Abstract
Background and purpose:
Annexin-A1 (ANXA1), a glucocorticoid-regulated protein, mediates several of the anti-inflammatory actions of the glucocorticoids. Previous studies demonstrated that ANXA1 is involved in pain modulation. The current study, using ANXA1 knockout mice (ANXA1−/−), is aimed at addressing the site and mechanism of the modulatory action of ANXA1 as well as possible involvement of ANXA1 in mediating the analgesic action of glucocorticoids.
Experimental approach:
The acetic acid-induced writhing response was performed in ANXA1−/− and wild-type (ANXA1+/+) mice with spinal and brain levels of prostaglandin E2 (PGE2) examined in both genotypes. The effect of the ANXA1 peptomimetic Ac2-26 as well as methylprednisolone on the writhing response and on spinal cord PGE2 of ANXA1+/+ and ANXA1−/− was compared. The expression of proteins involved in PGE2 synthesis, cytosolic phospholipase A2 (cPLA2) and cyclooxygenases (COXs), in the spinal cord of ANXA1+/+ and ANXA1−/− was also compared.
Key results:
ANXA1−/− mice exhibited a significantly greater writhing response and increased spinal cord levels of PGE2 compared with ANXA1+/+ mice. Ac2-26 produced analgesia and reduced spinal PGE2 levels in ANXA1+/+ and ANXA1−/− mice, whereas methylprednisolone reduced the writhing response and spinal PGE2 levels in ANXA1+/+, but not in ANXA1−/− mice. The expression of cPLA2, COX-1, COX-2 and COX-3 in spinal cord tissues was upregulated in ANXA1−/−compared with ANXA1+/+.
Conclusions and implications:
We conclude that ANXA1 protein modulates nociceptive processing at the spinal level, by reducing synthesis of PGE2 by modulating cPLA2 and/or COX activity. The analgesic activity of methylprednisolone is mediated by spinal ANXA1.
Keywords: annexin-A1, antinociceptive, cyclooxygenase, methylprednisolone, prostaglandin E2, spinal cord
Introduction
Annexin-A1 (ANXA1; formerly known as lipocortin-1), a 37 kDa glucocorticoid-regulated protein (Buckingham and Flower 1997), is a member of the annexin family of calcium and phospholipids-binding proteins and is found in the central nervous system (CNS) (Bolton et al., 1990). It has been demonstrated that ANXA1 mediates the anti-inflammatory actions of glucocorticoids in many experimental models (Podgorski et al., 1992; Yang et al., 1997, 2004) and also to possess antipyretic actions (Davidson et al., 1991).
Prostanoids are lipid mediators involved in transmission of nociceptive pain. Synthesis of the prostanoids is initiated by the generation of arachidonic acid by phospholipase A2 (PLA2), which is metabolized by cyclooxygenase (COX) enzymes to generate short-lived mediators that act as precursors for the synthesis of the biologically active prostanoids: prostaglandin E2 (PGE2), prostaglandin D2, prostaglandin I2, prostaglandin F2α or thromboxane A2. Two isoforms of COX are known to exist, COX-1 and COX-2. COX-1 is constitutively expressed and is generally responsible for the production of prostanoids involved in homoeostatic regulation of body functions (Crofford, 1997), whereas COX-2 is an inducible isoform (Xie et al., 1991) in most tissues (although substantial pools of the enzyme exist constitutively in some organs for example the CNS) and is responsible for production of prostanoids involved in pathological processes such as inflammation (Tomlinson et al., 1994). A third isoform, COX-3, was recently identified as a splice variant of COX-1 and is predominantly expressed in the CNS (Chandrasekharan et al., 2002).
In peripheral tissues, PGE2 and prostaglandin I2 were shown to be hyperalgesic, that is, they sensitize nerve endings to other pain producing agents such as bradykinin by lowering the activation threshold (Ferreira et al., 1974; Taiwo and Levine, 1990). At the spinal level, it was demonstrated that facilitation of nociception is mediated by PGE2 synthesized by constitutive COX-1 and COX-2 (Willingale et al., 1997; Yamamoto and Nozaki-Taguchi, 1997; Dirig et al., 1998). Within the brain, injection of PGE2 into the pre-optic area resulted in the generation of thermal hyperalgesia (Hori et al., 2000).
Few studies have addressed the question of whether ANXA1 is involved in the activation/modulation of pain pathways. Ferreira et al. (1997) showed that ANXA1 peptidomimetics produced analgesia in inflamed rat paws and that neutralizing antisera to ANXA1 prevented the antihyperalgesic activity of glucocorticoids. Using a rat model of C-fibre modulated bradykinin-induced plasma extravasation, Green et al. (1998) suggested that the inhibitory action of corticosterone on C-fibre activation was mediated by the release of ANXA1. More recently, Pieretti et al. (2004) demonstrated that inhibition of the formalin-induced nociceptive behaviour by ANXA1 peptidomimetics, administered locally or centrally, is dependent on activation of the receptors of the formylated peptide family.
