Abstract
Background and purpose:
The amelioration of insulin resistance by treatment with crocetin is closely related to the hypolipidaemic effect. The present study is designed to clarify the insulin-sensitizing mechanism of crocetin by elucidating the mechanism of regulation of lipid metabolism by crocetin.
Experimental approach:
Rats given a high-fat diet were treated with crocetin for 6 weeks before hyperinsulinaemic–euglycaemic clamp. 14C-palmitate was used as tracer to track the fate of non-esterified fatty acids or as substrate to measure β-oxidation rate. Triglyceride clearance in plasma and lipoprotein lipase activity in tissues were tested. Content of lipids in plasma and tissues was determined. Real-time PCR was used to assay the level of mRNA from genes involved in non-esterified fatty acid and triglyceride uptake and oxidation.
Key results:
Crocetin prevented high-fat-diet induced insulin resistance (increased clamp glucose infusion rate), raised hepatic non-esterified fatty acid uptake and oxidation, accelerated triglyceride clearance in plasma, enhanced lipoprotein lipase activity in liver, and reduced the accumulation of detrimental lipids (DAG and long-chain acyl CoA) in liver and muscle. Genes involved in hepatic lipid metabolism which are regulated by peroxisome proliferator-activated receptor-α, were modulated to accelerate lipid uptake and oxidation.
Conclusions and implications:
Through regulating genes involved in lipid metabolism, crocetin accelerated hepatic uptake and oxidation of non-esterified fatty acid and triglyceride, and reduced lipid availability to muscle, thus decreasing lipid accumulation in muscle and liver, and consequently improving sensitivity to insulin.
Keywords: crocetin, fatty-acid clearance, insulin resistance, lipoprotein lipase, peroxisome proliferator-activated receptor-α
Introduction
Increased availability of circulating lipids (non-esterified fatty acid, NEFA and triglyceride, TG), leading to raised TG accumulation within skeletal muscle and liver, is strongly associated with the development of insulin resistance. It is known that excessive TG accumulated in those tissues will produce some harmful fatty acid-derived metabolites, such as DAG and long-chain acyl CoA (LCACoA). Although the effect of LCACoA remains controversial, it is generally believed that DAG mediates the negative effects on insulin signalling (Turinsky et al., 1990; Schmitz et al., 1997; Chavez and Summers, 2003).
To ameliorate insulin resistance, much effort has been made to reduce the accumulation of detrimental lipids in muscle and liver, by decreasing the availability of TG and NEFA in the circulation. Agonists of the peroxisome proliferator-activated receptor-α (PPARα) are fairly successful in such attempts. PPARα is a transcription factor that has important effects on lipid homoeostasis via regulation of the expression of genes involved in lipid metabolism. It is predominantly expressed in the liver and regulates the transcription of genes involved in hepatic lipid uptake and oxidation, including fatty acid transport protein (FATP), CD36/fatty acid translocase (CD36/FAT), acyl CoA synthetase (ACS), acyl-CoA oxidase (ACO), carnitine palmitoyl transferase-1 (CPT-1) and lipoprotein lipase (LPL) (Escher and Wahli, 2000). Their activation is thought to cause sequestration of lipid into the liver for oxidation. These actions putatively reduce the amount of lipid available to skeletal muscle, the largest insulin-sensitive organ in the body (Oakes et al., 1997; Guerre-Millo et al., 2000; Ye et al., 2001).
Our group has recently found that crocetin, a carotenoid extracted from Gardenia jasminoides Ellis, has the ability to alleviate insulin resistance induced by high-fructose diet in rats (Xi et al., 2007) and that this effect is accompanied by a reduction in plasma TG and NEFA. Some previous reports also confirmed that crocetin ameliorated hypertriglyceridaemia in rats and quails (Deng et al., 2004; He et al., 2007) induced by high-lipid diets. Thus, it is reasonable to presume that the reversal of insulin resistance by crocetin is closely related to its hypolipidaemic action, which may reduce lipid accumulation in insulin-sensitive tissues. So far, the mechanism(s) by which crocetin lowers plasma NEFA and TG and its significance in alleviating insulin resistance have not been clear. Here we adopted a rodent model characterized by both insulin resistance and dyslipidaemia to clarify this question. In the present study, we demonstrated that crocetin may protect against disordered lipid metabolism and the subsequent insulin resistance induced by high-fat diet in rat, probably via its modulation on some PPARα-related genes.
Methods
Preparation of crocetin
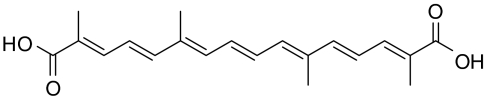
Crocetin was isolated from Gardenia jasminoides Ellis by our team. Briefly, comminuted dry fruit of Gardenia jasminoides Ellis was extracted by methanol–water. The extract was loaded onto a column of macroporous resin to eliminate saccharide and then was hydrolysed by acid. Crocetin was precipitated, collected and purified with ethanol–water. The purity of crocetin used in experiments is over 98% (assayed by HPLC) (chemical structure shown in Figure 1).
Figure 1.
Chemical structure of crocetin.
Experimental animals
All animal procedures were performed in accordance with the institutional guidelines for animal care of China Pharmaceutical University and approved by the local animal research committee. Male Wistar rats, aged 10–12 weeks from China Pharmaceutical University Animal Centre, were housed in a temperature-controlled (22±1 °C) environment with a 12-h light, 12-h dark cycle (lights on at 0600 h). After 1-week acclimatization period, 42 rats were assigned to three groups fed standard diet (normal group), high-fat diet (59% fat, 21% protein and 20% carbohydrate, expressed as a per cent of total dietary calories, control group) or high-fat diet supplemented with crocetin (crocetin group). Over the next 6 weeks, crocetin was administered in a daily dose of 50 mg kg−1, for this dosage regimen significantly ameliorated hyperlipidaemia and increased insulin sensitivity (Deng et al., 2004; He et al., 2007; Xi et al., 2007). Nine to ten days before the experiment, the left carotid artery and right jugular vein of some rats were cannulated under halothane anaesthesia as previously described (Clark et al., 1990). Rats were handled daily to minimize stress and only those rats with fully recovered body weight were used for the final study.
