CBD (0.01–100 μM) was evaluated as described above for the contractions evoked by ACh.
Statistics
Data are expressed as the mean±s.e.mean of experiments in n mice. To determine statistical significance, Student's t test was used for comparing a single treatment mean with a control mean, and a one-way analysis of variance followed by a Tukey–Kramer multiple comparisons test was used for analysis of multiple treatment means. P-values <0.05 were considered significant. The concentrations of CBD that produced 50% inhibition of ACh-induced contractions (IC50) or maximal inhibitory effect (Emax) were used to characterize its potency and efficacy, respectively. The IC50 and Emax values were calculated by nonlinear regression analysis using the equation for a sigmoid concentration–response curve (GraphPad Prism).
Drugs
CBD (purity by HPLC: 99.76%) was kindly supplied by GW Pharmaceuticals (Porton Down, Wiltshire, UK). ACh chloride, prostaglandin F2α, naloxone hydrochloride, loperamide hydrochloride, yohimbine hydrochloride and clonidine hydrochloride, were purchased from Sigma (Milan, Italy); JWH 015 was purchased from Tocris (Bristol, UK). AA-5-HT synthesized as previously described (Ortar et al., 2003), was a gift from Dr Vincenzo Di Marzo (CNR, Pozzuoli, Italy). Rimonabant and SR144528 (N-[-1S-endo-1,3,3-trimethyl bicyclo [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide) were a kind gift from Drs Madaleine Mossè and Francis Barth (Sanofi-Aventis, Montpellier, France).
CBD, rimonabant and SR144528 were dissolved in dimethyl sulphoxide (DMSO); AA-5-HT was dissolved in DMSO/Tween 80 (1:4), prostaglandin F2α in ethanol while the other drugs were dissolved in saline. The drug vehicles (DMSO, 4 μL per mouse; 20 μL of DMSO/Tween 80 per mouse; saline 0.1 mL per mouse; DMSO<0.01% in vitro) had no significant effect on the responses under study, both in vitro and in vivo.
Results
In vivo results
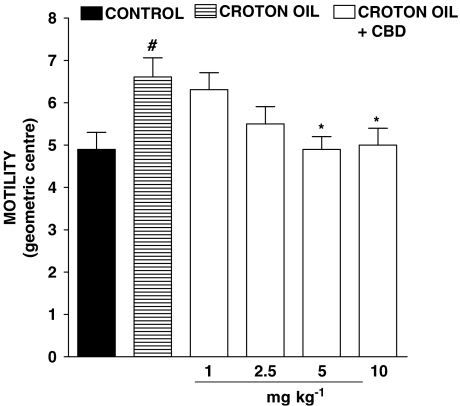
Oral administration of croton oil produced a significant increase in intestinal transit, shown as an increased value of the GC (Figure 1). Intraperitoneal administration of CBD caused a reduction in intestinal motility in croton oil-treated animals, which was statistically significant at doses of 5 and 10 mg kg−1 (Figure 1). However, CBD at these doses (5 and 10 mg kg−1, i.p.) did not modify transit in control mice, that is, in mice not treated with croton oil (GC: control: 5.12±0.24; CBD 5 mg kg−1 4.85±0.28; CBD 10 mg kg−1 5.14±0.30; n=8 for each experimental group, P>0.2).
Figure 1.
Inhibitory effect of cannabidiol (CBD, 1–10 mg kg−1, i.p.) on intestinal transit in croton oil-treated mice in vivo. Transit was expressed as the geometric centre (GC) of the distribution of a fluorescent marker along the small intestine. GC ranged from 1 (minimal motility) to 10 (maximal motility) (see Methods section). Bars represent the mean±s.e.mean of 8–10 animals for each experimental group. #P<0.05 vs control and *P<0.05 vs croton oil.