We have previously observed that ANXA1 null (ANXA1−/−) mice were more susceptible to nociceptive pain induced by the intraperitoneal (i.p.) injection of acetic acid compared with wild-type (ANXA1+/+), suggesting that ANXA1 modulates nociceptive pain. In addition, increased levels of PGE2 in the spinal cord of ANXA1−/− compared with ANXA1+/+ mice suggest that ANXA1 modulates nociceptive processing at the spinal level by downregulating PGE2 spinal nociceptive facilitation. Evidence is also presented to demonstrate that ANXA1 mediates the analgesic action of the glucocorticoid, methylpredinsolone.
Methods
Animals
All animal procedures were in accordance with UK Home Office regulations. Male and female ANXA1+/+ and ANXA1−/− mice on a C57BL/6 background (20–25 g body weight; Hannon et al., 2003), maintained on a standard chow pellet diet with tap water ad libitum, were used for all experiments. The animals were housed five per cage in a room with controlled lighting (lights on from 0800 to 2000 hours), in which the temperature was maintained at 21–23 °C.
Writhing test and tissue collection
Nociceptive responses were induced in ANXA1+/+ or ANXA1−/− mice by the i.p. injection of 0.6% (v/v) glacial acetic acid in 0.9% (w/v) saline. In response to acetic acid, the mice develop the ‘writhing' reaction, which is characterized by patterns of waves of constriction and elongation passing caudally along the abdominal wall followed by extension of the hind limbs (Collier et al., 1968). The number of writhes was counted for a 20-min period following the administration of the stimulus. In some experiments, the animals were pretreated with either acetyl 2–26 (Ac2-26; 200 μg, i.p., 10 min before) or 60 mg kg−1 6α-methylprednisolone 21-hemisuccinate (subcutaneously, 1 h before) to test for analgesic activity. At the end of the 20-min observation period, the animals were killed by cervical dislocation, the peritoneal cavity lavaged with 1 mL of 0.9% (w/v) saline and the samples frozen. The whole spinal cord was removed and immediately frozen. Whole brains were removed and dissected into the cerebral cortex, mid-brain, brain stem and cerebellum, and frozen immediately. PGE2 was measured in the peritoneal fluid, dissected brains and whole spinal cords using conventional enzyme immunoassay techniques after extraction with C18 Sep-Pak columns.
Extraction of PGE2 from peritoneal fluid, spinal cord and dissected brain
The procedure was carried out as a modification of the protocol described by Powell (1980). Frozen brain and spinal cord tissues were pulverized with a nitrogen bomb. Peritoneal fluid was centrifuged at 800 g for 10 min at 4 °C to remove cells. One millilitre of 15% (v/v) ethanol in distilled water (pH 3) was added to pulverized tissues or peritoneal supernatants and samples were stored at 4 °C for 10 min and then centrifuged at 375 g for 10 min at 4 °C. C-18 Sep-Pak columns were conditioned with 4 mL ethanol followed by 4 mL distilled water at a flow rate of 5–10 mL min−1. The supernatant from homogenates was then applied to the columns at a flow rate of 5 mL min−1. The columns were then washed with 4 mL distilled water followed by 4 mL of 15% (v/v) ethanol in distilled water. The samples were then eluted with 2 mL of ethyl acetate at a flow rate of 5 mL min−1. The samples were dried and then stored at −80 °C ready for prostaglandin measurement.
PGE2 enzyme immunoassay
Measurement of peritoneal, brain and spinal cord PGE2 was carried out by using a commercial enzyme immunoassay kit from Amersham Biosciences (Buckinghamshire, UK) according to the manufacturer's protocol. Briefly, extracted PGE2 was incubated on a goat anti-mouse IgG-coated plate along with anti-PGE2 antibody and horseradish peroxidase-labelled PGE2. The blue colour developed with 3,3′,5,5′-tetramethylbenzidine substrate was read colometrically at 630 nm. The concentration of PGE2 in the samples was determined by comparing the calculated percentage binding of PGE2 in the samples with a standard PGE2 curve (0.05–6.4 ng mL−1).
Western blotting
The protein concentration in pulverized spinal cord tissues was determined using the method developed by Bradford (1976). Briefly, spinal cord tissue homogenates from ANXA1+/+ and ANXA1−/− were pooled together (n=5), then sonicated for 40 s after reconstitution in 200 μL of protease inhibitory cocktail containing 104 mM 4-(2-aminoethyl) benzenesulphonylflouride, 0.08 mM aprotinin, 2.1 mM leupeptin, 3.6 mM bestatin, 1.5 mM pepstatin A and 1.4 mM E-64, in 50 mM Tris buffer (pH 7.4). The protein concentration was quantified using the Bradford reagent (Bio Rad, Hemel Hempstead, Hertfordshire, UK).
Protein samples (15 μg) were analysed with 10 or 7% sodium dodecyl sulphate-polyacryamide gels using the Bio-Rad mini protean II gel electrophoresis system. The proteins were separated electrophoretically at 180 V and then transferred to nitrocellulose membranes electrophoretically at 100 V for 1 h. The blots were blocked with 5% (w/v) milk and 0.2% (w/v) BSA in Tris-buffered saline containing 0.1% (v/v) Triton X-100 (TTBS) overnight at 4 °C. They were then washed for 20 min in TTBS and incubated with one of the following primary antibodies: monoclonal anti-cytosolic PLA2 (cPLA2, 0.2 μg mL−1), monoclonal anti-COX-1 (0.2 μg mL−1), polyclonal anti-COX-2 (2 μg mL−1), polyclonal anti-COX-3 (2 μg mL−1) or monoclonal anti-β-actin (1:10 000 dilution) at room temperature for 1 h, washed again and incubated with 0.2 μg mL−1 of anti-rabbit (used with polyclonal primary antibodies) or anti-mouse (used with monoclonal primary antibodies) horse-radish peroxidase-conjugated secondary antibodies. The blots were finally washed and developed on X-ray films using the western blotting ECL plus reagent.