Hyperinsulinaemic–euglycaemic clamping experiments
Conscious rats undergoing hyperinsulinaemic–euglycaemic clamp were infused with insulin (China Wanbang Biopharma, Xuzhou, Jiangsu, China) via the jugular cannula at a constant rate of 250 mU kg−1 h−1 together with a variable rate of 30% glucose to maintain euglycaemia (Kraegen et al., 1983).
General tracer methodology
Arterial blood samples (∼400 μL) were collected at baseline and directly before tracer administration. A CPT-1 inhibitor, etomoxir (Sigma-Aldrich Inc., Shanghai, China; 20 μmol kg−1), was administered i.v. 20 min before tracer infusion to terminate the β-oxidation of 14C-palmitate (Beijing Atom HighTech Co. Ltd, Beijing, China), so that the absolute magnitude of 14C-palmitate taken up by tissue could be measured. The tracer, as the form of albumin-palmitate–14C-palmitate complex (Oakes et al., 1999), was infused at a constant rate (∼1.67 × 104 Bq min−1, 17 μL min−1) into the jugular vein of conscious rats under postabsorptive (basal) conditions. To obtain plasma NEFA and 14C-palmitate concentrations, 150 μL arterial blood samples were collected 10, 20, 40, 60, 80, 100 and 120 min after the start of tracer infusion. At 120 min, tracer infusion was stopped and additional blood samples were collected at 124, 128, 132 and 140 min. Plasma was rapidly separated in a refrigerated centrifuge, and a 25-μL aliquot was placed directly into 2 mL lipid extraction mixture (described below). After collection of the 140-min blood sample, rats were given a lethal dose of Na-thiobutabarbitol. Samples (∼100 mg) of the following tissues were collected: liver and red gastrocnemius muscle (‘skeletal muscle' hereafter), and epididymal fat was rapidly dissected, freeze-clamped with aluminum tongs pre-cooled in liquid nitrogen and stored at −70 °C awaiting analysis.
Calculations
Whole-body clearance of 14C-palmitate (MCRp), tissue-clearance rates of 14C-palmitate (Kf) and the rate of appearance of plasma NEFA (Ra) were calculated from tissue content and arterial plasma profile of radiolabelled tracer as previously described (Oakes et al., 1999, 2001). Because high-fat feeding of rats tends to increase the mass of both the liver and fat, NEFA clearance into these tissues was expressed per whole liver and adipose depot, respectively, to reflect their influence on whole-body uptake.
Determination of plasma and tissue tracer concentrations
The method of isolation of 14C-palmitate from total 14C plasma radioactivity has been described previously (Oakes et al., 1999). Briefly, an initial acid lipid extraction, using a mixture of isopropanol/hexane/0.5 M H2SO4 (40:10:1), was followed by a polarity separation step, under alkaline conditions. The latter procedure predominantly partitioned esterified fatty acids into a hexane phase, and NEFAs in anionic form into an alcohol phase. Small corrections (<10%), based on separation of NEFA and esterified fatty acid standards, were applied for incomplete partitioning of tracer.
Tissue samples were homogenized in chloroform–methanol (2:1) using a glass hand-held homogenizer. An aliquot of this homogenate was taken to determine the total activity of the 14C label.
Activities of 14C in appropriate plasma and tissue fractions were measured on a Beckman LS 6000SC liquid-scintillation counter (Beckman Coulter Inc., Fullerton, CA, USA).
Plasma parameter measurements
Plasma NEFA concentration was determined using Cu-NEFA coextraction-based colorimetric kit (Nanjing Jiancheng Bioengineering Inc., Nanjing, China). Very-low-density lipoprotein (VLDL) and low-density lipoprotein (LDL) were precipitated by phosphotungstic acid/Mg2+ reagent (Demacker et al., 1997), whereas LDL was selectively precipitated by polyvinylsulphate/polyethyleneglycol methyl ether (Demacker et al., 1984). The content of TG in plasma and corresponding supernatant containing high-density lipoprotein or high-density lipoprotein plus VLDL was determined enzymatically with commercial assay kits (GPO-PAP; Dongou Bioengineering Co. Ltd, Wenzhou, China), thus the content of TG in VLDL, LDL and high-density lipoprotein could be calculated. Plasma glucose was determined using a glucose analyser (GT-1640; ARKRAY Inc., Kyoto, Japan). Plasma insulin was determined by radioimmunoassay using commercial kits for the rat (Beijing Atom HighTech Co. Ltd).
Lipoprotein analysis
The distribution of lipoproteins in serum from individual rats was analysed by agarose–starch gel electrophoresis (Chalvardjian, 1970). Briefly, 10 μL of samples were separated by electrophoresis for about 40 min at a constant voltage of 140 V in a glycine-sodium barbitone buffer, pH 8.6. The wet gels were put into five volumes of 1.5% picric acid in 12% acetic acid for protein precipitation. After washing and drying, staining began in the mixed solution of Fat Red 7B and Oil Red O overnight. The gels were scanned using the Bio-Rad Gel-Doc XR system, and images were analysed by Quantity One (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Tissue lipid content
Triglyceride and DAG in liver and muscle were extracted and separated using the method of Kaluzny et al. (1985). Briefly, the lipid extract of tissue samples was separated on aminopropyl solid-phase extraction cartridges (LC-NH2, 500 mg; Supelco, Bellefonte, PA, USA) using five eluants in sequence: (1) chloroform–isopropanol 2:1; (2) hexane; (3) 1% diethyl ether, 10% methylene chloride in hexane; (4) 5% ethyl acetate in hexane; and (5) 15% ethyl acetate in hexane. Samples of TG and DAG, eluted by eluant (3) and (5), respectively, were evaporated to dryness under N2 and reconstituted with isopropanol for assay using TG testing kit.