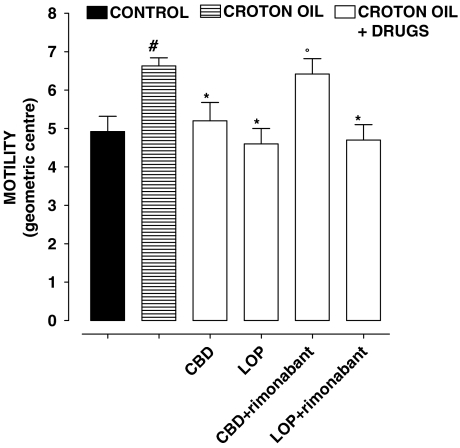
The cannabinoid CB1 receptor antagonist rimonabant, at a dose (0.1 mg kg−1) which per se did not modify intestinal motility in croton oil-treated animals (GC: croton oil 6.58±0.42; croton oil+rimonabant 6.89±0.58, n=8, P>0.2) counteracted the inhibitory effect of CBD (5 mg kg−1) but not that of loperamide (0.075 mg kg−1) on intestinal transit (Figure 2). However, the inhibitory effect of CBD (5 mg kg−1) on motility was not significantly modified by the cannabinoid CB2 antagonist SR144528 (1 mg kg−1), by the opioid receptor antagonist naloxone (2 mg kg−1) or by the α2-adrenoceptor antagonist yohimbine (1 mg kg−1) (Table 1). At the doses used, these antagonists significantly (P<0.05, n=8–10 for each experimental group) counteracted the inhibitory effect on motility of the corresponding agonists, (that is, SR144528 (1 mg kg−1) counteracted the inhibitory effect of JWH 015 10 mg kg−1 (GC values in control 4.91±0.43, croton oil 6.65±0.39, croton oil+JWH 015 5.11±0.36, CO+JWH 015+SR144528 6.60±0.37), naloxone (2 mg kg−1) counteracted the inhibitory effect of loperamide 0.075 mg kg−1 (control 4.90±0.44, croton oil 6.65±0.45; croton oil+loperamide 4.55±0.36, croton oil+loperamide+naloxone 6.60±0.44) and yohimbine (1 mg kg−1) counteracted the inhibitory effect of clonidine 0.075 mg kg−1 on motility (control 4.98±0.42, croton oil 6.67±0.36, croton oil+clonidine 4.50±0.37, croton oil+clonidine+yohimbine 6.58±0.38). In the absence of any agonist, SR144528, naloxone or yohimbine did not modify significantly motility in croton oil-treated animals (croton oil 6.70±0.52; croton oil+SR144528 6.49±0.62; croton oil+naloxone 6.65±0.49; croton oil+yohimbine 6.79±0.55, n=7–8, P>0.2).
Figure 2.
Croton oil-treated mice: effect of i.p.-injected cannabidiol (CBD, 5 mg kg−1) and loperamide (LOP, 0.075 mg kg−1) (alone or in the presence of the cannabinoid CB1 receptor antagonist rimonabant (0.1 mg kg−1, i.p.)) on intestinal transit in vivo. Transit was expressed as the geometric centre (GC) of the distribution of a fluorescent marker along the small intestine. GC ranged from 1 (minimal motility) to 10 (maximal motility) (see Methods section). Bars represent the mean±s.e.mean of 8–10 animals. #P<0.05 vs control, *P<0.05 vs croton oil and °P<0.05 vs CBD.
Table 1.
Croton oil (CO)-treated mice: effect of i.p.-injected cannabidiol (CBD, 5 mg kg−1) alone or in the presence of the CB2 antagonist SR144528 (1 mg kg−1), the opioid antagonist naloxone (2 mg kg−1) or the α2-adrenoceptor antagonist yohimbine (1 mg kg−1) on intestinal transit in vivo
| Treatment | Motility (geometric centre) |
|---|---|
| Control (no croton oil) | 4.91±0.43 |
| Croton oil (CO) | 6.65±0.41# |
| CO+CBD | 5.01±0.36* |
| CO+CBD+SR144528 | 4.99±0.38* |
| CO+CBD+naloxone | 4.98±0.44* |
| CO+CBD+yohimbine | 4.97±0.43* |
#P<0.05 vs control.
*P<0.05 vs croton oil.
N=8–10 animals for each experimental group. Transit was expressed as the geometric centre (GC) of the distribution of a fluorescent marker along the small intestine. GC ranged from 1 (minimal motility) to 10 (maximal motility) (see Methods section).
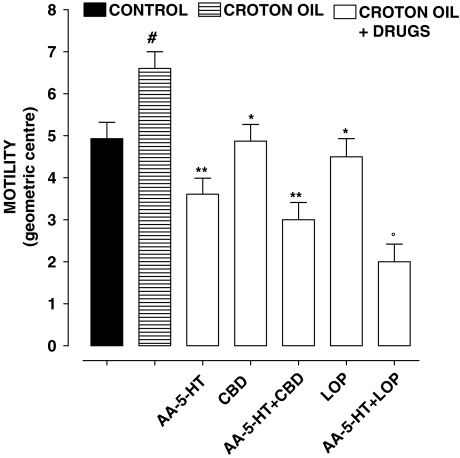
Figure 3 shows the effect of CBD (5 mg kg−1), loperamide (0.075 mg kg−1) or AA-5-HT (7.5 mg kg−1 (administered alone or in combination) in croton oil-treated mice. CBD, loperamide and AA-5-HT significantly reduced motility in croton oil-treated animals; however, the effects of CBD and AA-5-HT were not additive, while the effects of loperamide and AA-5-HT were additive (that is, loperamide (but not CBD) still inhibited motility in animals pretreated with AA-5-HT).