The bands were scanned and quantified using Image J software (NIH Image) and expressed as the ratio of β-actin protein.
Statistical analysis
The results were analysed using Graph Pad Prism 3.0 (San Diego, CA, USA) and results expressed and presented graphically as means±s.e.mean. Statistical analysis was performed by comparing the vehicle-treated group with the drug-treated group using a one-tailed unpaired t-test. A P-value ⩽0.05 was considered to be statistically significant.
Materials
The following drugs, reagents and kits were used: 6α-methylprednisolone 21-hemisuccinate and anti-β-actin antibody (Sigma, Dorset, Poole, UK), Ac2-26 peptide, which corresponds to amino acids 2–26 of the N-terminus region of ANXA1 protein, was synthesized at Imperial College London, glacial acetic acid (VWR, Leicestershire, UK), C18 Sep-Pak columns (Waters, Milford, MA, USA), PGE2 EIA kit (GE Healthcare, Buckinghamshire, UK), anti-ANXA1 antibody (Zymed Laboratories, San Francisco, CA, USA), anti-cPLA2 antibody, goat anti-rabbit and goat anti-mouse secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-COX-1 and anti-COX-2 antibodies (Cayman Chemicals, Ann Arbor, MI, USA), anti-COX-3 antibody (Autogen Bioclear, Mile Elm, Wiltshire, UK).
Results
ANXA1 modulates nociceptive pain in mice
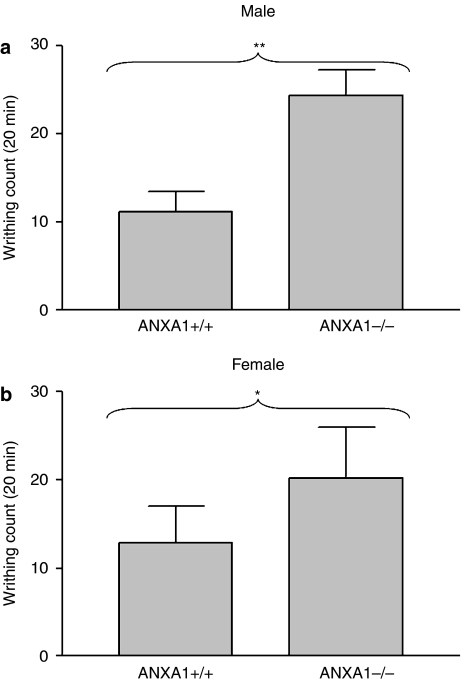
The writhing response induced by acetic acid in ANXA1−/− mice, lacking a functional ANXA1 protein, was significantly greater compared with ANXA1+/+ mice (Figures 1a and b). This effect was observed in both male ANXA1+/+ compared with male ANXA1−/− mice (P<0.01) and in female ANXA1+/+ compared with female ANXA1−/− mice (P<0.05).
Figure 1.
The acetic acid-induced writhing response in male (a) and female (b) ANXA1−/− mice compared with ANXA1+/+ mice. The writhing response was induced by the i.p. administration of 0.6% (v/v) of acetic acid and the writhing behaviour count for 20 min. *P<0.05, **P<0.01, n=6. ANXA1, annexin-A1.
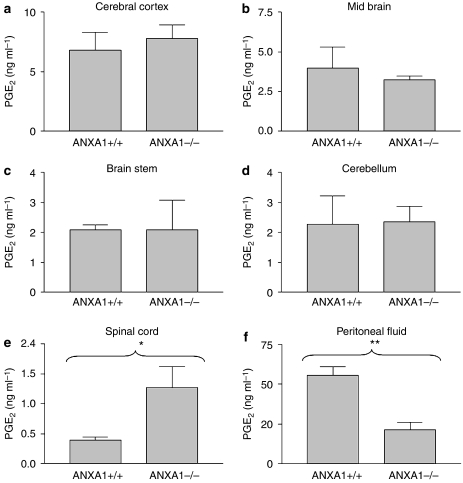
In an attempt to investigate the observed difference in the writhing response between ANXA1+/+ and ANXA1−/− mice, the levels of PGE2 were measured in the cerebral cortex, mid-brain, cerebellum, brain stem, spinal cord and peritoneal washouts. In spinal cord tissues of ANXA1−/− mice, the levels of PGE2 were significantly greater (P<0.05) compared with ANXA1+/+ mice (Figure 2e). There was no significant difference in the levels of PGE2 between ANXA1+/+ and ANXA1−/− in any brain tissue studied (Figures 2a–d). In peritoneal washouts, PGE2 was significantly higher in ANXA1+/+ compared with ANXA1−/− mice (Figure 2f).
Figure 2.
