Abstract
Background and purpose:
Allergen-induced airways oedema in actively sensitized rats has been studied earlier by magnetic resonance imaging (MRI). We used MRI to follow the consequences of non-immunological mast cell activation induced by compound 48/80 in the rat lungs in vivo.
Experimental approach:
Male naïve rats were scanned by MRI prior to and at several time points following intratracheal administration of the mast cell secretagogue, compound 48/80. The effects of a range of drugs on the response induced by compound 48/80 were studied.
Key results:
Strong fluid signals were detected by MRI in the lungs at 24 h after compound 48/80, correlating with increased protein concentration and inflammatory cell infiltration in bronchoalveolar lavage, and with perivascular oedema observed histologically. Pharmacological intervention demonstrated that the increase in MRI signal volume induced by compound 48/80 24 h after challenge was blocked by disodium cromoglycate and the glucocorticoid, budesonide. Pretreatment with wortmannin, capsazepine, DNK333 (a dual neurokinin (NK) 1 and NK2 antagonist) or the anti-allergy drug CGS8515, but not indomethacin, resulted in partial inhibition.
Conclusions and implications:
Compound 48/80 induced a complex inflammatory reaction which did not solely involve mast cell degranulation but also activation of sensory nerves and was qualitatively similar to allergen challenge. Changes observed by MRI correlated with decreases in protein concentration in BAL fluid. However, the magnitude of the changes detected was greater using MRI. Our results demonstrate that MRI is a sensitive and efficient tool to assess the effects of drugs on lung inflammation.
Keywords: prostaglandin, leukotriene, cromoglycate, wortmannin, PI3-kinase, capsazepine, imaging, magnetic resonance imaging (MRI), lung, rat
Introduction
Mast cells are regarded as critical players in orchestrating inflammatory and physiological processes (Metcalfe et al., 1997), and are considered central to immediate-type allergic responses (Holgate, 1999). In type-I allergic responses, mast cells are typically activated by crosslinking of FcɛR1 (high-affinity receptor for IgE (immunoglobulin-E)) receptors bound to IgE resulting in degranulation via an intracellular cascade in which phosphorylation by PI-3-kinase (PI-3K) is an obligatory step preceding mast cell degranulation (Yano et al., 1993).
Alternatively, mast cells can be stimulated by a large number of polycationic molecules known as basic secretagogues (Lagunoff et al., 1983). This family of molecules includes positively charged neuropeptides such as SP (substance P), bradykinin, and naturally occurring polyamines and synthetic amines such as compound 48/80, a condensation product of N-methyl-p-methoxyphenethylamine with formaldehyde (Lagunoff et al., 1983). The basic secretagogues stimulate mast cell degranulation through an IgE-independent mechanism. Mast cell degranulation is caused by direct activation of Gi and stimulation of the PI-3K (Shefler et al., 1999), the p42/p44 mitogen-activated protein kinase (Shefler et al., 1999), and the Syk kinase (Shefler and Sagi-Eisenberg, 2001) intracellular pathways.
Compound 48/80, like other basic secretagogues, causes mast cell degranulation via direct activation of Gi proteins (sensitive to Pertussis toxin). Degranulation of mast cells results in the release of stored autacoids, such as histamine and 5-HT, as well as de novo synthesis of arachidonic acid (AA), cytokines and chemokines (Stevens and Austen, 1989). Upon mast cell degranulation, AA is released and metabolized through the COX or the 5-lipoxygenase (5-LO) pathways to yield, respectively, prostaglandins (PGs) and thromboxanes, or leukotrienes (Barnes et al., 1998).
Non-immunological activation of mast cells has been implicated in the inflammatory process observed in asthmatic patients in response to exercise, cold air or hypertonic saline inhalation (Carroll et al., 2002). Recently, Liu et al. (2006) have described a model of non-immunological airway responses in rats using compound 48/80, where early and late-phase responses up to 8 h after treatment were observed by utilizing plethysmography.
The aims of the present work were (i) to investigate non-invasively by magnetic resonance imaging (MRI) the effects in the lung induced by compound 48/80 at time points ranging from 10 min to 96 h after treatment; and (ii) to examine the pharmacological effects of a range of drugs on the compound 48/80-induced response and to compare the magnitude of the changes seen by MRI with those of protein concentration in the bronchoalveolar lavage (BAL) fluid.
Materials and methods
All animal procedures and experiments were carried out with the approval of the Veterinary Authority of the City of Basel (license number 567).
Animals
Male Brown Norway (BN) rats (n=142), weighing 270–300 g, were supplied by IFFA CREDO (L'Arbresle, France). Animals were kept at an ambient temperature of 22±2 °C under a 12-h normal phase light–dark cycle and fed NAFAG pellets (Nahr- und Futtermittel AG, Gossau, Switzerland) for at least 1 week before starting the experiments. Drinking water was freely available.
Intratracheal administration of substances
Rats were anaesthetized in a chamber with isoflurane (4%; Abbott, Cham, Switzerland) and then suspended at an angle of approximately 45° by the two front upper teeth using a rubber band attached to a metal support. A curved cannula with a diameter of 1.6 mm attached to a 1-ml syringe was inserted into the trachea and a volume not greater of 0.2 mL was instilled. The animals were allowed to recover from the anaesthesia following i.t. treatment and were re-anaesthetized again for MRI scanning as described in the MRI acquisition protocols section.
Experimental protocol
In an initial experiment, rats were treated with compound 48/80 (1 mg kg−1 i.t; n=20) or vehicle (0.2 mL saline i.t.; n=14), and imaging was performed longitudinally at 10 and 30 min, and at 3, 6, 12, 24, 48, 72 and 96 h after compound 48/80 treatment. At 24 h after treatment, some animals were killed for BAL fluid (compound 48/80 treated: n=10; saline treated: n=8) or histological examination (compound 48/80 treated: n=4; saline treated: n=4).
In subsequent studies aimed at dissecting pharmacologically the response induced in the lungs by compound 48/80, animals (n=108) were treated with a range of drugs either prophylactically or 3 h after administration of the secretagogue. Imaging was performed at baseline (prior to compound 48/80) and at 24 h after administration of compound 48/80. Following imaging at 24 h, animals were killed and BAL fluid taken for analysis. The drug studies are described in detail below.