Figure 3.
Croton oil-treated mice: effect of i.p.-injected cannabidiol (CBD, 5 mg kg−1) and the fatty acid amide hydrolase inhibitor N-arachidonoyl-5-hydroxytryptamine (AA-5-HT, 7.5 mg kg−1) (alone or in combination) on intestinal transit in vivo. Transit was expressed as the geometric centre (GC) of the distribution of a fluorescent marker along the small intestine. GC ranged from 1 (minimal motility) to 10 (maximal motility) (see Methods section). Bars represent the mean±s.e.mean of 8–10 animals. #P<0.05 vs control, *P<0.05 and **P<0.05 vs croton oil and °P<0.05 vs LOP.
In vitro results
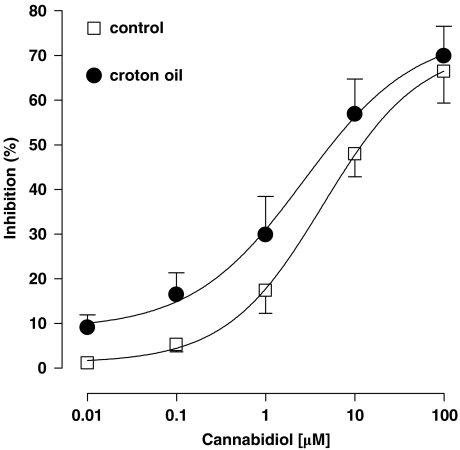
ACh (1 μM) evoked a contractile response that was 66±5% (in control tissues) or 81±3% (in the ileum from croton oil-treated mice, P<0. 05 vs control, n=7–9) of the contraction produced by ACh 1 mM. This concentration of ACh (1 mM) produced a maximal contractile response in the ileum (100% contraction). CBD (0.01–100 μM) had no effect on the baseline mechanical activity of the intestine, but it significantly and in a concentration-dependent manner, inhibited the contractions induced by ACh (Figure 4). The IC50 values of CBD were 4.39±1.55 μM in control tissues and 2.66±1.99 μM in inflamed tissues (no significant differences between the two IC50 values, n=7–9). The Emax values were 72±10% in control tissues and 75±14% in the inflamed gut (no significant difference between the two Emax values, n=7–9). CBD (0.01–10 μM) also reduced the contractions induced by prostaglandin F2α (0.2 μM) (data not shown).
Figure 4.
Inhibitory effect of cannabidiol (0.01–100 μM) on the contractions induced by ACh (1 μM) in the isolated mouse ileum of control and croton oil-treated mice. Each point represents mean±s.e.mean of 7–8 experiments.
Discussion
The presence of motility changes in inflammatory diseases of small or large intestine is a well-recognized and clinically accepted phenomenon (Ohama et al., 2007). The croton oil model of intestinal hypermotility has been extensively used to evaluated drugs with clinical or potential clinical use. Intestinal inflammation induced by croton oil is characterized by disruption of the mucosa and an infiltration of lymphocytes into the submucosa associated with an increase of intestinal transit (Pol and Puig, 1997). Motility in the croton oil model of ileitis may be attenuated by a number of drugs, including cannabinoid CB1 (Izzo et al., 2001b), α2-adrenoceptor (Pol et al., 1996) and opioid (Puig and Pol, 1998; Capasso et al., 2008) receptor agonists. In the present study, we have shown that CBD, a non-psychotropic component of the marijuana plant C. sativa, reduced motility in this experimental model of intestinal ileitis. Two points should be considered here: first, our method to evaluate motility does not distinguish between an effect on gastric emptying and transit through the small intestine and, second, we used a liquid non-nutrient meal and thus there is the possibility that our results will not translate to the transit of solid and/or caloric meals. Interestingly, CBD did not affect transit (present results) and defecation (Fride et al., 2005) in control mice, suggesting that this compound is pharmacologically active only when intestinal homoeostasis is perturbed by an inflammatory stimulus. Although we cannot exclude the possibility that in vivo CBD attenuates the systemic inflammatory response to croton oil rather than having direct effects on intestinal transit (see also below) and although there is evidence that rodent data on cannabinoids might not translate to humans (Sanger, 2007), the present results make CBD an attractive compound for possible therapeutic use to reduce motility during inflammation.