Comparison of the levels of PGE2 between ANXA1+/+ and ANXA1−/− mice writhing to 0.6% acetic acid in cerebral cortex (a), mid-brain (b), brain stem (c), cerebellum (d), spinal cord (e) and peritoneal washouts (f). Following the i.p. administration of acetic acid, the writhing response was observed for 20 min, after which the animals were killed by cervical dislocation, brain and spinal cord tissues dissected out and snap-frozen in liquid nitrogen. PGE2 was extracted using C18 columns and measured with a PGE2 EIA kit. *P<0.05, **P<0.01, n=6. ANXA1, annexin-A1; PGE2, prostaglandin E2.
The levels of PGE2 were significantly higher in the spinal cord tissues of ANXA1−/− compared with ANXA1+/+ mice; hence, we proposed that ANXA1 protein modulates nociception by downregulation of PGE2 synthesis in the cord. Therefore, for the rest of the current investigation, we only examined differences in PGE2 in at the spinal level.
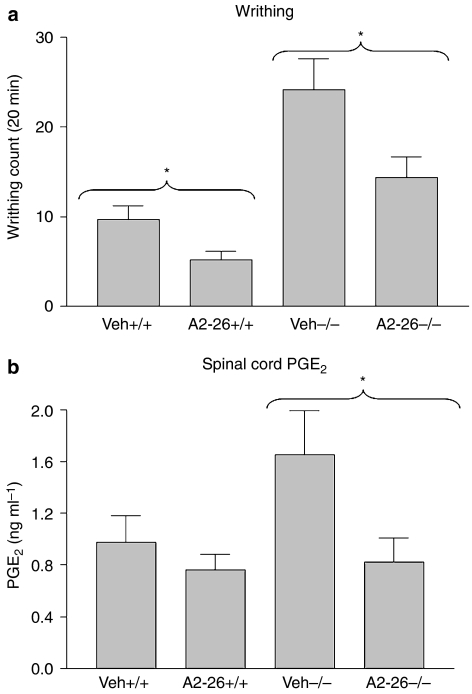
The ANXA1 peptidomimetic, Ac2-26, which corresponds to amino acids 2–26 of the N-terminal region of ANXA1 protein, has been shown to mimic the anti-inflammatory actions of the full-length protein. Ac2-26 (200 μg) administered i.p. to ANXA1+/+ and ANXA1−/− mice, 10 min before the induction of writhing with acetic acid, produced a significant analgesic effect (P<0.05) in both genotypes (Figure 3a). The analgesic effect of Ac2-26 was coupled with significant reduction in the levels of PGE2 in spinal cord tissues of ANXA1−/− mice and a nonsignificant reduction in ANXA1+/+ mice (Figure 3b).
Figure 3.
The effect of the ANXA1 peptidomimetic Ac2-26 on the writhing response induced with acetic acid (a) and on spinal cord PGE2 levels (b) of ANXA1+/+ and ANXA1−/−. Acetic acid (0.6%) was administered intraperitoneally 10 min after the administration of 200 μg of Ac2-26 and the writhing response observed for 20 min. The animals were killed by cervical dislocation, whole spinal cord tissues dissected and snap-frozen in liquid nitrogen. Spinal cord PGE2 levels were measured using a PGE2 EIA kit after extraction with C18 columns. *P<0.05, n=6. ANXA1, annexin-A1; PGE2, prostaglandin E2.
ANXA1 mediates the analgesic action of methylprednisolone
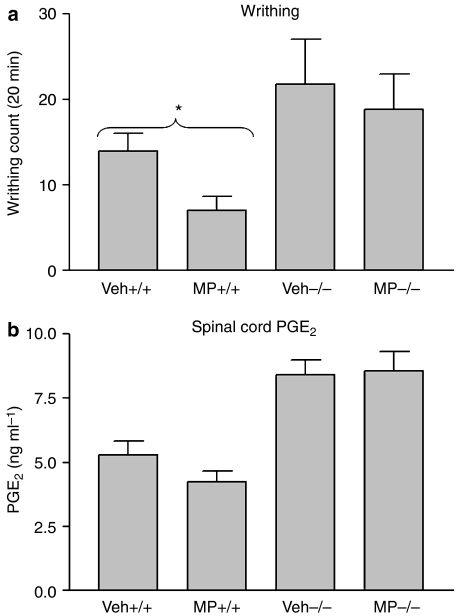
Methylprednisolone is a synthetic glucocorticoid used in the treatment of inflammatory conditions. Some of its anti-inflammatory actions were shown to be mediated through the induction of ANXA1 (Podgorski et al., 1992; Solito et al., 1993). The aim of the present experiments was to test whether ANXA1 also mediates the analgesic actions of this glucocorticoid. Thus, at the therapeutic dose of 60 mg kg−1 in rodents (Kim and Jahng, 2004), methylpredinsolone administered 1 h before the induction of the writhing response significantly reduced the writhing counts (P<0.05) in ANXA1+/+ mice but not in ANXA1−/− mice (Figure 4a). Methylprednisolone produced an inhibitory trend on spinal cord PGE2 levels (P>0.05) in ANXA1+/+, whereas no such trend was seen in tissues from ANXA1−/− (Figure 4b).
Figure 4.