Long-chain acyl CoA in liver and muscle was extracted according to the method of Hegarty et al., 2004. Briefly, tissue samples (50 mg) were homogenized in 10% trichloroacetic acid and washed with diethyl ether and water in sequence. Then, LCACoA in tissue was hydrolysed by KOH in the presence of dithiothreitol. The liberated CoA was measured by HPLC (Hosokawa et al., 1989). LCACoA content was calculated using the peak area and comparison to CoA standards (Sigma-Aldrich, Shanghai, China).
β-oxidation and CPT-1 activity
For quantification of β-oxidation in liver and skeletal muscle homogenates, 14C-labelled acid-soluble metabolites and CO2 produced from 14C-palmitate were counted as described previously (Doha et al., 2005).
Carnitine palmitoyl transferase-1 activity in liver and skeletal muscle was measured with isolated mitochondria with u.v. spectrophotometry described by Bieber et al. (1972). Proteins were determined by the procedure of Lowry et al. (1951), with BSA as standard.
Intravenous fat tolerance test and lipoprotein lipase/hepatic lipase activity test in tissues
According to the method of Chakrabarti et al. (2003), 20% intralipid was administered i.v. (5 mL kg−1), and plasma TG levels were measured at 0, 1, 10, 30, 60 and 120 min after injection.
The enzyme extracts from liver, muscle and epididymal fat were prepared according to the method of Iverius and Lindqvist (1986), and the LPL/hepatic lipase (HL) activity was measured with commercial kits (Nanjing Jiancheng Bioengineering Inc.).
RNA isolation and real-time detection PCR
For the determination of mRNA expression levels of PPARα, FATP, CD36/FAT, ACS, L-CPT-1, ACO, LPL, apoCIII in liver, total RNA isolation, cDNA synthesis and relative quantification of target gene mRNA compared with the housekeeping gene (glyceraldehyde-3-phosphate dehydrogenase) mRNA was determined by real-time PCR as described previously (Duran-Sandoval et al., 2004; Achouri et al., 2005; Ringseis et al., 2007). Relative quantification was performed using the ΔΔCt method (Livak and Schmittgen, 2001). Relative-expression ratios are expressed as fold changes of mRNA abundance in the crocetin group compared with the control group.
Statistical analysis
All results are presented as means±s.e.mean. Comparisons of two groups were performed by Student's t-test. Comparison of three groups was performed by one-way ANOVA incorporating a Fisher's protected least-significant-difference post hoc test. Group sizes ranged from five to seven rats unless otherwise specified. P<0.05 was considered statistically significant for all comparisons.
Results
General parameters
Crocetin administration improved insulin action, which had been impaired by high-fat diets, shown as an increase in the glucose infusion rate necessary to maintain euglycaemia under hyperinsulinaemic conditions (Table 1). This beneficial effect of crocetin on insulin sensitivity was associated with a marked reduction in basal plasma insulin levels (Table 1). This last variable, a marker to judge insulin resistance, significantly increased in the control group. The normoglycaemic plasma glucose levels were unaffected (Table 1). As shown in Table 2, crocetin did not affect body weight, but did substantially decrease the ratio of fat mass to body weight. Although epididymal and mesenteric fat, classified as visceral fat, was significantly decreased, retroperitoneal fat and the subcutaneous fat, inguinal fat, did not change after crocetin treatment. The finding that the ratio of liver weight to body weight increased in the crocetin group but that the liver-weight size did not change suggests that the altered weight ratio was mostly due to the decreased weight of fat. Food intake showed no significant difference between control and crocetin groups (25.7±1.5 vs 25.2±1.4 g day−1 per rat; P>0.05), which excludes the possibility that crocetin reduces fat mass by inhibiting feeding.
Table 1.
Effect of crocetin treatment on insulin sensitivity, plasma parameters and whole-body NEFA metabolism
| Normal | Control | Crocetin | |
|---|---|---|---|
| GIR (mg min−1 kg−1) | 29.39±3.52 | 17.37±2.60†† | 21.80±3.05* |
| Insulin (mU L−1) | 25.56±3.23 | 36.94±4.89†† | 28.99±5.93* |
| Glucose (mM) | 6.15±1.42 | 7.61±1.62 | 6.95±1.59 |
| TG (mM) | 0.74±0.13 | 1.18±0.36† | 0.79±0.11* |
| VLDL-TG (mM) | 0.11±0.06 | 0.57±0.27†† | 0.23±0.07* |
| LDL-TG (mM) | 0.17±0.06 | 0.32±0.11† | 0.37±0.11 |
| HDL-TG (mM) | 0.46±0.10 | 0.28±0.10† | 0.19±0.08 |
| NEFA (mM) | 0.37±0.10 | 0.35±0.09 | 0.29±0.08 |
| MCRp(mL min−1 per 100 g) | 7.91±0.73 | 9.77±0.71†† | 9.99±0.99 |
| Ra (μmol min−1) | 9.31±1.64 | 12.79±1.76† | 10.48±1.85 |
Abbreviations: GIR, glucose-infusion rate; HDL, high-density lipoprotein; LPL, lipoprotein lipase; MCRp, whole-body NEFA clearance; NEFA, non-esterified fatty acid; Ra, rate of appearance of plasma NEFA; TG, triglyceride; VLDL, very-low-density lipoprotein.
Data are means±s.e.mean. n=6–7 per group. †P<0.05, ††P<0.01 vs normal; *P<0.05 vs control (ANOVA).
Table 2.
Effect of crocetin on the body weight and the weight ratio of liver and adipose tissue to body
| Normal | Control | Crocetin | |
|---|---|---|---|
| Body weight (g) | 391.9±38.7 | 417.7±49.5 | 400.6±31.9 |
| Tissue weight (% of body weight) | |||
| Liver | 3.78±0.14 | 3.62±0.23 | 3.91±0.13* |
| Epididymal fat | 1.14±0.21 | 1.95±0.34†† | 1.51±0.30* |
| Mesenteric fat | 0.73±0.18 | 1.22±0.18†† | 0.95±0.18* |
| Retroperitoneal fat | 2.01±0.3 | 2.47±0.53 | 1.98±0.25 |
| Inguinal fat | 1.73±0.47 | 2.15±0.18 | 2.06±0.40 |
Data are means±s.e.mean. n=6–7 per group. ††P<0.01 vs normal, *P<0.05 vs control (ANOVA).