To investigate the mechanism of action of CBD-induced delay in motility, we considered the possible involvement of FAAH, that is, the enzyme involved in endocannabinoid degradation, for several reasons. Thus, FAAH mRNA has been detected in the mouse small intestine and its inhibition resulted in increased intestinal anandamide and 2-arachidonoylglycerol levels and reduction of transit along the small intestine in mice (Capasso et al., 2005). Intestinal FAAH activity is increased in the croton oil model of ileitis (Izzo et al., 2001a, 2001b) and, more importantly, CBD has been shown to inhibit anandamide hydrolysis (Watanabe et al., 1998; Bisogno et al., 2001). In the present study, we have shown that CBD, in contrast to loperamide, did not further reduce transit in animals treated with the FAAH inhibitor, AA-5-HT. The fact that the effects of CBD and AA-5-HT were not additive suggests that the mechanism of CBD-induced delay in motility may involve FAAH. Others have shown that FAAH mediates the antitumour activity of CBD in cultured cells (Massi et al., 2008).
It is now well known that activation of enteric cannabinoid CB1 receptors results in inhibition of intestinal motility in mice in vivo (Izzo et al., 2001a; Carai et al., 2006; Yuece et al., 2007). Previous studies have shown that the inhibitory effect of FAAH inhibitors on gastric and intestinal motility involves, at least in part, indirect activation of cannabinoid CB1 receptors (via enhanced production of intestinal endocannabinoids) (Capasso et al., 2005; Di Marzo et al., 2008). Indeed, the cannabinoid CB1 receptor antagonist rimonabant partially reduced the inhibitory effect of the FAAH inhibitor AA-5-HT on gastric (Di Marzo et al., 2008) and intestinal (Capasso et al., 2005) motility. In the present study, we have shown that a dose of rimonabant, ineffective per se, counteracted the inhibitory effect of CBD (but not the effect of the opioid agonist loperamide) on motility in croton oil-treated mice. On the basis of our experimental data and those previously published which showed the inhibitory effect of CBD on anandamide hydrolysis (Watanabe et al., 1998; Bisogno et al., 2001), we hypothesize that CBD may indirectly activate (via FAAH inhibition) enteric cannabinoid CB1 receptors and thus reduce motility. A direct activation of cannabinoid CB1 receptors seems unlikely as this Cannabis-derived compound has very little affinity for cannabinoid CB1 receptors (McPartland et al., 2007). Interestingly, increased intestinal FAAH activity and increased cannabinoid CB1 receptor expression have been observed in the intestine of croton oil-treated mice (Izzo et al., 2001b). This observation could explain why CBD reduced motility in pathophysiological states, whereas it was without effect in control mice. During the preparation of our paper, others have shown that CBD inhibited FAAH expression in the inflamed—but not in the normal—mouse gut (De Filippis et al., 2008), thus further supporting the involvement of this enzyme in CBD-mediated intestinal effects.
Another possible target of the CBD action is the cannabinoid CB2 receptor. In the gut, this receptor has been found to be expressed by inflammatory/immune cells and also identified on epithelial cells and neurons (Coutts and Izzo, 2004; Di Marzo and Izzo, 2006; Wright et al., 2008). Thomas et al. (2007) have recently shown that the ability of CBD to behave as a cannabinoid CB2 inverse agonist may contribute to its anti-inflammatory properties. Our results demonstrate that the cannabinoid CB2 receptor is functionally active in reducing motility during ileitis, as the selective cannabinoid CB2 receptor agonist JWH 015 (in a cannabinoid CB2 antagonist-sensitive manner) reduced motility in mice treated with croton oil (but not in control animals). However, blockade of the cannabinoid CB2 receptor with the selective antagonist SR144528 did not modify the inhibitory effect of CBD on motility, suggesting that CBD-mediated inhibition of transit is independent of the activation of cannabinoid CB2 receptors. Nevertheless, the cannabinoid CB2-mediated inhibition of intestinal motility, which has been previously documented in the model of intestinal inflammation induced by an endotoxic agent (Mathison et al., 2004), is relevant in the light of the observation that cannabinoid CB2 receptor agonists are devoid of the characteristic psychotropic effects associated with cannabis use (Izzo, 2007; Wright et al., 2008).