Inhibition of COX and 5-LO
The dual COX-1 and COX-2 inhibitor, indomethacin (n=5), was administered at a dose of 10 mg kg−1 by gavage (p.o.) 1 h prior to compound 48/80. The dose was chosen based on the experiments of Boughton-Smith et al. (1993), where oral administration of indomethacin inhibited carrageenin-induced rat paw oedema. The selective 5-LO inhibitor, CGS8515 (25 mg kg−1; n=5), was administered orally 2 h prior to compound 48/80. The dose was selected based on the experiments by Ku et al. (1988), in which CGS8515 reduced the formation of leukotrienes in the lung by 90%.
DSCG and wortmannin
Disodium cromoglycate (DSCG; n=10) was given i.t. at a dose of 10 mg kg−1 immediately before challenge with compound 48/80 (pretreatment regimen). Imaging took place 24 h after compound 48/80. In subsequent experiments, DSCG (10 mg kg−1 i.t.; n=8) was administered 3 h after challenge, and imaging performed 21 h later. The dose of DSCG was chosen based on the experiments of Shin et al. (2004), who showed that administration of 10 mg kg−1 DSCG, 10 min prior to compound 48/80 (8 mg kg−1 i.p.), inhibited by 50% the serum histamine levels in mice and that concentrations of 1 and 10 mg mL−1 DSCG were able to inhibit the peritoneal mast cell degranulation induced by compound 48/80 in rats by 60 and 80%, respectively. The PI-3K inhibitor, wortmannin (Ui et al., 1995), was administered i.t. at a dose of 100 μg kg−1 1 h prior to compound 48/80 (n=8). Tigani et al. (2001) demonstrated that this treatment regimen inhibited leukocyte infiltration and activation of eosinophils, 24 h following allergen challenge of actively sensitized rats.
Inhibition of sensory nerves
Capsazepine (3.5 mg kg−1 i.t.; n=5), a transient receptor potential vanilloid (TRPV)-1 antagonist, was administered i.t. 1 h before compound 48/80 challenge. This dose of capsazepine has been used previously to inhibit capsaicin-induced sensory nerve stimulation (Lee and Lundberg, 1994). DNK333 (10 mg kg−1;n=5), a dual neurokinin (NK) 1 and NK2 receptor antagonist, was administered i.p. 1 h before compound 48/80 challenge. In guinea pigs, intraduodenal administration of this dose of DNK333 has been shown to inhibit the SP-induced bronchoconstriction when administered 30 min prior to endotoxin challenge (Lewis et al., 2004).
Combination of wortmannin and DNK333
Animals (n=5) received wortmannin (100 μg kg−1 i.t.) 1 h prior to compound 48/80 followed by DNK333 (10 mg kg−1 i.p.) 30 min later. Imaging was performed 24 h after administration of the secretagogue.
The effects of a corticosteroid
Rats were pretreated 1 h before compound 48/80 with budesonide (3 mg kg−1 i.t.; n=8). In a post-treatment regimen, animals received budesonide (3 mg kg−1 i.t.; n=10) 3 h after compound 48/80. The dose of budesonide was based on the experiments performed by Tigani et al. (2003), in which pretreatment with a dose of 1 mg kg−1 i.t. resulted in a decrease of MRI signals and of BAL fluid parameters of inflammation induced by allergen challenge in actively sensitized BN rats.
Vehicle groups
In addition to the substance groups described above, the following vehicle groups were also studied: (i) saline (0.2 mL i.t.; n=8) administered before compound 48/80 challenge as vehicle for DSCG; (ii) a solution of 4% dimethyl sulphoxide (DMSO) in saline administered i.t. (0.2 mL; n=6) served as a vehicle for wortmannin and budesonide, and was applied 1 h prior to the secretagogue; (iii) a 10% TRIS buffer pH 8.4 was administered by gavage (2 mL kg−1; n=5) 1 h prior to compound 48/80 and was the vehicle for indomethacin; (iv) a solution of 2% 1-methyl-2-pyrrolidone in ‘Neoral placebo' served as the diluent for CGS8515 and was applied by gavage (2 mL kg−1, n=5) 2 h before compound 48/80; (v) 0.2 mL of a 0.5% ethanol solution was administered i.t. (n=5) 1 h before compound 48/80 and served as the vehicle for capsazepine; (vi) a solution of 2% DMSO in Neoral placebo was administered i.p. (2 mL kg−1, n=5) and served as a vehicle for DNK333; (vii) a separate group of rats (n=5) received both 4% DMSO (in saline) i.t. 1 h prior to compound 48/80 and 2% DMSO (in Neoral placebo) i.p. 30 min prior to the secretagogue and served as a control group for the dual administration of wortmannin and DNK333.
‘Neoral placebo' refers to the solution for oral use that is used to prepare a solution of cyclosporin (Neoral). Its components are corn oil-mono-di-triglycerides, polyoxyl 40 hydrogenated castor oil, DL-α-tocopherol and propylene glycol.
MRI acquisition protocols
During image acquisitions, rats were placed in a supine position in a cradle made of Plexiglas. Body temperature was maintained at 37±1 °C using warm air, regulated by a rectal temperature probe (DM 852; Ellab, Copenhagen, Denmark). Anaesthesia was maintained with 2% isoflurane, in a mixture of O2/N2O (2:1), administered via a nose cone. All measurements were performed on spontaneously breathing animals; neither cardiac nor respiratory triggering was applied. As demonstrated earlier, (Beckmann et al., 2001) averaging over several respiratory cycles suppressed artefacts caused by movements of the chest and the heart without the necessity of triggering the data acquisition. Measurements were carried out with a Biospec 47/40 spectrometer (Bruker Medical Systems, Ettlingen, Germany) operating at 4.7 T and equipped with an actively shielded gradient system capable of generating a gradient of 200 mT m−1. The operational software of the scanner was Paravision (version 3.2 Bruker).
For detection of fluid signals in the lungs (Beckmann et al., 2001), a gradient-echo sequence with the following parameters was applied: repetition time, 5.6 ms; echo time, 2.7 ms; flip angle of the excitation pulse, approximately 15°; field-of-view, 6 × 6 cm2; matrix size, 256 × 128; and slice thickness, 1.5 mm. A single slice image was obtained by computing the two-dimensional Fourier transformation of the averaged signal from 45 individual image acquisitions and interpolating the data set to 256 × 256 pixels. There was an interval of 530 ms between individual image acquisitions, resulting in a total acquisition time of 59 s for a single slice. The entire lung was covered by 18 consecutive transverse slices.