Plasma lipids
In the crocetin group, circulating TG level was significantly reduced but plasma NEFA concentration had no obvious change compared with control rats (Table 1). By chemical precipitation, we found that crocetin significantly reduced the TG content in VLDL. To assess whether the decrease of VLDL-TG played any role in the reduction in plasma TG concentration, the overall relationships between VLDL-TG and plasma TG concentrations were tested. The result showed a significant positive relationship between VLDL-TG and plasma TG concentration (r2=0.8558; P<0.01). Thus, in crocetin-treated rats, the reduction of TG content in VLDL accounted for the decrease in plasma TG level.
Tissue-specific and whole-body NEFA metabolism
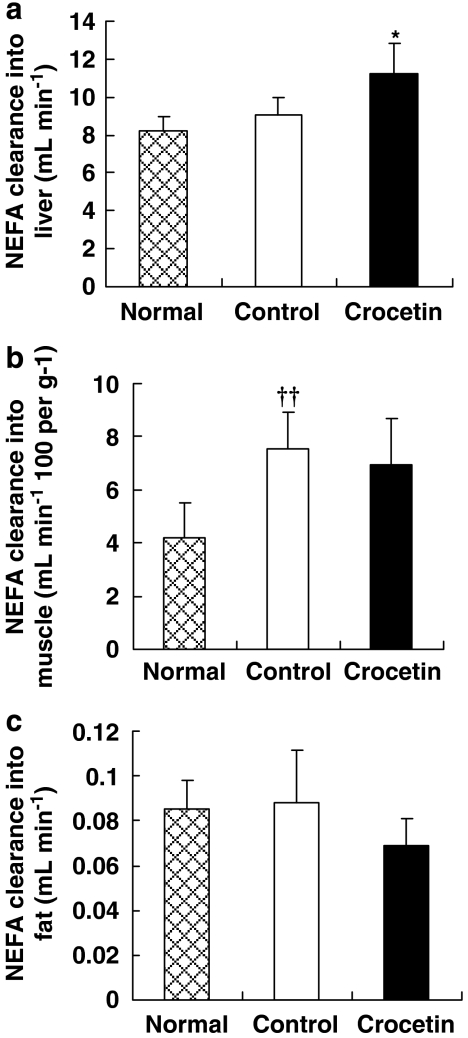
The intrinsic ability of a tissue to take up a circulating substrate is described by the so-called tissue clearance. This parameter depends on factors such as substrate transporters in the plasma membrane and substrate sequestration due to intracellular enzyme activity. NEFA clearance (Kf) into liver, epididymal fat and skeletal muscle was measured in this study using the accumulation of fatty acid tracer, 14C-palmitate, under the inhibition of CPT-1 by etomoxir. Crocetin-treated animals showed a substantially increased NEFA clearance into liver compared with untreated animals (Figure 2a), accompanied by a decreased tendency in NEFA clearance into muscle and fat (Figures 2b and c). This may explain why the whole-body NEFA clearance (MCRp) did not change after crocetin treatment (Table 1). Whole-body NEFA mobilization (Ra) was estimated based on steady-state tracer dilution using a constant infusion of 14C-palmitate. High-fat-fed rats showed substantially elevated Ra than the rats fed with standard diet (Table 1). Crocetin treatment had no effect on Ra, although there was a nonsignificant tendency for Ra to be decreased in the crocetin group.
Figure 2.
Effect of crocetin treatment of rats on a high-fat diet for 6 weeks on NEFA clearance (Kf) into (a) liver, (b) muscle and (c) fat. Normal, standard diet; control, high-fat diet alone; crocetin, high-fat diet+crocetin. Data are means±s.e.mean. n=6–7 per group. ††P<0.01 vs normal; *P<0.05 vs control (ANOVA). NEFA, non-esterified fatty acid.
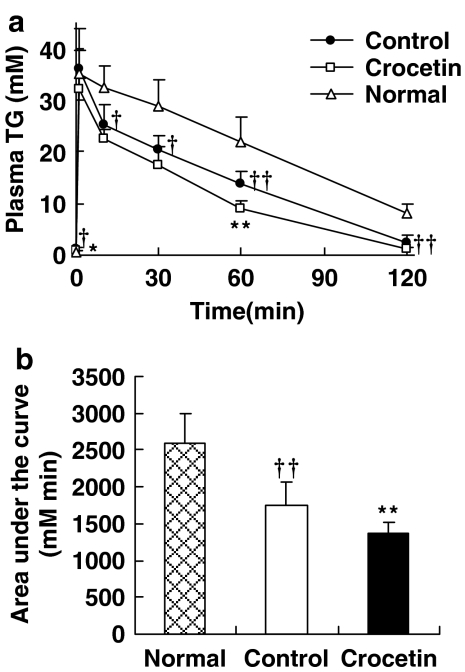
Triglyceride clearance
Rats on a high-fat diet, treated with crocetin, showed a significant enhancement (22%) in TG clearance (Figure 3) compared with control rats when challenged i.v. with a fat emulsion (20% intralipid).
Figure 3.
Effect of crocetin on plasma TG clearance in rats on a high-fat diet. (a) The concentration of TG in plasma at varied time; (b) The area under the cure, Animals fed with standard diet, high-fat diet or high-fat diet plus crocetin were injected with 20% intralipid, and then blood was collected at various times (as shown) for TG assay. Normal, standard diet; control, high-fat diet alone; crocetin, high-fat diet+crocetin. Data are mean±s.e.mean. n=5–6 per group. †P<0.05, ††P<0.01 vs normal; *P<0.05, **P<0.01 vs control (ANOVA). TG, triglyceride.