The effect of 6α-methylprednisolone 21-hemi-succinate (MP) on the writhing response induced with acetic acid (a) and on spinal cord PGE2 levels (b) in ANXA1+/+ and ANXA1−/− mice. Acetic acid (0.6%) was administered intraperitoneally 1 h after the administration of 60 mg kg−1 of MP and the writhing response observed for 20 min. The animals were killed by cervical dislocation, whole spinal cord tissues dissected and snap-frozen in liquid nitrogen. Spinal cord PGE2 levels were measured using a PGE2 EIA kit after extraction with C18 columns. *P<0.05, n=6. ANXA1, annexin-A1; PGE2, prostaglandin E2.
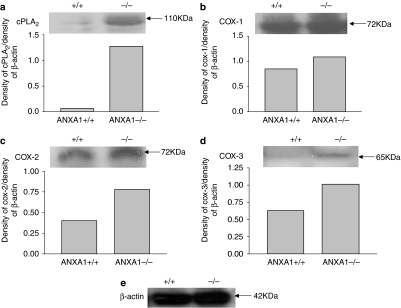
cPLA2, COX-1, COX-2 and COX-3 proteins are upregulated in spinal cord tissues of ANXA1−/− mice
The expression of some of the enzymes involved in synthesis of PGE2 namely cPLA2, COX-1, COX-2 and COX-3 was examined in spinal cord tissues of ANXA1+/+ and ANXA1−/− mice. The expression of all four proteins increased in the spinal cords of ANXA1−/− mice compared with ANXA1+/+ animals. cPLA2 increased by almost 100% (Figure 5a; 95%), whereas COX-1 increased by 21% (Figure 5b), COX-2 increased by 48% (Figure 5c) and COX-3 by 38% (Figure 5d).
Figure 5.
The expression profile of cPLA2 (a), COX-1 (b), COX-2 (c) and COX-3 (d) in the spinal cord tissues of ANXA1+/+ and ANXA1−/− mice expressed as density units and normalized to the density of β-actin (e) acting as a housekeeping protein. Whole spinal cord tissues were dissected from untreated mice and snap-frozen in liquid nitrogen. Tissues with the same genotype were pooled together (n=5) and crushed using a nitrogen gun. The protein concentration was determined and samples were constituted in Lammelli buffer. The proteins were detected using standard electrophoresis and immunoblotting techniques. ANXA1, annexin-A1.
Discussion
The present study demonstrated that ANXA1 is an endogenous modulator of acute nociceptive pain, as loss of a fully functional ANXA1 protein in ANXA1 null mice resulted in an exacerbation in the acetic acid-induced writhing pain compared with wild-type mice. The increase in the writhing response in ANXA1 null mice was coupled with an increase in the spinal levels of PGE2, suggesting that the observed antinociceptive action of ANXA1 is dependent on downregulation of the pathway leading to synthesis of PGE2. This eicosanoid is an important nociceptive mediator within the dorsal horn of the spinal cord (Willingale et al., 1997) and is involved in mediating the writhing response. Classical and antipyretic COX inhibitors have been shown to inhibit significantly the writhing response coupled with reduction in PGE2 synthesis in the CNS (Ayoub et al., 2006). The reduction of the writhing response and spinal cord PGE2 in ANXA1+/+ and ANXA1−/− animals by the ANXA1 N-terminal peptide Ac2-26 provides further support for the idea that the antinociceptive action of ANXA1 is dependent on reduction of PGE2 synthesis at the spinal level.
The observed reduction in the levels of PGE2 in the peritoneal washouts of ANXA1−/− compared with ANXA1+/+ mice could be due to the leukopaenia observed in the peritoneum of ANXA1−/− mice challenged with zymosan (Hannon et al., 2003).
Methylprednisolone is a synthetic anti-inflammatory glucocorticoid classified as a steroidal anti-inflammatory drug. It is commonly used topically for the treatment of inflammatory conditions of the eye, ear and nose and also for rheumatic diseases (British National Formulary 49, 2005). Methylprednisolone exhibited an analgesic action in ANXA1+/+ mice writhing to acetic acid and showed a tendency to reduce the spinal cord levels of PGE2, both effects believed to be mediated through ANXA1, as loss of the protein in ANXA1−/− mice resulted in loss of the analgesic action and in the PGE2 inhibitory action.
At the molecular level, the glucocorticoids possess genomic and non-genomic actions (Saklatvala, 2002). At the genomic level, the glucocorticoids repress transcription of a number of pro-inflammatory gene products that include the pro-inflammatory cytokines, interleukins 1–6 (Cavaillon, 1995) and pro-inflammatory enzymes such as inducible NOS (Matsumura et al., 2001), cPLA2 (Croxtall et al., 1996) and COX-2 (Hay and de Belleroche, 1998). Glucocorticoids are also involved in the induction of anti-inflammatory products such as interleukin-10 (Mozo et al., 2004) and ANXA1 (Podgorski et al., 1992; Croxtall and Flower, 1994). These genomic actions of glucocorticoids are dependent on binding, activation and nuclear localization of the glucocorticoid receptor expressed in most cells, which is a time-dependent process. The non-genomic actions of the glucocorticoids are very rapid occurring within minutes and are dependent on cytoplasmic glucocorticoid receptor (Buckingham, 2006).