The volume of fluid signals was quantified using a semiautomatic segmentation procedure implemented in the IDL (Interactive Data Language Research Systems, Boulder, CO, USA) environment (version 5.1) on a Linux system. The procedure has been extensively described earlier (Beckmann et al., 2001). Segmentation parameters were the same for all analysed images, chosen to select regions corresponding to high intensity signals. Because the fluid signals and those from vessels were of comparable intensities, the volume corresponding to the vessels was assessed on baseline images and then subtracted from the volumes determined on post-treatment images.
BAL fluid analysis
A detailed description of the BAL fluid procedure and the analysis of the parameters of inflammation has been provided earlier (Beckmann et al., 2001; Tigani et al., 2003). Briefly, animals were killed with sodium pentobarbital (250 mg kg−1 i.p.), The lungs were lavaged using three 4 mL aliquots of solution A (HBSS (Hank's balanced salt solution) × 10, 100 mL; 100 mM EDTA, 100 mL; 1 M HEPES, 10 mL; distilled water, 790 mL). The recovered solution was pooled and the total volume of recovered fluid adjusted to 12 mL by addition of solution A. An automatic cell analysing system was utilized (Cobas Helios 5Diff; Hoffmann-La Roche, Axon Lab, Basel, Switzerland) for the assessment of leukocyte numbers and cell differentiation. The level of protein in the BAL fluid supernatants was measured by a photometric assay, based on the reaction of protein with an alkaline copper tartrate solution and Folin reagent.
Histology
Rats were killed by an overdose of pentothal (250 mg kg−1 i.p.) administered after the last MRI session, namely at 24 h after compound 48/80 (n=4) or saline (n=4) challenge. Lungs were inflated with 5 mL of 10% phosphate-buffered neutral formalin via a cannula inserted into the trachea. The lungs were then removed from the thorax and immersed in formalin between 24 and 72 h. The lung tissue was sectioned transversally through the left lobe, the right apical, the right median and the right caudal lobes so as to include the main bronchi as well as the pulmonary alveoli. After processing to paraffin wax, sections were embedded in blocks. Slices of 3 μm thickness were cut from these blocks and then stained using Verhoeff's reaction for quantification of perivascular oedema.
Quantification of perivascular oedema
Five to eight pictures of arteries from each section of the left, right caudal and right median lobes were captured at × 10 magnification on Verhoeff-stained slides. Morphometric analyses were performed with the software ‘Image Access 4.0' (IMAGIC, Glattbrugg, Switzerland). The areas of external oedema and external elastic lamina were manually circumscribed and perivascular oedema was calculated as a percentage of external elastic lamina area. Only vessels with internal diameters between 35 and 150 μm were analysed. Approximately, 25 vessels were assessed per animal. The total oedema determined histologically (sum of the oedema in the left, right caudal and right median lobes) is shown as a percentage.
Statistics
Mean values (±s.e.mean) from n individual animals are presented. A two-way ANOVA was performed for comparisons between different time points. For studies involving drugs, comparisons using the Student's t-test (two tailed) between drug-treated and vehicle-treated groups were made; except for instances where more than two drugs were compared, a one-way ANOVA was performed.
Materials
The suppliers for the drugs used in these experiments were as follows: compound 48/80, CGS8515 and capsazepine (Sigma-Aldrich, Buchs, Switzerland); DSCG, DNK333, indomethacin and wortmannin (Novartis Pharma, Basel, Switzerland); budesonide (Sicor, Milano, Italy); and isoflurane (Abbott).
Results
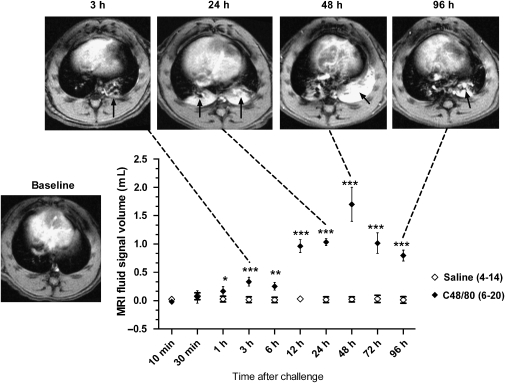
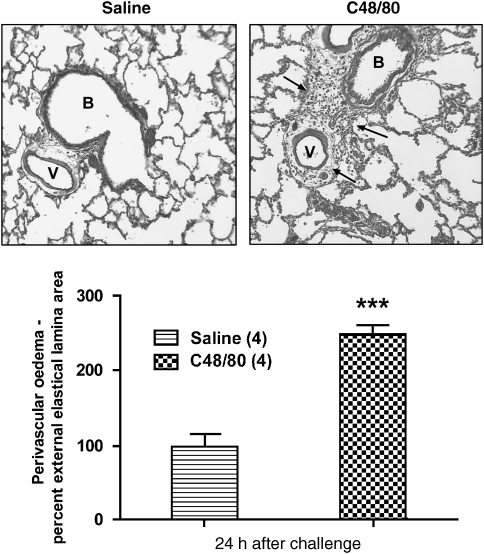
Intratracheal administration of compound 48/80 resulted in the appearance of signals in the lungs detected by MRI as early as 1 h, with significant signals being still apparent 96 h after challenge (Figure 1). Signals peaked at 48 h following compound 48/80. At 24 and 48 h after challenge, the signals were reminiscent in terms of magnitude and intensity of those observed following allergen challenge in actively sensitized BN rats (Beckmann et al., 2001). Administration of vehicle (saline 0.2 mL i.t) did not result in the appearance of signals in the lungs at the time points measured (Figure 1). Analysis of BAL fluid at 24 h following compound 48/80 showed an increase of inflammatory cell recruitment and activation, and a significant increase in protein concentration (Table 1) compared with vehicle-treated animals. Furthermore, histology revealed perivascular oedema following compound 48/80 but not saline (Figure 2).
Figure 1.
Time course following compound 48/80 (1 mg kg−1 i.t.) or saline (0.2 mL i.t.) administration. The number of animals used is shown in parenthesis. The significance levels *P<0.05, **0.001<P<0.01 and ***P<0.001 refer to statistical comparisons between compound 48/80 and vehicle-treated animals for each time point (two-way ANOVA).Transversal sections through the thorax of rats treated with compound 48/80 (1 mg kg−1 i.t.) are shown at baseline and 3, 24, 48 and 96 h after treatment are shown. The black arrows denote fluid signals related to compound 48/80 induced airways oedema as evidenced by post-mortem bronchoalveolar lavage (BAL) fluid and histological analyses.
Table 1.