We also investigated other mechanisms as potential contributors of the inhibitory effect of CBD on intestinal motility. Specifically, we investigated the possible involvement of α2-adrenoceptors and opioid receptors, because such receptors are upregulated in the intestinal model of ileitis induced by croton oil (Pol et al., 1996, 2001, 2003). Moreover, CBD has been recently shown to be an allosteric modulator at μ- and δ-opioid receptors (Kathmann et al., 2006). However, our experimental data did not support the involvement of α2-adrenoceptors or opioid receptors as specific antagonists of these receptors (namely naloxone and yohimbine) did not modify the inhibitory effect of CBD on motility.
Finally, to verify whether or not CBD may affect directly intestinal contractility, that is, to exert actions in the gut independently from possible systemic anti-inflammatory effects, we evaluated the effect of CBD on the contractions evoked by ACh in the isolated ileum. We found that CBD reduced, in a concentration-dependent manner, ACh-induced contractions, both in control and in croton oil-treated animals. The IC50 values found in our study (2.66–4.39 μM) were in the range of concentrations previously shown to reduce noradrenaline-induced contractions in the vas deferens (Thomas et al., 2004) and to exert neuroprotective (Esposito et al., 2006) and antitumour effects (Ligresti et al., 2006; Vaccani et al., 2006). In contrast to in vivo results, CBD inhibited ACh-induced contractions both in the healthy and in the inflamed intestine (no significant differences in potency or in efficacy were observed, although CBD showed a trend towards a greater potency in the intestine from croton oil-treated mice). Discrepancies between in vitro and in vivo actions of cannabinoids have been previously documented in the digestive tract (Coruzzi et al., 2006). It is very unlikely that the antispasmodic effect of CBD observed here was due to antimuscarinic actions, as CBD also inhibited the contractions induced by prostaglandin F2α.
In conclusion, we have shown that the marijuana component CBD normalize intestinal motility in an experimental model of ileitis. In vitro results showed antispasmodic actions of CBD on intestinal ileal segments. The inhibitory effect of CBD involves, at least in vivo, cannabinoid CB1 receptors and FAAH. In view of its safety records in humans (an average daily dose of about 700 mg/day for 6 weeks was found to be non-toxic, relative to placebo, in clinical trials; Cunha et al., 1980; Consroe et al., 1991) and because CBD reduced motility during inflammation and not in physiological conditions, CBD might be considered as a good candidate to be clinically evaluated for the treatment of hypermotility associated with inflammatory bowel disease.
Acknowledgments
This study was in part supported by Prin, Regione Campania and ‘Fondazione Enrico and Enrica Sovena'. We are grateful to Dr Vincenzo Di Marzo (CNR, Pozzuoli, Italy) and to GW Pharmaceuticals (Porton Down, Wiltshire, UK) for providing us AA-5-HT and CBD, respectively.
Abbreviations
- AA-5-HT
N-arachidonoyl-5-hydroxytryptamine
- CBD
cannabidiol
- FAAH
fatty acid amide hydrolase
- GC
geometric centre
- JWH 015
2-methyl-1-propyl-1H indol-3-yl)-1-naphthalenymethanone
- SR144528
N-[-1S-endo-1,3,3-trimethyl bicyclo [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153 Suppl 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular target for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli F, Capasso F, Capasso R, Ascione V, Aviello G, Longo R, et al. Effect of Boswellia serrata on intestinal motility in rodents: inhibition of diarrhoea without constipation. Br J Pharmacol. 2006;148:553–560. doi: 10.1038/sj.bjp.0706740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso R, Borrelli F, Capasso F, Siebert DJ, Stewart DJ, Zjawiony JK, et al. The hallucinogenic herb Salvia divinorum and its active ingredient salvinorin A inhibit enteric cholinergic transmission in the guinea-pig ileum. Neurogastroenterol Motil. 2006;18:69–75. doi: 10.1111/j.1365-2982.2005.00725.x. [DOI] [PubMed] [Google Scholar]