Composition of serum lipoproteins
Separation of the different lipoprotein fractions by electrophoresis followed by lipostaining indicated a decrease in VLDL accompanied by a rise in LDL, for the ratio of LDL to VLDL was markedly increased by crocetin treatment (0.45±0.11 vs 0.26±0.02 in control group; P<0.05).
LPL/HL activity in tissues
Results in vitro of LPL activity in adipose and muscle and HL activity in liver showed no significant difference between crocetin and control groups, whereas a pronounced rise in hepatic LPL activity was observed after crocetin treatment, which may account for the increased LPL activity in vivo reflected by the i.v. fat tolerance test (Table 3).
Table 3.
Effect of crocetin on lipase (LPL and HL) activity in tissues
| NEFA production (μmol h−1 g−1) | Normal | Control | Crocetin |
|---|---|---|---|
| Liver-LPL | 51.62±7.75 | 59.78±6.64 | 72.08±5.58** |
| Liver-HL | 62.40±8.52 | 71.04±15.98 | 82.89±17.19 |
| Muscle-LPL | 42.77±4.91 | 51.04±5.73† | 46.12±11.30 |
| Fat-LPL | 48.56±3.44 | 44.60±2.18† | 45.02±6.61 |
Abbreviations: HL, hepatic lipase; LPL, lipoprotein lipase.
Data are means±s.e.mean. n=6–7 per group. †P<0.05 vs normal; **P<0.01 vs control (ANOVA).
β-oxidation and CPT-1 activity in liver and muscle
As seen in Table 4, rats on a high-fat diet showed no obvious change in NEFA β-oxidation rate or mitochondrial CPT-1 activity in liver and skeletal muscle compared with rats fed with standard diet. In the crocetin group, hepatic β-oxidation rate together with CPT-1 activity was significantly higher than that in control rats, but a similar increase was not observed in skeletal muscle.
Table 4.
Effect of crocetin on (a) β-oxidation and (b) CPT-1 activity in liver and muscle
| Normal | Control | Crocetin | |
|---|---|---|---|
| (a) β-oxidation | |||
| Palmitate-oxidation rate (μmol h−1 g−1) | |||
| Liver | 3.49±0.54 | 3.25±0.49 | 4.00±0.59* |
| Muscle | 0.77±0.14 | 0.73±0.17 | 0.80±0.15 |
| (b) CPT-1 activity | |||
| CoA production (nmol min−1 per mg protein) | |||
| Liver | 10.27±1.08 | 9.85±1.55 | 13.46±2.11** |
| Muscle | 4.11±1.06 | 3.96±1.10 | 4.50±1.28 |
Abbreviations: CPT-1, carnitine palmitoyl transferase-1; NEFA, non-esterified fatty acid.
Data are means±s.e.mean. n=6–7 per group.
*P<0.05, **P<0.01 vs control (ANOVA).
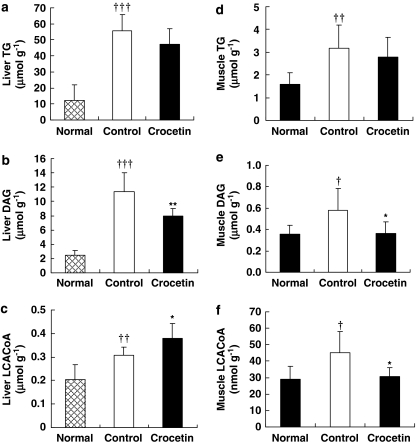
Tissue lipid content
High-fat diet raised the hepatic (Figures 4a–c) and muscular (Figure 4d–f) content of TG, DAG and LCACoA. Crocetin treatment normalized DAG and LCACoA levels but did not significantly lower TG in skeletal muscle (Figures 4d–f). In liver, the accumulation of DAG (Figure 4b) was reduced by crocetin but no obvious change was seen on TG content (Figure 4a). In contrast to the effects in skeletal muscle, crocetin increased the hepatic LCACoA content of high-fat-fed rats (Figure 4c).
Figure 4.
Tissue lipid contents of liver (a–c) and skeletal muscle (d–f). Normal, standard diet; control, high-fat diet alone; crocetin, high-fat diet+crocetin. Data are means±s.e.mean. n=6–9 per group. †P<0.05, ††P<0.01, †††P<0.001 vs normal; *P<0.05, **P<0.01 vs control (ANOVA).
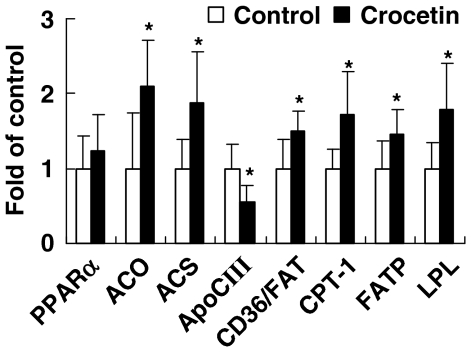
Relative mRNA level in the liver of high-fat-fed rats
Relative mRNA levels of ACO, ACS, apoCIII, CD36/FAT, CPT-1, FATP and LPL in liver of crocetin-treated rats were altered significantly compared with control values (P<0.05; Figure 5). But PPARα expression did not show significant difference between two groups.
Figure 5.
Effect of crocetin on hepatic abundance of mRNA from genes involved in oxidation and uptake of NEFA and TG, PPARα, ACO, ACS, apoCIII, CD36/FAT, CPT-1, FATP and LPL in rats on a high-fat diet. Control, high-fat diet alone; crocetin, high-fat diet+crocetin. Bars represent means±s.e.mean and are expressed as fold changes of mRNA abundance in the crocetin group compared with the control group. n=5–7 per group. *P<0.05 vs control (t-test). ACO, acyl CoA oxidase; ACS, acyl CoA synthetase; ApoCIII, apolipoprotein CIII; CPT-1, carnitine palmitoyl transferase-1; FATP, fatty acid transport protein; LPL, lipoprotein lipase; NEFA, non-esterified fatty acid; PPARα, peroxisome proliferator-activated receptor-α; TG, triglyceride.