Inflammatory cell infiltration and activation (mean values±s.e.mean) in the BAL fluid of BN rats treated with vehicle (saline; 0.2 mL i.t.; n=4) or compound 48/80 (1 mg kg−1 i.t; n=10)
| BAL 24 h after treatment | Saline 0.2 mL i.t. (4) | Compound 48/80 1 mg kg−1 i.t. (10) |
|---|---|---|
| Protein (μg mL−1) | 0.21±0.01 | 1.1±0.1*** |
| Eosinophils × 106 | 0.20±0.07 | 2.3±0.4*** |
| EPO (mU mL−1) | 2.7±0.4 | 12±3* |
| Neutrophils × 106 | 0.32±0.03 | 2.0±0.2*** |
| MPO (mU mL−1) | 29±5 | 76±13* |
| Macrophages × 106 | 1.4±0.1 | 3.2±0.4*** |
| Lymphocytes × 106 | 0.16±0.04 | 0.73±0.12*** |
| Total cells × 106 | 2.1±0.1 | 9.0±1.0*** |
Abbreviations: BAL, bronchoalveolar lavage; BN, Brown Norway; EPO, eosinophil peroxidase; MPO, myeloperoxidase.
Animals were killed 24 h after challenge with either substance. The significance levels *P<0.05 and ***P<0.001 refer to comparisons between vehicle- and compound 48/80-treated animals (Student's t-test (two- tailed)). The recovered solution was pooled and the total volume of recovered fluid adjusted to 12 mL by addition of solution A.
Figure 2.
(Top)—Verhoeff-stained slices of rat lungs previously treated with saline (0.2 mL i.t.; left) or compound 48/80 (1 mg kg−1 i.t.; right) and killed 24 h after either challenge. The black arrows denote the presence of oedema around the vessel (V) and the bronchi (B) following compound 48/80 treatment. (Bottom)—Perivascular oedema assessed from the left and right caudal lobes. Values (means±s.e.mean for four rats in each group) represent oedema percent. The oedema value 24 h after compound 48/80 (1 mg kg−1 i.t.) treated rats was significantly greater than saline-treated animals (0.2 mL i.t.; ***P<0.001; Student's t-test (two tailed)).
COX and 5-lipoxygenase inhibitors
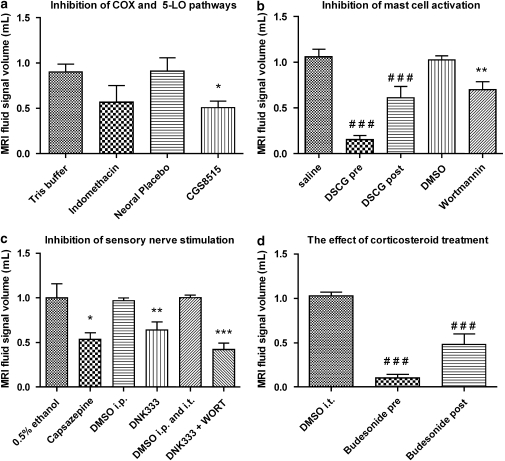
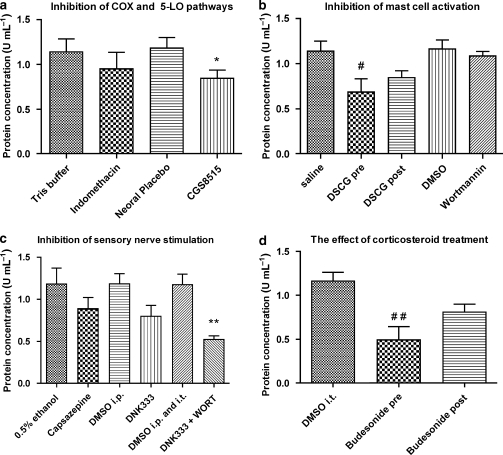
The 5-LO inhibitor, CGS8515 (25 mg kg−1 p.o.), but not the COX-1 and COX-2 inhibitor, indomethacin (10 mg kg−1 p.o.), was able to reduce the volume of fluid signals observed by MRI at 24 h after the secretagogue, when compared with their corresponding vehicle-treated groups (Figure 3). The inhibition observed by MRI correlated with a significant decrease in protein concentration in the BAL fluid collected immediately after imaging (Figure 4).
Figure 3.
Magnetic resonance imaging lung fluid signal volumes (means±s.e.mean) determined from MR images at 24 h after compound 48/80 (1 mg kg−1 i.t.) and after treatment with (a) indomethacin (10 mg kg−1 p.o.; n=5) or its vehicle (10% TRIS buffer 2 mL kg−1 p.o.; n=5) 1 h prior to compound 48/80; CGS8515 (25 mg kg−1 p.o., n=5) or its vehicle (2% MP in Neoral placebo p.o.; n=5) 2 h prior compound 48/80. (b) Disodium cromoglycate (DSCG; 10 mg kg−1 i.t.) administered immediately prior to (n=12) or 3 h (n=8) after compound 48/80. The vehicle for DSCG was saline i.t. (n=8); wortmannin (100 μg kg−1 i.t; n=8) or its vehicle (4% dimethyl sulphoxide (DMSO) i.t.; n=6) was applied 1 h prior to compound 48/80. (c) Capsazepine (3.5 mg kg−1 i.t.; n=5) or its vehicle (0.5% ethanol i.t.; n=5); DNK333 (10 mg kg−1 i.p., n=5) or its vehicle (2% DMSO in Neoral placebo 2 mL kg−1 i.p; n=5); a dual therapy with wortmannin (100 μg kg−1 i.t.) and DNK333 (10 mg kg−1 i.p; n=5) or both vehicle groups (4% DMSO i.t. and 2% DMSO i.p; n=5). Compound 48/80 was administered 30 min after treatment with capsazepine, DNK333 or their corresponding vehicles and 1 h after wortmannin or its vehicle. (d) budesonide (3 mg kg−1 i.t.) administered immediately prior to (n=10) or 3 h (n=8) after compound 48/80. The vehicle for budesonide was 4% DMSO in saline i.t. (n=6). The significance levels *P<0.05; **0.001<P<0.01 and ***P<0.001 refer to differences between the drug substance and their corresponding vehicle groups derived from the Student's t-test (two tailed). The significance levels ###P<0.05 refer to one-way ANOVA analysis of variance with a Bonferroni correction.
Figure 4.