Methylprednisolone was pre-administered 1 h before the induction of writhing pain, suggesting that the analgesic effect of this drug is unlikely to be explained by de novo synthesis of ANXA1 protein as it characteristically takes glucocorticoids more than 2 h to induce synthesis of ANXA1 (Solito et al., 1993; Parente and Solito, 1994). In addition, ANXA1 was shown to be constitutively expressed in CNS tissues (including spinal cord) of ANXA1+/+ mice, as also previously demonstrated by Strijbos et al. (1990), and that expression was reduced dramatically in ANXA1−/− mice. Croxtall et al. (2002) showed that in the human lung carcinoma cell line, A549, glucocorticoids reduced PGE2 biosynthesis by either inhibition of cPLA2 activity or inhibition of COX-2 protein induction, the former effect considered to be a non-genomic, and the latter, a genomic effect. Some of the drugs tested were able to do both (Croxtall et al., 2002). Methylprednisolone was shown to reduce cPLA2 activity, but did not inhibit COX-2 protein induction. Therefore, it is possible that direct inhibition of cPLA2 activity by methylprednisolone could explain its analgesic action in this model.
However, as we have shown that the analgesic action of methylpredinsolone is dependent on ANXA1, we postulate that methylprednisolone is more likely to be working through the increasing externalization of ANXA1. This phenomenon of exportation of stored ANXA1 through the plasma membrane from the intracellular pool of cytoplasmic ANXA1 into the pericellular pool (attached to the outer surface of the cell membrane) by glucocorticoids occurs within minutes and contributes to the anti-inflammatory effects of the glucocorticoids (Buckingham, 1996; Philip et al., 1997).
In an attempt to dissect out the mechanism by which ANXA1 reduces spinal cord PGE2 biosynthesis, we examined the expression of enzymes involved in the biosynthesis of PGE2. Within the spinal cord, the expression of cPLA2, COX-1, COX-2 and COX-3 proteins was higher in ANXA1−/− mice compared with ANXA1+/+ mice, which correlates with the observed pattern of PGE2 release. Thus, the higher levels of PGE2 in the spinal cords of ANXA1−/− mice could be the result of increased activity of these enzymes as a result of their increased expression. These results suggest that ANXA1 modulates spinal nociceptive processing by reducing activity of one or some of these enzymes. The current investigation does not provide evidence into which enzyme is inhibited by ANXA1; however, it has already been shown that ANXA1 reduces cPLA2 activity (Solito et al., 1998; Tommasini and Cantoni, 2004). The increased expression of cPLA2 is likely to be a consequence of ANXA1 deletion, as shown previously (Hannon et al., 2003).
In this model, we have already shown that both COX-1 and COX-3, but not COX-2, are involved in nociceptive transmission at the spinal level; hence, it is also possible that ANXA1 inhibits activity or synthesis of these enzymes (Ayoub et al., 2006).
The current findings demonstrate that ANXA1 plays an antinociceptive role within the spinal cord, which provides a novel drug target for the development of analgesics. In addition, the current study provides a possible mechanism for the analgesic action of the glucocorticoids, which are routinely given to patients with postoperative pain associated with procedures such as third molar extraction, tonsillectomy and breast augmentation (Schultze-Mosgau et al., 1995; Stewart et al., 2002; Bamgbose et al., 2005; Romundstad et al., 2006).
Acknowledgments
Financial support was provided by the Leverhulme Trust and the William Harvey Research Foundation (SSA), St Bartholomew's and the London Charitable Foundation (SY) and the Wellcome Trust (RJF). We thank Miss Prescilla Sawmynaden for technical assistance.
Abbreviations
- ANXA1
annexin-A1
- COX
cyclooxygenase
- cPLA2
cytosolic PLA2
- PGE2
prostaglandin E2
Conflict of interest
The authors state no conflict of interest.