- Capasso R, Borrelli F, Zjawiony J, Kutrzeba L, Aviello G, Sarnelli G, et al. The hallucinogenic herb Salvia divinorum and its active ingredient salvinorin A reduce inflammation-induced hypermotility in mice. Neurogastroenterol Motil. 2008;20:142–148. doi: 10.1111/j.1365-2982.2007.00994.x. [DOI] [PubMed] [Google Scholar]
- Capasso R, Izzo AA, Fezza F, Pinto A, Capasso F, Mascolo N, et al. Inhibitory effect of palmitoylethanolamide on gastrointestinal motility in mice. Br J Pharmacol. 2001;134:945–950. doi: 10.1038/sj.bjp.0704339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso R, Matias I, Lutz B, Borrelli F, Capasso F, Marsicano G, et al. Fatty acid amide hydrolase controls mouse intestinal motility in vivo. Gastroenterology. 2005;129:941–951. doi: 10.1053/j.gastro.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Carai MA, Colombo G, Gessa GL, Yalamanchili R, Basavarajappa BS, Hungund BL. Investigation on the relationship between cannabinoid CB1 and opioid receptors in gastrointestinal motility in mice. Br J Pharmacol. 2006;148:1043–1050. doi: 10.1038/sj.bjp.0706824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlini EA, Cunha JM. Hypnotic and antiepileptic effects of cannabidiol. J Clin Pharmacol. 1981;21:417S–427S. doi: 10.1002/j.1552-4604.1981.tb02622.x. [DOI] [PubMed] [Google Scholar]
- Chesher GB, Dahl CJ, Everingham M, Jackson DM, Marchant-Williams H, Starmer GA. The effect of cannabinoids on intestinal motility and their antinociceptive effect in mice. Br J Pharmacol. 1973;49:588–594. doi: 10.1111/j.1476-5381.1973.tb08534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consroe P, Laguna J, Allender J, Snider S, Stern L, Sandyk R, et al. Controlled clinical trial of cannabidiol in Huntington's disease. Pharmacol Biochem Behav. 1991;40:701–708. doi: 10.1016/0091-3057(91)90386-g. [DOI] [PubMed] [Google Scholar]
- Coruzzi G, Adami M, Guaita E, Menozzi A, Bertini S, Giovannini E, et al. Effects of cannabinoid receptor agonists on rat gastric acid secretion: discrepancy between in vitro and in vivo data. Dig Dis Sci. 2006;51:310–317. doi: 10.1007/s10620-006-3130-2. [DOI] [PubMed] [Google Scholar]
- Costa B, Giagnoni G, Franke C, Trovato AE, Colleoni M. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol. 2004;143:247–250. doi: 10.1038/sj.bjp.0705920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Trovato AE, Comelli F, Giagnoni G, Colleoni M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol. 2007;556:75–83. doi: 10.1016/j.ejphar.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Coutts AA, Izzo AA. The gastrointestinal pharmacology of cannabinoids: an update. Curr Opin Pharmacol. 2004;4:572–579. doi: 10.1016/j.coph.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21:175–185. doi: 10.1159/000137430. [DOI] [PubMed] [Google Scholar]
- De Filippis D, Iuvone T, D'Amico A, Esposito G, Steardo G, Herman AG, et al. Effect of cannabidiol on sepsis-induced motility disturbances in mice: involvement of CB1 receptors and fatty acid amide hydrolase Neurogastroenterol Motil 2008. 2008; e-pub ahead of print 27 March 2008 [DOI] [PubMed]
- Di Marzo V, Capasso R, Matias I, Aviello G, Petrosino S, Borrelli F, et al. The role of endocannabinoids in the regulation of gastric emptying: alterations in mice fed a high fat diet. Br J Pharmacol. 2008;153:1272–1280. doi: 10.1038/sj.bjp.0707682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Izzo AA. Endocannabinoid overactivity and intestinal inflammation. Gut. 2006;55:1373–1376. doi: 10.1136/gut.2005.090472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirikoc S, Priola SA, Marella M, Zsürger N, Chabry J. Nonpsychoactive cannabidiol prevents prion accumulation and protects neurons against prion toxicity. J Neurosci. 2007;27:9537–9544. doi: 10.1523/JNEUROSCI.1942-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst R, Danenberg H, Gallily R, Mechoulam R, Meir K, Grad E, et al. Cannabidiol, a nonpsychoactive Cannabis constituent, protects against myocardial ischemic reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;293:H3602–H3607. doi: 10.1152/ajpheart.00098.2007. [DOI] [PubMed] [Google Scholar]
- Esposito G, De Filippis D, Carnuccio R, Izzo AA, Iuvone T. The marijuana component cannabidiol inhibits beta-amyloid-induced tau protein hyperphosphorylation through Wnt/beta-catenin pathway rescue in PC12 cells. J Mol Med. 2006;84:253–258. doi: 10.1007/s00109-005-0025-1. [DOI] [PubMed] [Google Scholar]