Protein concentration in bronchoalveolar lavage (BAL) fluid (means±s.e.mean) determined at 24 h after compound 48/80 (1 mg kg−1 i.t.) and after treatment with (a) indomethacin (10 mg kg−1 p.o.; n=5) or its vehicle (10% TRIS buffer 2 mL kg−1 p.o.; n=5) 1 h prior to compound 48/80; CGS8515 (25 mg kg−1 p.o., n=5) or its vehicle (2% MP in Neoral placebo p.o.; n=5) 2 h prior compound 48/80. (b) Disodium cromoglycate (DSCG; 10 mg kg−1 i.t.) administered immediately prior to (n=12) or 3 h (n=8) after compound 48/80. The vehicle for DSCG was saline i.t. (n=8). Wortmannin (100 μg kg−1 i.t; n=8) or its vehicle (4% dimethyl sulphoxide (DMSO) i.t.; n=6) was applied 1 h prior to compound 48/80. (c) Capsazepine (3.5 mg kg−1 i.t.; n=5) or its vehicle (0.5% ethanol i.t.; n=5); DNK333 (10 mg kg−1 i.p., n=5) or its vehicle (2% DMSO in Neoral placebo 2 mL kg−1 i.p; n=5); a dual therapy with wortmannin (100 μg kg−1 i.t.) and DNK333 (10 mg kg−1 i.p; n=5) or both vehicle groups (4% DMSO i.t. and 2% DMSO i.p; n=5). Compound 48/80 was administered 30 min after treatment with capsazepine, DNK333 or their corresponding vehicles and 1 h after wortmannin or its vehicle. (d) Budesonide (3 mg kg−1 i.t.) administered immediately prior to (n=10) or 3 h (n=8) after compound 48/80. The vehicle for budesonide was 4% DMSO in saline i.t. (n=6). The significance levels *P<0.05 and **0.001<P<0.01 refer to differences between the drug substance and their corresponding vehicle groups derived from the Student's t-test (two tailed). The significance levels #P<0.05 and ##0.001 <P<0.01 refer to one-way ANOVA analysis of variance with a Bonferroni correction.
Targeting mast cell degranulation
Assessment of fluid signal volumes by MRI at 24 h after compound 48/80 showed that DSCG (10 mg kg−1 i.t.) administered immediately prior to compound 48/80 was able to block the effects of the secretagogue in the lung, compared with vehicle (saline)-treated rats. When administered 3 h after compound 48/80, DSCG suppressed the appearance of MRI fluid signals detected by MRI 24 h later (Figure 3). In comparison to its vehicle, the PI-3K inhibitor, wortmannin (100 μg kg−1 i.t.), administered 1 h prior to the secretagogue, reduced the volume of MRI signals at 24 h after compound 48/80 (Figure 3). These changes were accompanied by a reduction in protein concentration in BAL fluid collected 24 h after compound 48/80 from rats pretreated with DSCG, but not in animals post treated with DSCG or pretreated with wortmannin (Figure 4). It is important to mention that, immediately following i.t. administration of compound 48/80, all rats stopped breathing and respiratory support by means of a small animal respiration pump was required until the end of the recovery phase from isoflurane (2–3 min). The animals were observed for a period of 1–2 h after treatment during which no breathing difficulties were noted. Of the compounds used in these studies, DSCG, administered immediately before compound 48/80, was the only compound that was able to prevent the compound 48/80-induced apnoea.
Inhibition of sensory nerve activity
Treatment with the TRPV-1 antagonist, capsazepine (3.5 mg kg−1 i.t.), or the dual NK1 and NK2 receptor antagonist, DNK333 (10 mg kg−1 i.p.), 30 min prior to compound 48/80 (1 mg kg−1 i.t.) significantly, reduced the effects of compound 48/80 as detected by MRI 24 h after challenge, compared with their corresponding vehicle groups (Figure 3). Even though not significant, a tendency for lower levels of protein concentration was observed in BAL fluid at the same time points following treatment with capsazepine or DNK333 (Figure 4).
Combined treatment with wortmannin and DNK333
Administration of wortmannin (100 μg kg−1 i.t.) 1 h prior to compound 48/80 (1 mg kg−1 i.t.) followed by DNK333 (10 mg kg−1 i.p.) 30 min later resulted in a reduction of the volume of MRI signals 24 h after compound 48/80, compared with administration of both vehicles prior to the secretagogue (Figure 3). However, DSCG or budesonide treatment was more effective. BAL fluid analysis revealed reductions in protein concentration, following dual therapy with wortmannin and DNK333 (Figure 4). Both MRI and the BAL fluid analysis suggest that the effects of the combined treatment with wortmannin and DNK333 were additive, not synergistic.
Effects of budesonide
Budesonide (3 mg kg−1 i.t.) administered 1 h prior to compound 48/80 completely blocked its effects, whereas treatment 3 h after compound 48/80 significantly inhibited the appearance of MRI fluid signals measured 24 h after administration of the secretagogue (Figure 3). BAL fluid analysis 24 h after compound 48/80, demonstrated a significantly decreased level of protein concentration for pretreatment with budesonide, but not for the post-treatment condition.
Discussion
The present results show that i.t. administration of compound 48/80 (1 mg kg−1) to naïve BN rats leads to the appearance of oedematous fluid signals detected by MRI in the lungs as early as 1 h, reaching a maximum 48 h after instillation of the secretagogue. BAL fluid studies revealed an increase in protein concentration, cellular infiltration and eosinophil activation (eosinophil peroxidase; EPO) 24 h after instillation. Histological analysis performed at the same time point showed the presence of perivascular oedema in compound 48/80-treated, but not in vehicle-treated animals. These results demonstrate that the presence of MRI fluid signals following compound 48/80 is as a result of microvasculature leakage of fluid into the lung parenchyma. These observations are in line with those of Fingar et al. (1994) demonstrating inflammation in the lung following administration of compound 48/80.
Pharmacological intervention to investigate the nature of the response induced by compound 48/80 demonstrated that assessment of MRI fluid signal volume is a valid read-out to assess the effect of blocking various targets that may be involved in mediating the response to compound 48/80. Furthermore, assessment of fluid signal volume in the lungs by MRI appeared to be more sensitive as a marker for airways oedema than determination of protein concentration in BAL fluid (as illustrated in Table 2). This demonstrates the increased capability of MRI for the detection of inflammation compared with the determination of protein concentration in the BAL fluid. Protein was chosen as a comparator to MRI fluid signals as it is widely used as a read-out of inflammation. Additionally, protein concentration in BAL fluid has been shown to correlate with lung oedema (i) following treatment with OA in sensitized BN rats (Beckmann et al., 2001; Tigani et al., 2003); (ii) 6 h after treatment with OA in sensitized or endotoxin in naïve rats (Quintana et al., 2006); or (iii) 1 week after bleomycin administration to naïve rats (Karmouty-Quintana et al., 2007).