References
- Ayoub SS, Colville-Nash PR, Willoughby DA, Botting RM. The involvement of a cyclooxygenase 1 gene-derived protein in the antinociceptive action of paracetamol in mice. Eur J Pharmacol. 2006;538:57–65. doi: 10.1016/j.ejphar.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Bamgbose BO, Akinwande JA, Adeyemo WL, Ladeinde AL, Arotiba GT, Ogunlewe MO. Effects of co-administered dexamethasone and diclofenac potassium on pain, swelling and trismus following third molar surgery. Head Face Med. 2005;1:11. doi: 10.1186/1746-160X-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton C, Elderfield AJ, Flower RJ. The detection of lipocortins 1, 2 and 5 in central nervous system tissues from Lewis rats with acute experimental allergic encephalomyelitis. J Neuroimmunol. 1990;29:173–181. doi: 10.1016/0165-5728(90)90160-o. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- British National Formulary 49 2005. Published by British Medical Association and Royal Pharmaceutical Society of Great Britain. ISSN: 0260-535X
- Buckingham JC. Fifteenth Gaddum Memorial Lecture December 1994. Stress and the neuroendocrine-immune axis: the pivotal role of glucocorticoids and lipocortin 1. Br J Pharmacol. 1996;118:1–19. doi: 10.1111/j.1476-5381.1996.tb15360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham JC. Glucocorticoids: exemplars of multi-tasking. Br J Pharmacol. 2006;47 Suppl 1:S258–S268. doi: 10.1038/sj.bjp.0706456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham JC, Flower RJ. Lipocortin 1: a second messenger of glucocorticoid action in the hypothalamo-pituitary-adrenocortical axis. Mol Med Today. 1997;3:296–302. doi: 10.1016/S1357-4310(97)88908-3. [DOI] [PubMed] [Google Scholar]
- Cavaillon JM. Cytokines in inflammation. C R Sciences Soc Biol Fil. 1995;189:531–544. [PubMed] [Google Scholar]
- Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure and expression. Proc Natl Acad Sci USA. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier HOJ, Dinneen LC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofford LJ. COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol. 1997;24:15–19. [PubMed] [Google Scholar]
- Croxtall JD, Choudhury Q, Newman S, Flower RJ. Lipocortin 1 and the control of cPLA2 activity in A549 cells. Glucocorticoids block EGF stimulation of cPLA2 phosphorylation. Biochem Pharmacol. 1996;52:351–356. doi: 10.1016/0006-2952(95)02442-5. [DOI] [PubMed] [Google Scholar]
- Croxtall JD, Flower RJ. Antisense oligonucleotides to human lipocortin-1 inhibit glucocorticoid-induced inhibition of A549 cell growth and eicosanoid release. Biochem Pharmacol. 1994;48:1729–1734. doi: 10.1016/0006-2952(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Croxtall JD, van Hal PT, Choudhury Q, Gilroy DW, Flower RJ. Different glucocorticoids vary in their genomic and non-genomic mechanism of action in A549 cells. Br J Pharmacol. 2002;135:511–519. doi: 10.1038/sj.bjp.0704474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J, Flower RJ, Milton AS, Peers SH, Rotondo D. Antipyretic actions of human recombinant lipocortin-1. Br J Pharmacol. 1991;102:7–9. doi: 10.1111/j.1476-5381.1991.tb12122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirig DM, Isakson PC, Yaksh TL. Effect of COX-1 and COX-2 inhibition on induction and maintenance of carrageenan-evoked thermal hyperalgesia in rats. J Pharmacol Exp Ther. 1998;285:1031–1038. [PubMed] [Google Scholar]
- Ferreira SH, Cunha FQ, Lorenzetti BB, Michelin MA, Perretti M, Flower RJ, et al. Role of lipocortin-1 in the anti-hyperalgesic actions of dexamethasone. Br J Pharmacol. 1997;121:883–888. doi: 10.1038/sj.bjp.0701211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SH, Moncada S, Vane JR. Proceedings: Potentiation by prostaglandins of the nociceptive activity of bradykinin in the dog knee joint. Br J Pharmacol. 1974;50:461P. [PMC free article] [PubMed] [Google Scholar]
- Green PG, Strausbaugh HJ, Levine JD. Annexin I is a local mediator in neural-endocrine feedback control of inflammation. J Neurophysiol. 1998;80:3120–3126. doi: 10.1152/jn.1998.80.6.3120. [DOI] [PubMed] [Google Scholar]
- Hay CH, de Belleroche JS. Dexamethasone prevents the induction of COX-2 mRNA and prostaglandins in the lumbar spinal cord following intraplantar FCA in parallel with inhibition of oedema. Neuropharmacology. 1998;37:739–744. doi: 10.1016/s0028-3908(98)00073-2. [DOI] [PubMed] [Google Scholar]
- Hannon R, Croxtall JD, Getting SJ, Roviezzo F, Yona S, Paul-Clark MJ, et al. Aberrant inflammation and resistance to glucocorticoids in annexin 1−/− mouse. FASEB J. 2003;17:253–255. doi: 10.1096/fj.02-0239fje. [DOI] [PubMed] [Google Scholar]
- Hori T, Oka M, Hosoi M, Abe M, Oka K. Hypothalamic mechanisms of pain modulatory actions of cytokines and prostaglandin E2. Ann NY Acad Sci. 2000;917:106–120. doi: 10.1111/j.1749-6632.2000.tb05375.x. [DOI] [PubMed] [Google Scholar]
- Kim DH, Jahng TA. Continuous brain-derived neurotrophic factor (BDNF) infusion after methylprednisolone treatment in severe spinal cord injury. J Korean Med Sci. 2004;19:113–122. doi: 10.3346/jkms.2004.19.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M, Kakishita H, Suzuki M, Banba N, Hattori Y. Dexamethasone suppresses iNOS gene expression by inhibiting NF-kappaB in vascular smooth muscle cells. Life Sci. 2001;69:1067–1077. doi: 10.1016/s0024-3205(01)01196-1. [DOI] [PubMed] [Google Scholar]