Discussion and conclusions
In this study, we have shown, for the first time, that crocetin, a natural carotenoid, is an effective insulin-sensitizing agent in a non-genetic rat model of insulin resistance induced by high-fat diet. We have also provided evidence that the effect of crocetin in TG and NEFA metabolism plays a causative role in the amelioration of insulin resistance. The clearance of plasma TG and NEFA to liver was significantly elevated after crocetin treatment, and this impact may reduce the availability of lipids to skeletal muscle as the largest peripheral insulin-sensitive tissue.
The hypotriglyceridaemic action of crocetin may be explained by the increased plasma TG clearance, assayed by the i.v. fat tolerance test, which reflects the elevated total lipolytic activity of LPL and HL. LPL is the key enzyme in the hydrolysis of TGs in chylomicrons and VLDL (Applebaum-Bowden, 1995), and HL is known to be important in the final steps in the lipolytic conversion of VLDL to LDL by hydrolysis of the LDL TG (Connelly, 1999). The increased lipolytic activity in crocetin-treated rats induced more rapid stripping of TG from VLDL particles, leading to lowered TG content in VLDL and an accelerated conversion of VLDL to TG-poor remnants, LDL. Study on LPL/HL activity in tissues indicated that this increase in total lipolytic activity was mainly due to the elevation in hepatic LPL activity. Changes in LPL activity in different tissues determine, in part, not only plasma TG levels, but also the uptake of TG by different tissues (Jansen et al., 1998). Thus, despite the lack of direct kinetic evidence to demonstrate the turnover of plasma TG, it can be deduced that liver, with the increase in LPL activity after crocetin treatment, would take up more plasma TG.
In basal conditions, neither whole-body NEFA clearance nor whole-body NEFA mobilization were obviously changed by crocetin treatment, which helped to explain the nonsignificant variations in plasma NEFA level. However, tissue-specific clearance of NEFA was redistributed after crocetin treatment, that is NEFA clearance into liver significantly increased, accompanied by a tendency towards a decreased clearance rate in skeletal muscle and adipose tissue. This may suggest that liver had taken up the part of NEFA that should have been transported into skeletal muscle and adipose tissue by the increased capacity for NEFA uptake after crocetin treatment.
To illuminate the fate of TG and NEFA transported to liver, hepatic β-oxidation, as the formation of acid-soluble metabolites and CO2 production, was measured, as well as the activity of CPT-1, the rate-limiting enzyme in NEFA mitochondrial β-oxidation. The elevation in these two parameters suggests that more of the NEFA and TG taken up into the liver in the crocetin group were subject to oxidation.
Elevation in hepatic β-oxidation may surpass the elevation in hepatic uptake of NEFA and TG, as crocetin reduced the hepatic DAG content and demonstrated a decreased trend in TG. Unexpectedly, LCACoA content in liver was increased by crocetin treatment. This increase could result from expanded peroxisome population as shown by elevated ACO expression (Moody et al., 1991). But as long as LCACoAs are confined within the peroxisomes, these lipid moieties might not be able to interfere with insulin signalling or carbohydrate metabolism in cytosol (Hegarty et al., 2004).
Although crocetin did not significantly reduce the accumulation of TG in skeletal muscle of high-fat-fed rats, a substantial decrement was observed in the content of DAG and LCACoA. Intramuscular TG is often used as a marker of insulin resistance, but there is no demonstration that TG per se interferes with insulin action. In contrast, DAG and LCACoA have been shown to be important molecules that interfere with various aspects of insulin signalling and carbohydrate metabolism and are likely to contribute to the detrimental effects of intramuscular lipid accumulation on insulin action (Shulman, 2000; Cooney et al., 2002). Besides, based on the primary importance of skeletal muscle in insulin-stimulated glucose disposal (it accounts for more than 80% of whole-body glucose disposal) (DeFronzo et al., 1981), the reduction of these lipid intermediates is believed to contribute greatly to the amelioration of whole-body insulin sensitivity. In fact, insulin resistance induced by high-fat diet is associated with an increase in NEFA clearance into skeletal muscle (Hegarty et al., 2002), but crocetin failed to correct this effect. As well, crocetin exerted no effect on muscular NEFA β-oxidation and LPL activity in skeletal muscle. Thus, the reduction of muscular lipid content by crocetin is presumably due to the lowered availability of TG. It may suggest that the main target organ of crocetin is liver, and the improvement of lipid profiles in skeletal muscle is a side effect of these hepatic effects of crocetin.
PPARα, once activated, would heterodimerize with the retinoid X receptor and bind to peroxisome proliferator response elements in the promoters of genes including LPL (Schoonjans et al., 1996), ACO (Kliewer et al., 1992), ACS (Schoonjans et al., 1995) and CPT-1 (Louet et al., 2001), the transcription of which would be activated by the complex. The gene for apoCIII is suppressed by PPARα activation due to competition on the same response element between PPARα–retinoid X receptor heterodimers and the liver-enriched transcriptional activator, hepatocyte nuclear factor-4 (Hertz et al., 1995). Although peroxisome proliferator response elements have not been identified in CD36/FAT or FATP genes to date, study on PPARα-null mouse indicates that PPARα has an obligatory role in induction of CD36/FAT and FATP mRNAs in liver (Motojima et al., 1998). On the basis of the organ selectivity of crocetin, only the hepatic expression of those PPARα-related genes was tested. The result showed that crocetin treatment increased hepatic mRNA level of LPL and reduced that of apoCIII, the apolipoprotein that inhibits the hydrolysing action of LPL, which is consistent with the elevated LPL activity in liver. The enhancement of hepatic NEFA uptake by crocetin fits well with the upregulation of FATP, CD36/FAT and ACS, the genes involved in NEFA transport (Martin et al., 1997; Motojima et al., 1998). The fact that crocetin raised mRNA level of hepatic CPT-1 may account for the increased β-oxidation in liver. Although crocetin did not directly enhance the expression of PPARα, it did increase the mRNA level of hepatic ACO, the well-known marker of PPARα activation. Furthermore, like typical PPARα activators, crocetin reduced the mass of visceral fat. All this evidence suggests that crocetin may act as a PPARα agonist.