Table 2.
Comparison between MRI signal volume and protein concentration in BAL fluid
| Treatment | MRI fluid volume | Protein concentration in BAL |
|---|---|---|
| Indomethacin | No changes | No changes |
| CGS8515 | Decreased | Decreased |
| DSCG pre treatment | Decreased | Decreased |
| DSCG post treatment | Decreased | No changes |
| Wortmannin | Decreased | No changes |
| Capsazepine | Decreased | No changes |
| DNK333 | Decreased | No changes |
| DNK333 and wortmannin | Decreased | No changes |
| Budesonide pretreatment | Decreased | Decreased |
| Budesonide post treatment | Decreased | No changes |
Abbreviations: BAL, bronchoalveolar lavage; DSCG, disodium cromoglycate; MRI, magnetic resonance imaging.
Pretreatment with CGS8515, a selective 5-LO inhibitor, but not with indomethacin, a dual COX-1 and COX-2 inhibitor, reduced the volume of fluid signals detected by MRI as well as the level of protein in BAL fluid at 24 h after compound 48/80. These results demonstrate that inflammation in the lungs induced by compound 48/80 involves the generation of AA metabolites, consistent with the in vitro experiments of Shefler and Sagi-Eisenberg (2001). The lack of inhibition following indomethacin treatment is possibly as a result of suppression of PGE2 synthesis by the dual COX-1 and COX-2 inhibitor. PGE2 is an important endogenous inhibitory mediator in asthma (Barnes, 2000), reducing bronchoconstriction and inflammation (Pavord and Tattersfield, 1995).
Disodium cromoglycate has been shown to inhibit mast cell activation by altering the function of delayed chloride channels in the cell membrane, preventing cellular activation and subsequent degranulation (Barnes et al., 1995). In our experiments, DSCG administered immediately before compound 48/80 inhibited the effects of the secretagogue as detected by MRI and BAL fluid analysis (protein concentration) 24 h after the challenge. These results are fully consistent with findings first published by Orr et al. (1971), showing that DSCG is able to prevent compound 48/80-induced mast cell degranulation. Administration of DSCG 3 h after compound 48/80 was also able to considerably reduce the effects of the secretagogue, an observation that is in agreement with a clinical study involving 14 asthmatic patients, where DSCG administered 2 h after allergen challenge prevented the appearance of the late asthmatic response (Pelikan and Knottnerus, 1993). This observation suggests that DSCG may also act through a different mechanism other than preventing mast cell degranulation.
Wortmannin prevents mast cell degranulation through inhibition of the PI-3K pathway, an obligatory step in the signalling cascade that follows Gi protein activation by allergen (Yano et al., 1993) or compound 48/80 (Shefler et al., 1999). Pretreatment with a high dose of wortmannin resulted in a partial reduction of the MRI fluid signal volume, compared with an almost complete reduction following pretreatment with DSCG, as detected at 24 h after instillation of compound 48/80. Similarly, the reduction in BAL fluid protein concentration observed in DSCG pretreatment was not apparent following treatment with wortmannin. Our MRI findings are consistent with those of Tigani et al. (2001) who demonstrated that wortmannin (100 μg kg−1 i.t.) inhibits inflammatory cell infiltration and EPO activity in actively sensitized BN rats, as revealed by BAL fluid analysis 24 h after challenge with allergen.
The striking difference between pretreatment with DSCG and wortmannin suggests that mast cell degranulation per se is not the sole cause of compound 48/80-induced airways oedema. The inhibitory potential of DSCG 3 h after compound 48/80 further supports this observation. Thus, it is important to consider that DSCG is not only a mast cell stabilizer, but is also capable of affecting a large variety of inflammatory cells including eosinophils, neutrophils, platelets, lymphocytes and macrophages (for a recent review see Storms and Kaliner, 2005). DSCG is also able to inhibit tachykinergic nerves (Page, 1994), activation of C-fibres by capsaicin (Dixon et al., 1980) and neurogenic oedema in the rat paw resulting from electrical stimulation of the saphenous nerve (Le Filliatre et al., 2001). Moreover, compound 48/80 is not only a mast cell degranulator, but it is also able to stimulate sensory nerves. Olgart and Gazelius (1978) have shown that local application of compound 48/80 resulted in prolonged activation of intradental sensory nerves in cats and dogs that was inhibited by DSCG. More recently, Eglezos et al. (1992) demonstrated that compound 48/80 resulted in the release of CGRP (calcitonin gene-related peptide) from the superfused rat urinary bladder that was inhibited by capsaicin desensitization of sensory nerves, but not by indomethacin, methysergide, ondansetron, chlorpheniramine, cimetidine or by systemic pretreatment with compound 48/80 (to deplete mast cells).
Activation of sensory nerves leads to the release of tachykinins such as SP, CGRP and NKA (Reynolds et al., 1997). These tachykinins are able to mediate diverse effects such as vasodilatation, oedema, mucus release and inflammatory cell activation (Reynolds et al., 1997), via activation of NK1, NK2 and NK3 receptors (Barnes et al., 1998). The participation of tachykinergic sensory nerves in the effects of compound 48/80-induced airways oedema was studied here with pretreatment with capsazepine, a TRPV-1 antagonist, or DNK333, a dual NK1 and NK2 antagonist. These antagonists inhibited the effects of compound 48/80 as detected by MRI 24 h after administration of the secretagogue, demonstrating the participation of sensory nerves in the inflammation mediated by compound 48/80.
Combined treatment with wortmannin and DNK333 resulted in an additive inhibitory rather than a synergistic effect on the oedematous response induced by compound 48/80. It is important to consider, that AA metabolites released following mast cell activation can also stimulate sensory nerves through direct interaction with TRPV-1 (Manzini et al., 1989), which may suggest that mast cell degranulation induced by compound 48/80 is the main cause of sensory nerve activation. However, the fact that (i) wortmannin alone did not achieve the same level of inhibition as DSCG (a mast cell and sensory nerve inhibitor), and that (ii) combined therapy of wortmannin and DNK333 led to an additive effect compared with wortmannin or DNK333 on their own, show that compound 48/80 is able to stimulate sensory nerves directly, in addition to via mast cells. A single i.t. application of compound 48/80 to BN rats resulted in an immediate respiratory arrest that required reanimation. The effect was exclusively blocked by pretreatment with DSCG. We interpret these results as further evidence in support of a direct stimulation of sensory nerves by compound 48/80, which can be prevented by DSCG. Curiously, pretreatment with capsazepine was not able to inhibit the respiratory arrest induced by compound 48/80. A possible explanation for this observation is the fact that not all sensory nerves are vanilloid sensitive (Szallasi and Blumberg, 1999), thus, it is plausible that this immediate effect of compound 48/80 was as a result of the stimulation of other, vanilloid-insensitive, neurons.