- Mozo L, Suarez A, Gutierrez C. Glucocorticoids up-regulate constitutive interleukin-10 production by human monocytes. Clin Exp Allergy. 2004;34:406–412. doi: 10.1111/j.1365-2222.2004.01824.x. [DOI] [PubMed] [Google Scholar]
- Parente L, Solito E. Association between glucocorticosteroids and lipocortin 1. Trends Pharmacol Sci. 1994;15:362. doi: 10.1016/0165-6147(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Pieretti S, Di Giannuario A, De Felice M, Perretti M, Cirino G. Stimulus-dependent specificity for annexin 1 inhibition of the inflammatory nociceptive response: the involvement of the receptor for formylated peptides. Pain. 2004;109:52–63. doi: 10.1016/j.pain.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Philip JG, Flower RJ, Buckingham JC. Glucocorticoids modulate the cellular disposition of lipocortin 1 in the rat brain in vivo and in vitro. Neuroreport. 1997;8:1871–1876. doi: 10.1097/00001756-199705260-00016. [DOI] [PubMed] [Google Scholar]
- Podgorski MR, Goulding NJ, Hall ND, Flower RJ, Maddison PJ. Autoantibodies to lipocortin-1 are associated with impaired glucocorticoid responsiveness in rheumatoid arthritis. J Rheumatol. 1992;19:1668–1671. [PubMed] [Google Scholar]
- Powell WS. Rapid extraction of oxygenated metabolites of arachidonic acid from biological samples using octadecylsilyl silica. Prostaglandins. 1980;20:947–957. doi: 10.1016/0090-6980(80)90144-6. [DOI] [PubMed] [Google Scholar]
- Romundstad L, Breivik H, Roald H, Skolleborg K, Haugen T, Narum J, et al. Methylprednisolone reduces pain, emesis, and fatigue after breast augmentation surgery: a single-dose, randomized, parallel-group study with methylprednisolone 125 mg, parecoxib 40 mg, and placebo. Anesth Analg. 2006;102:418–425. doi: 10.1213/01.ane.0000194358.46119.e1. [DOI] [PubMed] [Google Scholar]
- Saklatvala J. Glucocorticoids: do we know how they work. Arthritis Res. 2002;4:146–150. doi: 10.1186/ar398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze-Mosgau S, Schmelzeisen R, Frolich JC, Schmele H.Use of ibuprofen and methylprednisolone for the prevention of pain and swelling after removal of impacted third molars J Oral Maxillofac Surg 1995532–7.discussion 7–8 [DOI] [PubMed] [Google Scholar]
- Solito E, De Caterina R, Giannessi D, Paggiaro PL, Sicari R, Parente L. Studies on the induction of lipocortin-1 by glucocorticoids. Ann Ist Super Sanita. 1993;29:391–394. [PubMed] [Google Scholar]
- Solito E, Raguenes-Nicol C, de Coupade C, Bisagni-Faure A, Russo-Marie F. U937 cells deprived of endogenous annexin 1 demonstrate an increased PLA2 activity. Br J Pharmacol. 1998;124:1675–1683. doi: 10.1038/sj.bjp.0701991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R, Bill R, Ullah R, McConaghy P, Hall SJ. Dexamethasone reduces pain after tonsillectomy in adults. Clin Otolaryngol Allied Sci. 2002;27:321–326. doi: 10.1046/j.1365-2273.2002.00588.x. [DOI] [PubMed] [Google Scholar]
- Strijbos P, Tilders F, Carey F, Forder R, Rothwell N. Localization of lipocortin-1 in normal rat brain. Biochem Soc Trans. 1990;18:1234–1235. doi: 10.1042/bst0181234. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. Effects of cyclooxygenase products of arachidonic acid metabolism on cutaneous nociceptive threshold in the rat. Brain Res. 1990;537:372–374. doi: 10.1016/0006-8993(90)90389-s. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Appleton I, Moore AR, Gilroy DW, Willis D, Mitchell JA, et al. Cyclo-oxygenase and nitric oxide synthase isoforms in rat carrageenin-induced pleurisy. Br J Pharmacol. 1994;113:693–698. doi: 10.1111/j.1476-5381.1994.tb17048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasini I, Cantoni O. Dexamethasone promotes toxicity in U937 cells exposed to otherwise nontoxic concentrations of peroxynitrite: pivotal role for lipocortin 1-mediated inhibition of cytosolic phospholipase A2. Mol Pharmacol. 2004;65:964–972. doi: 10.1124/mol.65.4.964. [DOI] [PubMed] [Google Scholar]
- Willingale HL, Gardiner NJ, McLymont N, Giblett S, Grubb BD. Prostanoids synthesised by cyclo-oxygenase isoforms in the rat spinal cord and their contribution to the development of neuronal hyperexcitability. Br J Pharmacol. 1997;122:1593–1604. doi: 10.1038/sj.bjp.0701548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Chipman JG, Robertson DL, Erikson RL, Simmons DL. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci USA. 1991;88:2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N. Analysis of the roles of cyclooxygenase (COX)-1 and COX-2 spinal nociceptive transmission. Prog Pain Res Mngmnt. 1997;8:303–312. doi: 10.1016/s0006-8993(96)00817-7. [DOI] [PubMed] [Google Scholar]
- Yang Y, Leech M, Hutchinson P, Holdsworth SR, Morand EF. Antiinflammatory effect of lipocortin 1 in experimental arthritis. Inflammation. 1997;21:583–596. doi: 10.1023/a:1027330021479. [DOI] [PubMed] [Google Scholar]
- Yang YH, Morand EF, Getting SJ, Paul-Clark M, Liu DL, Yona S, et al. Modulation of inflammation and response to dexamethasone by Annexin 1 in antigen-induced arthritis. Arthritis Rheum. 2004;50:976–984. doi: 10.1002/art.20201. [DOI] [PubMed] [Google Scholar]