In summary, crocetin enhanced the ability of the liver to take up and oxidize NEFA and TG by modulating the PPARα-related genes in liver, leading to the redistribution of tissue-specific NEFA clearance and depressed TG availability. Consequently, the hepatic and skeletal muscle accumulation of detrimental lipids decreased, with consequent improvement of insulin resistance induced by high-fat diets in rats.
Acknowledgments
We thank Professor Boping Ye for providing real-time PCR system.
Abbreviations
- ACO
acyl CoA oxidase
- ACS
acyl CoA synthetase
- ApoCIII
apolipoprotein CIII
- CD36/FAT
CD36/fatty acid translocase
- CPT-1
carnitine palmitoyl transferase-1
- FATP
fatty acid transport protein
- HL
hepatic lipase
- LCACoA
long-chain acyl CoA
- LDL
low-density lipoprotein
- LPL
lipoprotein lipase
- NEFA
non-esterified fatty acid
- PPAR
peroxisome proliferator-activated receptor
- TG
triglyceride
- VLDL
very-low-density lipoprotein
Conflict of interest
The authors state no conflict of interest.
References
- Achouri Y, Hegarty BD, Allanic D. Long chain fatty acyl CoA synthetase 5 expression is induced by insulin and glucose: involvement of sterol regulatory element-binding protein-1c. Biochimie. 2005;87:1149–1155. doi: 10.1016/j.biochi.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Applebaum-Bowden D. Lipases and lecithin: cholesterol acyltransferase in the control of lipoprotein metabolism. Curr Opin Lipidol. 1995;6:130–135. doi: 10.1097/00041433-199506000-00004. [DOI] [PubMed] [Google Scholar]
- Bieber LL, Abraham T, Helmarath T. A rapid spectrophotometric assay for carnitine palmitoyltransferase. Anal Biochem. 1972;50:509–518. doi: 10.1016/0003-2697(72)90061-9. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R, Vikramadithyan RK, Misra P. Ragaglitazar: a novel PPARα and PPARγ agonist with potent lipid lowering and insulin-sensitizing efficacy in animal models. Br J Pharmacol. 2003;140:527–537. doi: 10.1038/sj.bjp.0705463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalvardjian A. Agarose–starch gel electrophoresis of rat serum lipoproteins. J Lipid Res. 1970;12:156–169. [PubMed] [Google Scholar]
- Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys. 2003;419:101–109. doi: 10.1016/j.abb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Clark PW, Jenkins AB, Kraegen EW. Pentobarbital reduces basal liver glucose output and its insulin suppression in rats. Am J Physiol. 1990;258:E701–E707. doi: 10.1152/ajpendo.1990.258.4.E701. [DOI] [PubMed] [Google Scholar]
- Connelly PW. The role of hepatic lipase in lipoprotein metabolism. Clin Chim Acta. 1999;286:243–255. doi: 10.1016/s0009-8981(99)00105-9. [DOI] [PubMed] [Google Scholar]
- Cooney GJ, Thompson AL, Furler SM, Ye J EW K. Muscle long-chain acyl CoA esters and insulin resistance. Ann NY Acad Sci. 2002;967:196–207. doi: 10.1111/j.1749-6632.2002.tb04276.x. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- Demacker PN, Hessels M, Toenhake-Dijkstra H, Baadenhuijsen H. Precipitation methods for high-density lipoprotein cholesterol measurement compared and final evaluation under routine operating conditions of a method with a low sample-to-reagent ratio. Clin Chem. 1997;43:663–668. [PubMed] [Google Scholar]
- Demacker PN, Hljmans AG, Brenninkmeljer BJ, Jansen AP, Laar AV. Five Methods for determining low-density lipoprotein cholesterol compared. Clin Chem. 1984;30:1797–1800. [PubMed] [Google Scholar]
- Deng Y, Qian Z, Tang F. Effects of crocetin on experimental atherosclerosis in rats. Chin Tradition Herbal Drugs. 2004;35:777–781. [Google Scholar]
- Doha KO, Kima YW, Parka SY, Leea SK, Parkb JS, Kim JY. Interrelation between long-chain fatty acid oxidation rate and carnitine palmitoyltransferase 1 activity with different isoforms in rat tissues. Life Sci. 2005;77:435–443. doi: 10.1016/j.lfs.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Duran-Sandoval D, Mautino G, Martin G, Percevault F, Barbier O, Fruchart JC, et al. Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes. 2004;53:890–898. doi: 10.2337/diabetes.53.4.890. [DOI] [PubMed] [Google Scholar]
- Escher P, Wahli W. Peroxisome proliferator-activated receptors: insight into multiple cellular functions. Mutat Res. 2000;448:121–138. doi: 10.1016/s0027-5107(99)00231-6. [DOI] [PubMed] [Google Scholar]
- Guerre-Millo M, Gervois P, Raspe E. Peroxisome proliferator-activated receptor α activators improve insulin sensitivity and reduce adiposity. J Biol Chem. 2000;275:16638–16642. doi: 10.1074/jbc.275.22.16638. [DOI] [PubMed] [Google Scholar]
- He S, Qian Z, Wen N, Tang F, Xu G, Zhou C. Influence of Crocetin on experimental atherosclerosis in hyperlipidaemic-diet quails. Eur J Pharmacol. 2007;554:191–195. doi: 10.1016/j.ejphar.2006.09.071. [DOI] [PubMed] [Google Scholar]
- Hegarty BD, Cooney GJ, Kraegen EW, Furler SM. Increased efficiency of fatty acid uptake contributes to lipid accumulation in skeletal muscle of high fat-fed insulin-resistant rats. Diabetes. 2002;51:1477–1484. doi: 10.2337/diabetes.51.5.1477. [DOI] [PubMed] [Google Scholar]