- Esposito G, Scuderi C, Savani C, Steardo L, Jr, De Filippis D, Cottone P, et al. Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br J Pharmacol. 2007;151:1272–1279. doi: 10.1038/sj.bjp.0707337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fride E, Ponde D, Breuer A, Hanus L. Peripheral, but not central effects of cannabidiol derivatives: mediation by CB(1) and unidentified receptors. Neuropharmacology. 2005;48:1117–1129. doi: 10.1016/j.neuropharm.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Guimarães FS, Chiaretti TM, Graeff FG, Zuardi AW. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology (Berl) 1990;100:558–559. doi: 10.1007/BF02244012. [DOI] [PubMed] [Google Scholar]
- Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (−)delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci USA. 1998;95:8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Nozako M, Ogata A, Hazekawa M, Liu AX, et al. Repeated treatment with cannabidiol but not delta9-tetrahydrocannabinol has a neuroprotective effect without the development of tolerance. Neuropharmacology. 2007;52:1079–1087. doi: 10.1016/j.neuropharm.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Iuvone T, Esposito G, Esposito R, Santamaria R, Di Rosa M, Izzo AA. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J Neurochem. 2004;89:134–141. doi: 10.1111/j.1471-4159.2003.02327.x. [DOI] [PubMed] [Google Scholar]
- Izzo AA. The cannabinoid CB(2) receptor: a good friend in the gut. Neurogastroenterol Motil. 2007;19:704–708. doi: 10.1111/j.1365-2982.2007.00977.x. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Capasso R, Pinto L, Di Carlo G, Mascolo N, Capasso F. Effect of vanilloid drugs on gastrointestinal transit in mice. Br J Pharmacol. 2001a;132:1411–1416. doi: 10.1038/sj.bjp.0703975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Fezza F, Capasso R, Bisogno T, Pinto L, Iuvone T, et al. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br J Pharmacol. 2001b;134:563–570. doi: 10.1038/sj.bjp.0704293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:354–361. doi: 10.1007/s00210-006-0033-x. [DOI] [PubMed] [Google Scholar]
- Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De Petrocellis L, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. 2006;318:1375–1387. doi: 10.1124/jpet.106.105247. [DOI] [PubMed] [Google Scholar]
- Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 2000;97:9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi P, Valenti M, Vaccani A, Gasperi V, Perletti G, Marras E, et al. 5-Lipoxygenase and anandamide hydrolase (FAAH) mediate the antitumor activity of cannabidiol, a non-psychoactive cannabinoid. J Neurochem. 2008;104:1091–1100. doi: 10.1111/j.1471-4159.2007.05073.x. [DOI] [PubMed] [Google Scholar]
- Mathison R, Ho W, Pittman QJ, Davison JS, Sharkey KA. Effects of cannabinoid receptor-2 activation on accelerated gastrointestinal transit in lipopolysaccharide-treated rats. Br J Pharmacol. 2004;142:1247–1254. doi: 10.1038/sj.bjp.0705889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland JM, Glass M, Pertwee RG. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br J Pharmacol. 2007;152:583–593. doi: 10.1038/sj.bjp.0707399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO. Cannabidiol—recent advances. Chem Biodivers. 2007;4:1678–1692. doi: 10.1002/cbdv.200790147. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA, Gallily R. Cannabidiol: an overview of some pharmacological aspects. J Clin Pharmacol. 2002;42:11S–19S. doi: 10.1002/j.1552-4604.2002.tb05998.x. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Guimarães FS. Anxiolytic-like effect of cannabidiol in the rat Vogel conflict test. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1466–1471. doi: 10.1016/j.pnpbp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Ohama T, Hori M, Ozaki H. Mechanism of abnormal intestinal motility in inflammatory bowel disease: how smooth muscle contraction is reduced. J Smooth Muscle Res. 2007;43:43–54. doi: 10.1540/jsmr.43.43. [DOI] [PubMed] [Google Scholar]
- Ortar G, Ligresti A, De Petrocellis L, Morera E, Di Marzo V. Novel selective and metabolically stable inhibitors of anandamide cellular uptake. Biochem Pharmacol. 2003;65:1473–1481. doi: 10.1016/s0006-2952(03)00109-6. [DOI] [PubMed] [Google Scholar]