Finally, the glucocorticosteroid, budesonide, administered prior to or after compound 48/80 challenge inhibited the effects of the secretagogue as detected by MRI. These results demonstrate once again the effectiveness of corticosteroids in the clearance of inflammation through their broad spectrum of activity (Tigani et al., 2003).
In summary, the inflammatory effects observed by MRI 24 h after compound 48/80 are consistent with the fact that the secretagogue leads to mast cell degranulation. Moreover, our data suggest that compound 48/80 is able to stimulate nerves directly and that vanilloid-sensitive sensory neurons play a role in the inflammatory effects of compound 48/80. On the basis of our observations, we postulate a series of positive feedback loops that (a) result in further sensory nerve activation via AA metabolites following mast cell degranulation and (b) lead to further mast cell activation through the release of SP from sensory nerves via direct action of compound 48/80 or AA metabolites. The continuous activation of both the nervous and immunological systems is the most likely mechanisms by which compound 48/80 leads to the strong and long-lasting oedematous response as shown by MRI and by histology, as well as by the inflammatory cell infiltration and activation observed in BAL fluid. Finally, the study demonstrates that the assessment of MRI fluid signal volume is sensitive enough to detect the effect of pharmacological intervention in a model of lung oedema that may not be apparent using conventional experimental read-outs based on BAL fluid analyses. Thus, proton MRI is a sensitive tool that has the capacity for real-time and non-invasive assessment of drug therapeutic properties that can be used for drug screening in pulmonary research (Beckmann et al., 2003, 2007).
Acknowledgments
We acknowledge Dr Eckhard Webber (Novartis Pharma) for providing the information concerning the affinity of DNK333 for rat receptors. This work was supported by Novartis and by the 3R Research Foundation Muensingen, Switzerland (project 82/02 to NB).
Abbreviations
- AA
arachidonic acid
- BAL
bronchoalveolar lavage
- DSCG
disodium cromoglycate
- EPO
eosinophil peroxidase
- LO
lipoxygenase
- 5-LO
5-lipoxygenase
- MRI
magnetic resonance imaging
- NK
neurokinin
- PG
prostaglandin
- PI-3K
PI-3-kinase
- TRPV
transient receptor potential vanilloid
Conflict of interest
The authors state no conflict of interest.
References
- Barnes PJ. Endogenous inhibitory mechanisms in asthma. Am J Respir Crit Care Med. 2000;161:S176–S181. doi: 10.1164/ajrccm.161.supplement_2.a1q4-6. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma: an update. Pharmacol Rev. 1998;50:515–596. [PubMed] [Google Scholar]
- Barnes PJ, Holgate ST, Laitinen LA, Pauwels R. Asthma mechanisms, determinants of severity and treatment: the role of nedocromil sodium. Clin Exp Allerg. 1995;25:771–787. doi: 10.1111/j.1365-2222.1995.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Beckmann N, Cannet C, Karmouty-Quintana H, Tigani B, Zurbruegg S, Blé FX, et al. Lung MRI for experimental drug research. Eur J Radiol. 2007;64:381–396. doi: 10.1016/j.ejrad.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Beckmann N, Tigani B, Ekatodramis D, Borer R, Mazzoni L, Fozard JR. Pulmonary oedema induced by allergen challenge in the rat: noninvasive assessment by magnetic resonance imaging. Magn Reson Med. 2001;45:88–95. doi: 10.1002/1522-2594(200101)45:1<88::aid-mrm1013>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Beckmann N, Tigani B, Mazzoni L, Fozard JR. Techniques: magnetic resonance imaging of the lung provides potential for non-invasive preclinical evaluation of drugs. Trends Pharmacol Sci. 2003;24:550–554. doi: 10.1016/j.tips.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Boughton-Smith NK, Deakin AM, Follenfant RL, Whittle BJ, Garland LG. Role of oxygen radicals and arachidonic acid metabolites in the reverse passive Arthus reaction and carrageenin paw oedema in the rat. Br J Pharmacol. 1993;110:896–902. doi: 10.1111/j.1476-5381.1993.tb13897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll NG, Mutavdzic S, James AL. Distribution and degranulation of airway mast cells in normal and asthmatic subjects. Eur Respir J. 2002;19:879–885. doi: 10.1183/09031936.02.00275802. [DOI] [PubMed] [Google Scholar]
- Dixon M, Jackson DM, Richards IM. The action of sodium cromoglycate on ‘C' fibre endings in the dog lung. Br J Pharmacol. 1980;70:11–13. doi: 10.1111/j.1476-5381.1980.tb10898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglezos A, Lecci A, Santicioli P, Giuliani S, Tramontana M, Del Bianco E, et al. Activation of capsaicin-sensitive primary afferents in the rat urinary bladder by compound 48/80: a direct action on sensory nerves. Arch Int Pharmacodyn Ther. 1992;315:96–109. [PubMed] [Google Scholar]
- Fingar VH, Taber SW, Wieman TJ. A new model for the study of pulmonary microcirculation: determination of pulmonary oedema in rats. J Surg Res. 1994;57:385–393. doi: 10.1006/jsre.1994.1159. [DOI] [PubMed] [Google Scholar]
- Holgate ST. The epidemic of allergy and asthma. Nature. 1999;402:B2–B4. doi: 10.1038/35037000. [DOI] [PubMed] [Google Scholar]
- Karmouty-Quintana H, Cannet C, Zurbruegg S, Blé FX, Fozard JR, Page CP, et al. Bleomycin-induced lung injury assessed noninvasively and in spontaneously breathing rats by proton MRI. J Magn Reson Imaging. 2007;26:941–949. doi: 10.1002/jmri.21100. [DOI] [PubMed] [Google Scholar]