- Hegarty BD, Furler SM, Oakes ND, Kraegen EW, Cooney GJ. Peroxisome proliferator-activated receptor (PPAR) activation induces tissue-specific effects on fatty acid uptake and metabolism in vivo—a study using the novel PPARα/γ agonist tesaglitazar. Endocrinology. 2004;145:3158–3164. doi: 10.1210/en.2004-0260. [DOI] [PubMed] [Google Scholar]
- Hertz R, Bishara-Shieban J, Bar-Tana J. Mode of action of peroxisome proliferators as hypolipidaemic drugs, suppression of apolipoprotein C-III. J Biol Chem. 1995;270:13470–13475. doi: 10.1074/jbc.270.22.13470. [DOI] [PubMed] [Google Scholar]
- Hosokawa Y, Shimomura Y, Harris RA. Determination of short-chain acyl CoA esters by high-performance liquid chromatography. Anal Biochem. 1989;153:45–49. doi: 10.1016/0003-2697(86)90058-8. [DOI] [PubMed] [Google Scholar]
- Iverius PH, Lindqvist AMO. Preparation, characterization and measurement of lipoprotein lipase. Methods Enzymol. 1986;129:691–704. doi: 10.1016/0076-6879(86)29099-0. [DOI] [PubMed] [Google Scholar]
- Jansen H, Breedveld B, Schoonderwoerd K. Role of lipoprotein lipases in postprandial lipid metabolism. Atherosclerosis. 1998;141 Suppl 1:S31–S34. doi: 10.1016/s0021-9150(98)00214-7. [DOI] [PubMed] [Google Scholar]
- Kaluzny MA, Duncan LA, Merritt MV, Eppse DE. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J Lipid Res. 1985;26:135–140. [PubMed] [Google Scholar]
- Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358:771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraegen EW, James DE, Bennett SP, Chisholm DJ. In vivo insulin sensitivity in the rat determined by euglycaemic clamp. Am J Physiol. 1983;245:E1–E7. doi: 10.1152/ajpendo.1983.245.1.E1. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Louet JF, Chatelain F, Decaux JF, Park EA, Kohl C, Pineau T, et al. Long-chain fatty acids regulate liver carnitine palmitoyltransferase 1 gene (L-CPT 1) expression through a peroxisome-proliferator-activated receptor α(PPARα)-independent pathway. Biochem J. 2001;354:189–197. doi: 10.1042/0264-6021:3540189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARα and PPARγ activators. J Biol Chem. 1997;272:28210–28217. doi: 10.1074/jbc.272.45.28210. [DOI] [PubMed] [Google Scholar]
- Moody DE, Reddy JK, Lake BG, Popp JA, Reese DH. Peroxisome proliferation and non-genotoxic carcinogenesis: commentary on a symposium. Fund Appl Toxicol. 1991;6:233–248. doi: 10.1016/0272-0590(91)90108-g. [DOI] [PubMed] [Google Scholar]
- Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor α and γ activators in a tissue- and inducer specific manner. J Biol Chem. 1998;273:16710–16714. doi: 10.1074/jbc.273.27.16710. [DOI] [PubMed] [Google Scholar]
- Oakes ND, Camilleri S, Furler SM, Chisholm DJ, Kraegen EW. The insulin sensitizer, BRL 49653, reduces systemic fatty acid supply and utilization and tissue lipid availability in the rat. Metabolism. 1997;46:935–942. doi: 10.1016/s0026-0495(97)90083-4. [DOI] [PubMed] [Google Scholar]
- Oakes ND, Kjellstedt A, Forsberg GB. Development and initial evaluation of a novel method for assessing tissue-specific plasma free fatty acid utilization in vivo using (R)-2-bromopalmitate tracer. J Lipid Res. 1999;40:1155–1169. [PubMed] [Google Scholar]
- Oakes ND, Thalén PG, Jacinto SM, Ljung B. Thiazolidinediones increase plasma-adipose tissue NEFA exchange capacity and enhance insulin-mediated control of systemic NEFA availability. Diabetes. 2001;50:1158–1165. doi: 10.2337/diabetes.50.5.1158. [DOI] [PubMed] [Google Scholar]
- Ringseis R, Muschick A, Eder K. Dietary oxidized fat prevents ethanolinduced triacylglycerol accumulation and increases expression of PPARα target genes in rat liver. J Nutr. 2007;137:77–83. doi: 10.1093/jn/137.1.77. [DOI] [PubMed] [Google Scholar]
- Schmitz PC, Browne CL, Oakes ND. Alterations in the expression and cellular localization of protein kinase C isozymes epsilon and theta are associated with insulin resistance in skeletal muscle of the high-fat-fed rat. Diabetes. 1997;46:169–178. doi: 10.2337/diab.46.2.169. [DOI] [PubMed] [Google Scholar]
- Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, Heyman RA, Briggs M, Deeb S, et al. PPARα and PPARγ activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996;15:5336–5348. [PMC free article] [PubMed] [Google Scholar]
- Schoonjans K, Watanabe M, Suzuki H, Mahfoudi A, Krey G, Wahli W, et al. Induction of the acyl-coenzyme A synthetase gene by fibrates and fatty acids is mediated by a peroxisome proliferator response element in the C promoter. J Biol Chem. 1995;270:19269–19276. doi: 10.1074/jbc.270.33.19269. [DOI] [PubMed] [Google Scholar]
- Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turinsky J, O'Sullivan DM, Bayly BP. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem. 1990;265:16880–16885. [PubMed] [Google Scholar]
- Xi L, Qian Z, Xu G, Zheng S, Sun S, Wen N, et al. Beneficial impact of crocetin, a carotenoid from saffron, on insulin sensitivity in fructose-fed rats. J Nutr Biochem. 2007;18:64–72. doi: 10.1016/j.jnutbio.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Ye JM, Doyle PJ, Iglesias MA, Watson DG, Cooney GJ, Kraegen EW. Peroxisome proliferator-activated receptor (PPAR)-α activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats: comparison with PPAR-γ activation. Diabetes. 2001;50:411–417. doi: 10.2337/diabetes.50.2.411. [DOI] [PubMed] [Google Scholar]