- Peluso I, Campolongo P, Valeri P, Romanelli L, Palmery M. Intestinal motility disorder induced by free radicals: a new model mimicking oxidative stress in gut. Pharmacol Res. 2002;46:533–538. doi: 10.1016/s1043661802002372. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB(1) and CB(2) receptor pharmacology of three plant cannabinoids: delta(9)-tetrahydrocannabinol, cannabidiol and delta(9)-tetrahydrocannabivarin. Br J Pharmacol. 2007;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol O, Alameda F, Puig MM. Inflammation enhances mu-opioid receptor transcription and expression in mice intestine. Mol Pharmacol. 2001;60:894–899. doi: 10.1124/mol.60.5.894. [DOI] [PubMed] [Google Scholar]
- Pol O, Palacio JR, Puig MM. The expression of delta- and kappa-opioid receptor is enhanced during intestinal inflammation in mice. J Pharmacol Exp Ther. 2003;306:455–462. doi: 10.1124/jpet.103.049346. [DOI] [PubMed] [Google Scholar]
- Pol O, Puig MM. Reversal of tolerance to the antitransit effects of morphine during acute intestinal inflammation in mice. Br J Pharmacol. 1997;122:1216–1222. doi: 10.1038/sj.bjp.0701472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol O, Valle L, Ferrer I, Puig MM. The inhibitory effects of alpha(2)-adrenoceptor agonists on gastrointestinal transit during croton oil-induced intestinal inflammation. Br J Pharmacol. 1996;119:1649–1655. doi: 10.1111/j.1476-5381.1996.tb16085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MM, Pol O. Peripheral effects of opioids in a model of chronic intestinal inflammation in mice. J Pharmacol Exp Ther. 1998;287:1068–1075. [PubMed] [Google Scholar]
- Rezaie A, Parker RD, Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause. Dig Dis Sci. 2007;52:2015–2021. doi: 10.1007/s10620-006-9622-2. [DOI] [PubMed] [Google Scholar]
- Rock EM, Limebeer CL, Mechoulam R, Piomelli D, Parker LA. The effect of cannabidiol and URB597 on conditioned gaping (a model of nausea) elicited by a lithium-paired context in the rat. Psychopharmacology. 2008;196:389–395. doi: 10.1007/s00213-007-0970-1. [DOI] [PubMed] [Google Scholar]
- Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses. 2006;66:234–246. doi: 10.1016/j.mehy.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Sanger GJ. Endocannabinoids and the gastrointestinal tract: what are the key questions. Br J Pharmacol. 2007;152:663–670. doi: 10.1038/sj.bjp.0707422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook JE, Burks TF. Psychoactive cannabinoids reduce gastrointestinal propulsion and motility in rodents. J Pharmacol Exp Ther. 1989;249:444–449. [PubMed] [Google Scholar]
- Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Ross RA, Saha B, Mahadevan A, Razdan RK, Pertwee RG. 6″-Azidohex-2″-yne-cannabidiol: a potential neutral, competitive cannabinoid CB1 receptor antagonist. Eur J Pharmacol. 2004;487:213–221. doi: 10.1016/j.ejphar.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Vaccani A, Massi P, Colombo A, Rubino T, Parolaro D. Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism. Br J Pharmacol. 2006;144:1032–1036. doi: 10.1038/sj.bjp.0706134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet A, Tuinstra TJ, Bast A. Modulation of oxidative stress in the gastrointestinal tract and effect on rat intestinal motility. Biochem Pharmacol. 1989;38:2807–2818. doi: 10.1016/0006-2952(89)90435-8. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ogi H, Nakamura S, Kayano Y, Matsunaga T, Yoshimura H, et al. Distribution and characterization of anandamide amidohydrolase in mouse brain and liver. Life Sci. 1998;62:1223–1229. doi: 10.1016/s0024-3205(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Wright KL, Duncan M, Sharkey KA. Cannabinoid CB(2) receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br J Pharmacol. 2008;153:263–270. doi: 10.1038/sj.bjp.0707486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuece B, Sibaev A, Broedl UC, Marsicano G, Göke B, Lutz B, et al. Cannabinoid type 1 receptor modulates intestinal propulsion by an attenuation of intestinal motor responses within the myenteric part of the peristaltic reflex. Neurogastroenterol Motil. 2007;19:744–753. doi: 10.1111/j.1365-2982.2007.00975.x. [DOI] [PubMed] [Google Scholar]