- Ku EC, Raychaudhuri A, Ghai G, Kimble EF, Lee WH, Colombo C, et al. Characterization of CGS 8515 as a selective 5-lipoxygenase inhibitor using in vitro and in vivo models. Biochim Biophys Acta. 1988;959:332–342. doi: 10.1016/0005-2760(88)90207-x. [DOI] [PubMed] [Google Scholar]
- Lagunoff D, Martin TW, Read G. Agents that release histamine from mast cells. Annu Rev Pharmacol Toxicol. 1983;23:331–351. doi: 10.1146/annurev.pa.23.040183.001555. [DOI] [PubMed] [Google Scholar]
- Le Filliatre G, Sayah S, Latournerie V, Renaud JF, Finet M, Hanf R. Cyclo-oxygenase and lipoxygenase pathways in mast cell dependent-neurogenic inflammation induced by electrical stimulation of the rat saphenous nerve. Br J Pharmacol. 2001;132:1581–1589. doi: 10.1038/sj.bjp.0703950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Lundberg JM. Capsazepine abolishes pulmonary chemoreflexes induced by capsaicin in anesthetized rats. J Appl Physiol. 1994;76:1848–1855. doi: 10.1152/jappl.1994.76.5.1848. [DOI] [PubMed] [Google Scholar]
- Lewis CA, El-Hashim AZ, Gerspacher M, Hoshiko K, Mazzoni L, Pfannkuche HJ, et al. The airways pharmacology of DNK333, a potent, selective, non-peptide dual NK1/NK2 receptor antagonist. Drug Dev Res. 2004;63:161–173. [Google Scholar]
- Liu S, Hiedayati N, Shudou M, Maeyama K. Activation of connective tissue-type and mucosal-type mast cells in compound 48/80-induced airway response. Eur J Pharmacol. 2006;530:128–135. doi: 10.1016/j.ejphar.2005.10.067. [DOI] [PubMed] [Google Scholar]
- Manzini S, Ballati L, Geppetti P, Rubini I, Meini S, Perretti F. Arachidonic acid-induced bronchomotor responses are partially mediated by release of sensory neuropeptides from capsaicin-sensitive structures. Br J Pharmacol. 1989;98:1077–1079. doi: 10.1111/j.1476-5381.1989.tb12649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Olgart L, Gazelius B. Inhibition of compound 48/80-induced intradental sensory nerve activity by disodium cromoglycate and serotonin antagonists. Acta Physiol Scand. 1978;104:415–421. doi: 10.1111/j.1748-1716.1978.tb06296.x. [DOI] [PubMed] [Google Scholar]
- Orr TS, Hall DE, Gwilliam JM, Cox OS. The effect of disodium cromoglycate on the release of histamine and degranulation of rat mast cells induced by compound 48-80. Life Sci I. 1971;10:805–812. doi: 10.1016/0024-3205(71)90035-x. [DOI] [PubMed] [Google Scholar]
- Page C. Sodium cromoglycate, a tachykinin antagonist. Lancet. 1994;343:70. doi: 10.1016/s0140-6736(94)90812-5. [DOI] [PubMed] [Google Scholar]
- Pavord ID, Tattersfield AE. Bronchoprotective role for endogenous prostaglandin E2. Lancet. 1995;344:436–438. doi: 10.1016/s0140-6736(95)90409-3. [DOI] [PubMed] [Google Scholar]
- Pelikan Z, Knottnerus I. Inhibition of the late asthmatic response by nedocromil sodium administered more than two hours after allergen challenge. J Allergy Clin Immunol. 1993;92:19–28. doi: 10.1016/0091-6749(93)90032-b. [DOI] [PubMed] [Google Scholar]
- Quintana HK, Cannet C, Schaeublin E, Zurbruegg S, Sugar R, Mazzoni L, et al. Identification with MRI of the pleura as a major site of the acute inflammatory effects induced by ovalbumin and endotoxin challenge in the airways of the rat. Am J Physiol Lung Cell Mol Physiol. 2006;291:L651–L657. doi: 10.1152/ajplung.00303.2005. [DOI] [PubMed] [Google Scholar]
- Reynolds PN, Holmes MD, Scicchitano L. Role of tachykinins in bronchial hyper-responsiveness. Clin Exp Pharmacol Physiol. 1997;24:273–280. doi: 10.1111/j.1440-1681.1997.tb01819.x. [DOI] [PubMed] [Google Scholar]
- Shefler I, Sagi-Eisenberg R. Gi-mediated activation of the Syk kinase by the receptor mimetic basic secretagogues of mast cells: role in mediating arachidonic acid/metabolites release. J Immunol. 2001;167:475–481. doi: 10.4049/jimmunol.167.1.475. [DOI] [PubMed] [Google Scholar]
- Shefler I, Seger R, Sagi-Eisenberg R. Gi-mediated activation of mitogen-activated protein kinase (MAPK) pathway by receptor mimetic basic secretagogues of connective tissue-type mast cells: bifurcation of arachidonic acid-induced release upstream of MAPK. J Pharmacol Exp Ther. 1999;289:1654–1661. [PubMed] [Google Scholar]
- Shin HY, Kim JS, An NH, Park RK, Kim HM. Effect of disodium cromoglycate on mast cell-mediated immediate-type allergic reactions. Life Sci. 2004;74:2877–2887. doi: 10.1016/j.lfs.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Stevens RL, Austen KF. Recent advance in the cellular and molecular biology of mast cells. Immunol Today. 1989;10:381–385. doi: 10.1016/0167-5699(89)90272-7. [DOI] [PubMed] [Google Scholar]
- Storms W, Kaliner MA. Cromolyn sodium: fitting an old friend into current asthma treatment. J Asthma. 2005;42:79–89. [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Tigani B, Cannet C, Zurbrugg S, Schaeublin E, Mazzoni L, Fozard JR, et al. Resolution of the oedema associated with allergic pulmonary inflammation in rats assessed noninvasively by magnetic resonance imaging. Br J Pharmacol. 2003;140:239–246. doi: 10.1038/sj.bjp.0705429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigani B, Hannon JP, Mazzoni L, Fozard JR. Effects of wortmannin on airways inflammation induced by allergen in actively sensitised Brown Norway rats. Eur J Pharmacol. 2001;433:217–223. doi: 10.1016/s0014-2999(01)01515-1. [DOI] [PubMed] [Google Scholar]
- Ui M, Okada T, Hazeki K, Hazeki O. Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem Sci. 1995;20:303–307. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- Yano H, Nakanishi S, Kimura K, Hanai N, Saitoh Y, Fukui Y, et al. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J Biol Chem. 1993;268:25846–25856. [PubMed] [Google Scholar]